Abstract
Background
In contrast to the wealth of data on isolated systolic hypertension involving the systemic circulation in the elderly, much less is known about age-related change in pulmonary artery systolic pressure (PASP) and its prognostic impact in the general population. We sought to define the relationship between PASP and age, evaluate which factors influence PASP and determine if PASP is independently predictive of mortality in the community.
Methods and Results
A random sample of Olmsted County, MN general population (N=2042) underwent echocardiography and spirometry and was followed for a median of 9 years. PASP was measured from the tricuspid regurgitation velocity. Left ventricular diastolic pressure was estimated using Doppler echocardiography (E/e' ratio) and arterial stiffening was assessed using the brachial artery pulse pressure. Among 1413 (69%) subjects with measurable PASP (63±11y; 43% male), PASP (median, 25th-75th percentile) was 26 (24-30) mmHg and increased with age (r=0.31; p<0.001). Independent predictors of PASP were age, pulse pressure and mitral E/e' (all p≤0.003). Increasing PASP was associated with higher mortality (hazard ratio 2.73 per 10 mmHg; p<0.001). In subjects without cardiopulmonary disease (any heart failure, coronary artery disease, hypertension, diabetes mellitus or chronic obstructive lung disease), the age-adjusted hazard ratio was 2.74 per 10 mmHg (p=0.016).
Conclusions
We provide the first population-based evidence of age-related increase in pulmonary artery pressure, its association with increasing left heart diastolic pressures and systemic vascular stiffening, as well as its negative impact on survival. Pulmonary artery pressure may serve as a novel cardiovascular risk factor and potential therapeutic target.
Keywords: hypertension, pulmonary, population, aging, vasculature
In the systemic circulation, age-related vascular stiffening contributes to isolated systolic hypertension in the elderly, promoting increased risk of cardiovascular morbidity and mortality 1-5. However, less is known about age-related changes in pulmonary artery systolic pressure (PASP), its determinants, and any prognostic impact of elevated PASP in the general community. As in the systemic circulation6, the pulmonary vasculature may be affected by age-associated arterial remodeling7-13, leading to pulmonary vascular stiffening and increases in PASP. Pulmonary artery pressure is also directly affected by downstream left heart filling pressures, which may increase with age-related left ventricular diastolic dysfunction14. The increasing prevalence of cardiopulmonary diseases with age may also contribute to increases in PASP. Previous studies examining the association between age and PASP have produced conflicting results and were conducted in patients referred for cardiac catheterization or echocardiography, leading to referral bias15-20. Importantly, population-based data are lacking, and no studies have reported data on PASP and mortality in the community.
Accordingly, the objectives of this study were to define the relationship between age and PASP in a large cross-sectional sample of the general community; to identify the factors associated with PASP; and to assess if PASP is independently predictive of mortality.
Methods
This study was conducted in Olmsted County, MN, with the approval of the Mayo Foundation Institutional Review Board. All subjects provided written informed consent. The unique features of Olmsted County favoring population-based research have been described21. Data from this patient population has been published 5,14,22, but previous studies have not included analysis of pulmonary pressure or its association with outcome.
Study design and subject recruitment
Using the resources of the Rochester Epidemiology Project21, a random sample of the Olmsted County, MN population aged ≥45 years on January 1, 1997 was enrolled and studied over 3 years ending September 30, 2000. Of 4203 invitees, 2042 (47%) subjects participated. As previously described, participants were uniformly white and participation rates were similar in men and women and among persons with and without cardiovascular disease, but were lower in persons with lung disease23. Each subject underwent medical review, Doppler echocardiography and pulmonary function testing.
The following cardiopulmonary diagnoses were recorded by trained nurses using established criteria 14,24: heart failure, coronary artery disease, hypertension, diabetes mellitus or chronic obstructive lung disease. Participants underwent focused physical examination including measurement of height, weight and brachial artery blood pressure. Pulse pressure, calculated as the difference between brachial systolic and diastolic blood pressure, was used as an index of systemic arterial stiffness4. Systemic arterial stiffening results in the earlier return of reflected wave and thus, increased systolic and decreased diastolic blood pressure, such that pulse pressure is increased 6,25.
Doppler Echocardiography
All echocardiograms were performed by registered diagnostic cardiac sonographers using standardized instruments and protocols, and interpreted by an echocardiologist (M.M.R.) blinded to clinical data. All parameters were measured in triplicate and averaged. In addition to standard M-mode, 2-dimensional and color Doppler imaging, continuous-wave Doppler examination of tricuspid flow, pulsed-wave Doppler examination of mitral inflow and Doppler tissue imaging of the medial mitral annulus were performed in each subject14. Left ventricular ejection fraction and cardiac output were derived by standard methods14.
Determination of pulmonary artery pressures
PASP was estimated by Doppler echocardiography from the systolic right ventricular to right atrial pressure gradient using the modified Bernoulli equation (4 * peak tricuspid regurgitant velocity2). Right atrial pressure was assumed to be 5 mmHg and was then added to the calculated gradient to yield PASP. None of the subjects had significant right ventricular outflow tract obstruction. Echocardiographic estimates of PASP obtained in this fashion have been shown to correlate well with invasively measured values over a wide range of values (correlation coefficients ranging between 0.89 and 0.97) in our institution 26,27 and others 28,29.
Determination of left ventricular diastolic pressures
The ratio of early transmitral flow velocity (E) to early mitral annular (medial) tissue velocity (e') was used as an echo-derived estimate of left ventricular diastolic pressure30. This index (E/e') has been shown to reliably detect elevated left ventricular diastolic pressure in patients with elevated echo-derived PASP undergoing right heart catheterization31.
Pulmonary function testing
Spirometry was performed in accordance with recommended techniques32 using an automated pulmonary function testing system (MultiSpiro SX Spirometer with Mayo Health Station Software). Measurement of forced expiratory volume in one second and forced vital capacity were standardized as percentages of predicted normal values33.
Follow up
Subjects were followed from baseline echocardiography and spirometry at enrollment to death (all-cause mortality) or last contact, at which time they were censored. Vital status (March 2008) was determined from the Mayo Clinic registration database and the Rochester Epidemiology Project death database, where mortality data on Olmsted County residents are routinely collected by reviewing community medical records, death certificates, and obituary notices14.
Statistical methods
PASP was log transformed to satisfy normality assumptions. The associations of PASP with age, body mass index, pulse pressure, echo-derived left ventricular diastolic pressure (E/e' ratio), ejection fraction, and lung function from spirometry were investigated by calculating Pearson's correlation coefficients in univariate analyses. For multivariate analysis, linear regression was used, where the dependent variable was logarithm of PASP and factors entered into the model included age and other covariates. The standardized coefficient of each significant variable was expressed as the % change in PASP for each standard deviation change in the variable. Any interaction between variables was also evaluated and accounted for as appropriate. Comparisons among age quartile groups were performed by one-way analysis of variance (continuous variables) or Pearson's Chi-square test (categorical variables). The effect of increasing PASP on survival was assessed by Kaplan-Meier analysis comparing PASP groups of at least 150 subjects using Log Rank statistics. In addition to the overall Log Rank statistic, each PASP group was compared to the reference group using a Log Rank test with Bonferroni adjustment for multiple comparisons. The association of PASP with mortality was adjusted for age and other covariates (pulse pressure, ejection fraction, echo-estimated left ventricular diastolic pressure and spirometry values) using Cox regression analysis with stepwise modeling (probability for stepwise as calculated by the likelihood ratio test: entry=0.05; removal=0.10). Statistical significance (2-sided) was judged at P<0.05.
Results
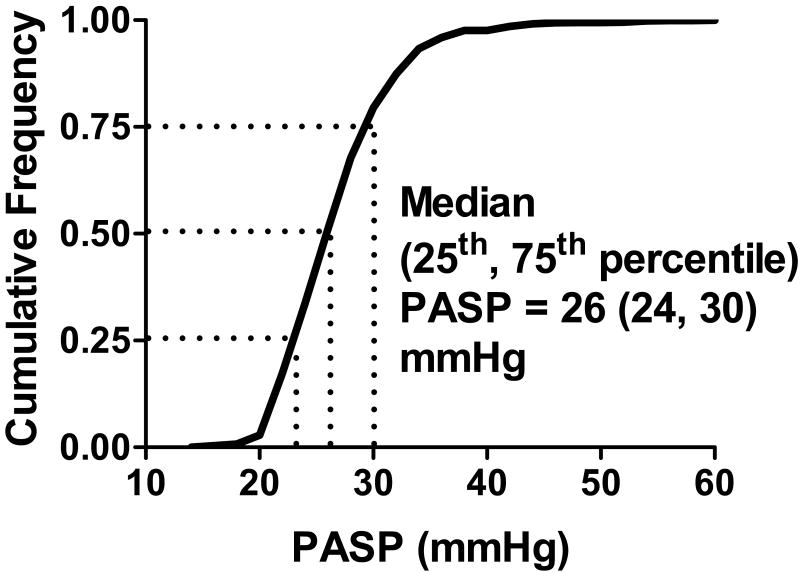
Tricuspid regurgitation jets were analyzable in 1413 (69%) subjects whose characteristics are summarized in Table 1. In these subjects, median (25th, 75th percentile) PASP was 26 (24, 30) mmHg (Figure 1).
Table 1. Characteristics of subjects with measurable PASP in the whole population.
| All | Quartiles of Age | P value* | ||||
|---|---|---|---|---|---|---|
| N | 1413 | 380 | 348 | 349 | 336 | |
| Age range, years | 45-96 | 45-54 | 55-62 | 63-71 | 72-96 | |
| Males, % | 43 | 44 | 44 | 46 | 37 | 0.106 |
| Height, m | 1.67±0.11 | 1.69±10.8 | 1.68±10.2 | 1.67±10.9 | 1.63±9.4 | <0.001 |
| Weight, kg | 77.2±16.2 | 79.2±17.2 | 79.4±16.6 | 78.0±15.1 | 72.0±14.7 | <0.001 |
| Body mass index, kg/m2 | 27.8±7.9 | 27.8±9.1 | 27.9±5.2 | 28.4±10.6 | 26.9±4.9 | 0.110 |
| Hypertension, % | 36 | 16 | 32 | 46 | 55 | <0.001 |
| Coronary artery disease, % | 14 | 2 | 8 | 20 | 28 | <0.001 |
| Diabetes mellitus, % | 7 | 3 | 3 | 10 | 11 | <0.001 |
| Heart failure, % | 3 | 1 | 1 | 1 | 8 | <0.001 |
| Chronic obstructive pulmonary disease, % | 4 | 1 | 2 | 4 | 7 | <0.001 |
| Medications, % | <0.001 | |||||
| • Beta-blockers | 17 | 7 | 14 | 24 | 23 | |
| • Calcium channel blockers | 7 | 2 | 5 | 9 | 13 | |
| • ACE inhibitors | 10 | 4 | 7 | 11 | 16 | |
| • Angiotensin receptor blockers | 2 | 1 | 1 | 2 | 4 | |
| • Diuretics | 18 | 7 | 13 | 18 | 34 | |
| Systemic blood pressure, mmHg | ||||||
| • Systolic | 131±22 | 121±15 | 128±19 | 135±21 | 143±25 | <0.001 |
| • Diastolic | 73±10 | 72±9 | 73±10 | 73±11 | 73±10 | 0.670 |
| • Pulse pressure | 59±18 | 49±12 | 56±14 | 62±16 | 70±20 | <0.001 |
| Heart rate, bpm | 65±11 | 64±9 | 65±10 | 64±11 | 67±12 | 0.008 |
| Doppler Echocardiography | ||||||
| Ejection fraction, % | 63±7 | 63±5 | 63±6 | 64±7 | 63±9 | 0.231 |
| Cardiac output, l/min | 5.6±1.4 | 5.5±1.2 | 5.6±1.3 | 5.7±1.4 | 5.7±1.5 | 0.355 |
| PASP, mmHg | 28±5 | 26±4 | 27±4 | 28±5 | 30±6 | <0.001 |
| Mitral E/e' ratio | 8.7±3.2 | 7.5±2.2 | 8.3±2.5 | 9.1±3.1 | 10.5±4.1 | <0.001 |
| Left atrial volume index, ml/m2 | 25.5±11.2 | 22.3±5.2 | 23.9±7.3 | 25.5±7.9 | 31.1±18.7 | <0.001 |
| Spirometry | ||||||
| Forced expiratory volume in one second, % predicted | 94±18 | 99±15 | 95±16 | 93±18 | 88±21 | <0.001 |
| Forced vital capacity, % predicted | 97±16 | 100±14 | 98±14 | 96±16 | 90±18 | <0.001 |
| Forced expiratory volume to vital capacity ratio, % | 77±8 | 79±6 | 77±7 | 77±8 | 76±10 | <0.001 |
p value for comparison among age quartiles
Data are mean±SD
PASP, pulmonary artery systolic pressure
Figure 1. Distribution of pulmonary artery systolic pressure in the general community.
The cumulative frequency distribution of pulmonary artery systolic pressure (PASP) in the population is shown. Median (25th, 75th percentile) PASP was 26 (24, 30) mmHg.
Compared to participants in whom jets could not be analyzed, those analyzed were older (61±10 vs 63±11 years; p<0.001), more often female (40 vs 57%; p<0.001), had lower body mass index (30.5±6.0 vs 27.8±7.9 kg/m2, p<0.001), were less likely to have diabetes (10 vs 7%; p=0.012), hypertension (45 vs 36%; p<0.001) or chronic obstructive lung disease (19 vs 14%; p=0.010), and were similarly likely to have coronary artery disease (13 vs 14%; p=0.43) or heart failure (2 vs 3%; p=0.15). Survival was similar in subjects with, versus subjects without, measurable PASP (p=0.69).
Association of PASP and brachial artery systolic blood pressure with age
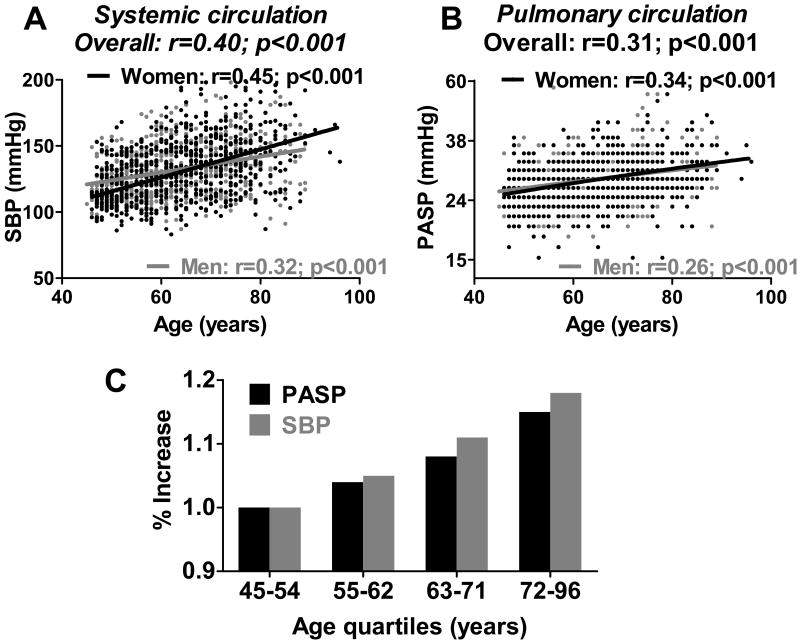
Consistent with prior studies 1-5, systolic blood pressure in the systemic circulation increased with age in the population (r=0.40; p<0.001) (Figure 2A). In the pulmonary circulation, PASP similarly increased with age (r=0.31; p<0.001) in both men and women (Figure 2B). Across age quartiles (Table 1), the absolute increase in PASP was smaller in the lower-pressured pulmonary circulation compared to that in the higher-pressured systemic circulation. To account for the baseline pressure differences in the two circulations (Figure 2C), the % increase in systolic blood pressure with age relative to the youngest quartile was calculated and found to be strikingly similar in the pulmonary and systemic circulations.
Figure 2. Association of systemic and pulmonary arterial systolic pressures with age.
In the systemic circulation (A), brachial artery systolic blood pressure (SBP) increased with age. In the pulmonary circulation (B), pulmonary artery systolic pressure (PASP) similarly increased with age in men (gray) and women (black) in the population. For each association, raw data points, linear regression line, Pearson's correlation coefficient, and probability value for the association are shown. Across age quartiles (C), the % increase in SBP (gray) and PASP (black) relative to the youngest quartile was strikingly similar in the systemic and pulmonary circulations.
Adjusting for age, there was no sex-related difference in PASP (p=0.41). PASP also correlated weakly with body mass index (r=0.07; p=0.008), even after adjusting for age (p=0.001).
Association of PASP with diastolic dysfunction and arterial stiffening
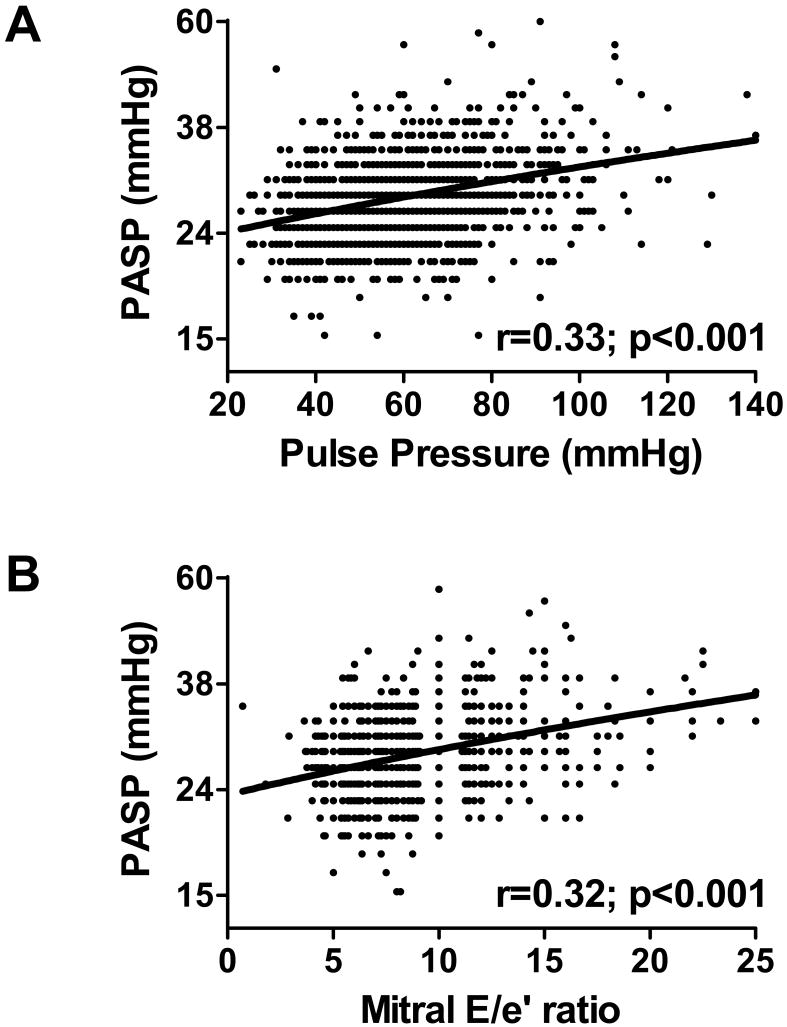
PASP increased with increasing pulse pressure (r=0.33; p<0.001) (Figure 3A) and increasing left ventricular diastolic pressure (r=0.32; p<0.001) (Figure 3B), even after adjusting for age (age-adjusted p<0.001 for both). There was no interaction between age and pulse pressure (p=0.58) or age and left ventricular diastolic pressure (p=0.07), implying a similar effect of pulse pressure or left ventricular diastolic pressure on PASP at all ages. Adjusting for medication use did not affect these results (not shown). PASP also varied directly albeit modestly with cardiac output (r=0.11; p<0.001), and inversely with forced expiratory volume (r=-0.17; p<0.001) and forced vital capacity (r=-0.20; p<0.001). There was no correlation with ejection fraction (p=0.18).
Figure 3. Association of pulmonary artery systolic pressure with systemic arterial stiffening and left ventricular diastolic dysfunction.
Pulmonary artery systolic pressure (PASP, shown in log scale) increased in association with evidence of systemic vascular stiffening (increasing pulse pressure, shown in A) and increasing diastolic pressures (increasing mitral E/e' ratio shown in B). For each association, raw data points, linear regression line, Pearson's correlation coefficient, and probability value for the association are shown.
In multivariate analysis, PASP was independently associated with age (28% increase for each 10.6 years; p=0.003), pulse pressure (42% increase for each 17.5 mmHg; p<0.001) and echo-estimated left ventricular diastolic pressure (58% increase for each 3.2 units of the mitral E/e' ratio; p<0.001), but not body mass index or lung function. There was no interaction between pulse pressure and left ventricular diastolic pressure (p=0.14). These associations remained highly significant (p<0.001) after adjusting for cardiac output. This suggests that age, vascular stiffening and diastolic dysfunction each contributed independently to increasing PASP in the population.
Subset of persons without cardiopulmonary disease
Cardiopulmonary disease, defined as the presence of any heart failure, coronary artery disease, hypertension, diabetes mellitus or chronic obstructive lung disease, was identified in 635 persons using established criteria14,24. When analysis was restricted to subjects without any cardiopulmonary disease (N=778), median (25th, 75th percentile) PASP was 26 (24, 30). PASP increased across age quartiles (Table 2), as did indices of systemic arterial stiffening, elevated left sided filling pressures and decreasing lung function, although absolute changes were smaller compared to findings in the whole population.
Table 2. Characteristics of subjects without cardiopulmonary disease.
| All | Quartiles of Age | P value* | ||||
|---|---|---|---|---|---|---|
| N | 778 | 173 | 201 | 199 | 205 | |
| Age range, years Systemic blood pressure, mmHg | 45-96 | 45-51 | 52-57 | 58-65 | 66-96 | |
| Systemic blood pressure, mmHg | ||||||
| • Systolic | 125±18 (86-198) |
117±12 (93-149) |
121±16 (86-155) |
125±17 (91-179) |
134±21 (90-198) |
<0.001 |
| • Diastolic | 71±9 (38-98) |
71±8 (38-84) |
72±9 (50-95) |
72±10 (45-98) |
72±10 (45-98) |
0.839 |
| • Pulse pressure | 53±15 (23-120) |
46±11 (23-82) |
50±12 (25-82) |
54±14 (25-99) |
63±16 (32-120) |
<0.001 |
| Heart rate, bpm | 65±10 (39-110) |
63±9 (39-93) |
65±9 (42-92) |
65±9 (44-98) |
67±12 (44-110) |
0.001 |
| Doppler Echocardiography | ||||||
| Ejection fraction, % | 64±5 (38-80) |
63±5 (48-75) |
63±5 (38-80) |
64±5 (43-75) |
65±6 (38-78) |
<0.001 |
| Cardiac output, l/min | 5.5±1.3 (2.3-10.9) |
5.5±1.3 (3.1-10.6) |
5.3±1.1 (3.0-8.8) |
5.6±1.3 (3.2-10.9) |
5.5±1.4 (2.3-10.9) |
0.164 |
| PASP, mmHg | 27±4 (15-57) |
26±4 (17-39) |
26±4 (15-57) |
27±4 (17-41) |
28±5 (15-43) |
<0.001 |
| Mitral E/e' ratio | 8.1±2.6 (1.8-22.5) |
7.2±1.8 (1.8-14.3) |
7.4±2.2 (3.8-16.0) |
8.1±2.5 (3.7-22.0) |
9.4±3.1 (3.6-22.5) |
<0.001 |
| Left atrial volume index, ml/m2 | 23.1±6.5 (10.4-70.5) |
22.4±5.2 (10.4-37.1) |
22.0±5.4 (11.1-48.5) |
23.1±6.7 (10.3-70.5) |
24.9±7.9 (11.6-69.0) |
<0.001 |
| Spirometry | ||||||
| Forced expiratory volume in one second, % predicted | 98±15 (47-175) |
100±16 (51-175) |
99±13 (51-135) |
97±16 (60-140) |
95±16 (47-139) |
0.002 |
| Forced vital capacity, % predicted | 100±15 (50-190) |
102±15 (72-190) |
102±12 (62-132) |
101±14 (64-149) |
96±16 (50-139) |
<0.001 |
| Forced expiratory volume to vital capacity ratio, % | 78±7 (51-94) |
79±6 (51-90) |
78±6 (63-94) |
77±7 (54-94) |
77±7 (56-94) |
0.002 |
p value for comparison among age quartiles
Data are mean±SD (range)
PASP, pulmonary artery systolic pressure
In univariate analysis, PASP still increased with age (r=0.25; p<0.001) and was positively correlated with pulse pressure (r=0.28; p<0.001), echo-estimated left ventricular diastolic pressure (r=0.25; p<0.001) and cardiac output (r=0.17; p<0.001); and negatively correlated, albeit weakly, with forced expiratory volume (r=-0.08; p=0.035) and forced vital capacity (r=-0.12; p=0.001). In multivariate analysis, similar to results in the whole population, age (38% increase for each 9.6 years; p=0.001), pulse pressure (38% increase for each 14.4 mmHg increase; p=0.001) and left ventricular end diastolic pressure (37% increase for each 2.6 units of the mitral E/e' ratio; p=0.001) were significant predictors of PASP, even after adjusting for cardiac output. None of the interaction terms were significant.
Association of PASP with all-cause mortality
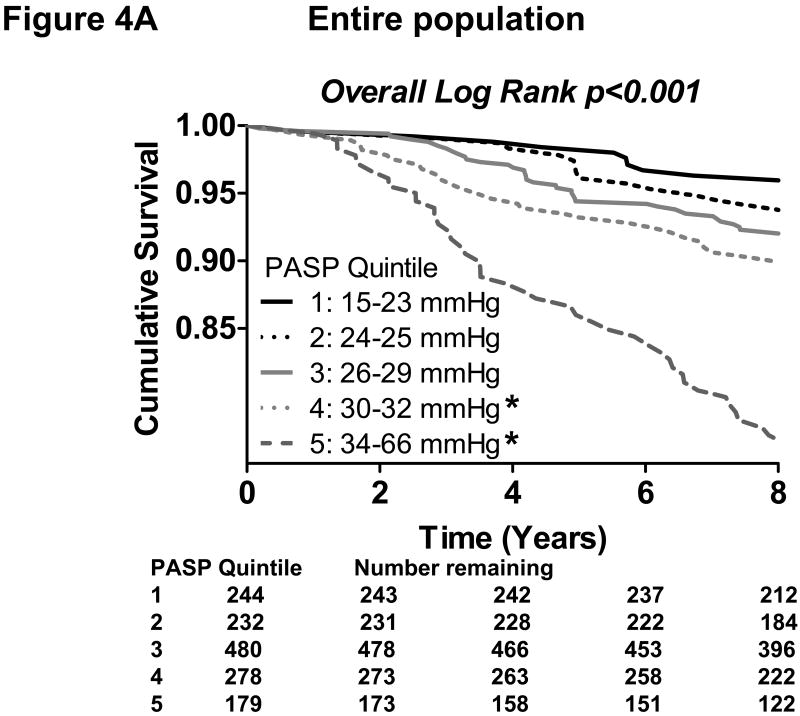
In the entire population, a total of 155 deaths occurred during a median follow up of 9.0 years. By Kaplan-Meier analysis, increasing quintiles of PASP predicted poorer survival in the general population (p<0.001). The pattern of increasing mortality risk was observed across the lower four quintiles and most strikingly in the highest two quintiles (Figure 4A). By Cox regression analysis, increasing PASP was strongly associated with mortality (unadjusted hazard ratio= 2.73 per 10 mmHg; p<0.001). When PASP, age, pulse pressure, ejection fraction, echo-estimated left ventricular diastolic pressure and spirometry values were included in stepwise modeling, PASP remained an independent predictor of mortality (adjusted hazard ratio=1.46 per 10 mmHg; p=0.017), along with age, pulse pressure, ejection fraction and forced expiratory volume. Forward and backward stepwise modeling gave the same results.
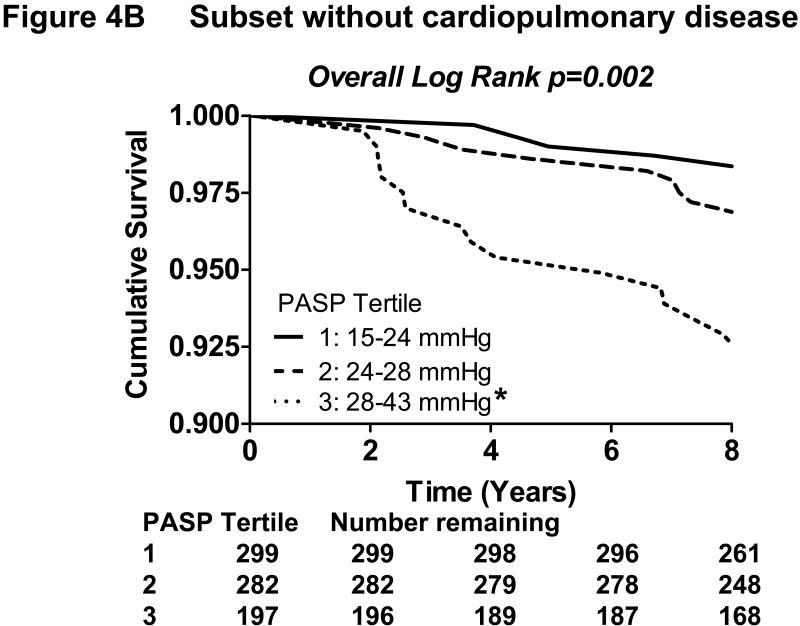
Figure 4. Kaplan-Meier curves showing the association of increasing pulmonary artery systolic pressure with decreasing survival in the community.
In the entire population (A), increasing quintiles of pulmonary artery systolic pressure (PASP) was associated with reduced survival by Kaplan-Meier analysis (overall Log Rank p<0.001). *Bonferroni-adjusted p<0.05 in pairwise comparison to the lowest quintile.
In the subset of the population without cardiopulmonary disease (B), increasing tertiles of PASP was similarly associated with reduced survival (overall Log Rank p=0.002). *Bonferroni-adjusted p<0.05 in pairwise comparison to the lowest tertile.
In the subset without cardiopulmonary disease, there were 36 deaths over a median follow up of 9.0 years. By Kaplan-Meier analysis, increasing tertiles of PASP similarly predicted poorer survival (p=0.002) (Figure 4B). Increasing PASP was also strongly associated with mortality by Cox regression analysis (unadjusted hazard ratio= 4.65 per 10 mmHg; p<0.001) and remained an independent predictor, along with age, in stepwise modeling (adjusted hazard ratio=2.74 per 10 mmHg; p=0.016). Forward and backward stepwise modeling yielded the same results.
Discussion
These are the first population-based data showing that pulmonary artery pressures increase with age in subjects from the general community. The increase in pulmonary artery pressure was coupled with increases in pulse pressure and estimated left heart filling pressures, suggesting that age-associated blood vessel stiffening and diastolic dysfunction contribute to changes in pulmonary artery pressure. Importantly, increasing pulmonary artery systolic pressure was associated with increased all-cause mortality, independent of both age and the presence of clinically-evident cardiopulmonary disease. As with systemic arterial hypertension, increasing blood pressure in the pulmonary circulation may serve as a cardiovascular risk factor and potentially, a novel therapeutic target.
Pulmonary artery pressure is determined by the amount of blood flowing through the pulmonary circulation (cardiac output), the intrinsic properties of the vasculature (resistance, compliance and impedance), and the left atrial pressure downstream of the pulmonary circuit (left ventricular diastolic pressure). Similar to the systemic circulation, high output states and vascular stiffening may contribute to increases in systolic pressure in the pulmonary circulation. In contrast to the systemic circulation where downstream venous pressure is very low compared with arterial pressure, pulmonary venous pressure is much greater in relation to pulmonary arterial pressures. Hence increases in pulmonary venous pressures, determined primarily by left ventricular diastolic function, may also lead to increases in PASP. Further, a reduction in left atrial compliance, as may occur in advanced left ventricular diastolic dysfunction 34, can also contribute to increasing PASP independent of left ventricular filling pressures 35.
Previous cardiac catheterization-based studies have generally shown increasing pulmonary artery pressures at rest and/or with exercise among older aged volunteers compared with younger subjects15-19, but these studies were relatively small-sized and subject to referral bias, as participants had by definition all been referred for cardiac catheterization. More recently, McQuillan et al20 reported echo-derived PASP values in 3790 normal subjects identified from a database of patients referred for echocardiography. In this large series, mean PASP was 28±5 mmHg and increased with age and body mass, similar to the current findings. Although left heart filling pressures were not formally assessed, the authors noted a direct relationship between PASP and left ventricular wall thickness and left atrial diameter, suggesting an association with diastolic dysfunction and elevated left heart filling pressures. However, similar to earlier cath-lab based studies, this series was limited by selection bias, as acknowledged by the authors in their call for population-based studies 19,20. The current data therefore extend the previous by providing the first population-based evidence of age-associated increases in pulmonary artery pressures and its association with age-dependent diastolic dysfunction in the general population.
This is also the first study to show an association between PASP and mortality in the general population. The age-associated increases in PASP appears analogous to increases in systemic systolic blood pressure with aging 1,3-5,25 – a phenomenon which is largely related to blood vessel stiffening in conduit arteries such as the aorta. Just as “isolated systolic hypertension” confers greater risk for cardiovascular events and mortality even with relatively small increases in systolic blood pressure 2, we show that even minor elevations in pulmonary artery pressure also predict greater risk. While the relative accuracy of echo PASP measurement compared to brachial blood pressure measurement must be considered, it is notable that the relative increase in pulmonary and systemic pressures across age quartiles was strikingly similar after accounting for the baseline pressure differences between the two circulations. Further, PASP may have been underestimated in the community due to the lower participation rates and lower feasibility of PASP measurements in persons with lung disease, as well as the uniform assignment of a right atrial pressure of 5 mmHg. While this minimum assumed right atrial pressure is appropriate for most community-dwelling adults with normal PASP, it may be inappropriately low for subjects with elevated pulmonary pressures. The current data cannot discern how much of the age-dependent increase in PASP was related to changes in vascular tone (resistive load) compared with vascular stiffening. In the systemic circulation, mean vascular resistance remains stable with aging5, and blood pressure elevations are predominantly related more to vascular stiffening. Age-associated arterial remodeling has similarly been reported in the pulmonary vasculature7-13, and the significant association between PASP and brachial pulse pressure noted in the current study supports the notion that blood vessel stiffening processes with aging contribute to the increase in blood pressure in both pulmonary and systemic vascular beds.
Besides being a marker of diastolic dysfunction and vascular stiffening, the independent association of PASP with increased mortality suggests that PASP could ultimately prove to be a novel cardiovascular risk factor. Whether or how elevations in pulmonary artery pressure should be treated in such patients remains unknown, but given the importance of diastolic dysfunction, it is likely that treatments will need to focus on both the left heart as well as the pulmonary vasculature. These notions merit further study. The ease of assessing PASP and pulmonary venous hypertension non-invasively, the availability of an increasing number pulmonary vasculature-modifying drugs 36, and ongoing trials involving agents targeting both diastolic dysfunction as well as pulmonary hypertension (Phosphodiesterase-5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure or RELAX trial; http://clinicaltrials.gov), all suggest that this may become possible.
Strengths and limitations
Strengths of this study include the population-based approach, comprehensive echocardiographic characterization of diastolic function, uniform assessment of pulmonary function by spirometry and outcome assessment in a large sample of the general community. The large number of subjects provided statistical power to detect even weaker (r<0.2) associations of potential physiological significance. Well-known examples of mathematically modest yet clinically important associations include the correlation between severity or duration of systemic hypertension and degree of left ventricular hypertrophy 37. In the current study, the large number of events also allowed adjustment for multiple covariates to yield clinically meaningful and robust results.
While Doppler estimates of PASP correlate excellently with invasive measures, systematic overestimation may occur in low risk populations 38 and invasive hemodynamic measurements remain the gold standard for verification of pulmonary hypertension. However, this study could not have been performed using an invasive approach. The contribution of hypoxic pulmonary vasoconstriction to increases in PASP in the population cannot be ascertained from these data, although we postulate that in this community sample the contribution was small. We similarly lack data regarding potential genetic or molecular determinants of pulmonary pressures. The single time point measurement in this study preclude the evaluation of time dependent covariance in survival analysis.
Conclusions
We provide the first population-based evidence of age-associated increases in pulmonary artery pressure, document the adverse prognostic implications of elevated pulmonary artery pressures in the general population and provide data suggesting that increasing pulmonary artery pressure with age is related, at least in part, to age-associated vascular stiffening and pulmonary venous hypertension from left ventricular diastolic dysfunction. These findings need to be confirmed in other populations, and if proven, suggest that PASP may be a novel cardiovascular risk factor. The observation of increasing mortality provides rationale for future investigations into treatments targeting factors which lead to increased pulmonary artery pressure.
Acknowledgments
Funding Sources: Funding provided in part by NIH grant HL 55502 (Rodeheffer) and HL 63281 (Redfield).
Footnotes
Disclosures: None.
Clinical Commentary: In contrast to the wealth of data on isolated systolic hypertension involving the systemic circulation in the elderly, much less is known about age-related change in pulmonary artery systolic pressure and its prognostic impact in the general population. Similar to the systemic circulation, vascular stiffening may contribute to increases in systolic pressure in the pulmonary circulation. Unlike the systemic circulation where downstream venous pressure is very low compared with arterial pressure, pulmonary venous pressure is much greater in relation to pulmonary arterial pressures. Hence increases in pulmonary venous pressures, determined primarily by left ventricular diastolic function, may also lead to increases in pulmonary arterial pressures. We provide the first population-based data showing that pulmonary artery pressures increase with age in subjects from the general community. The increase in pulmonary artery pressure was coupled with increases in pulse pressure and estimated left heart filling pressures, suggesting that age-associated blood vessel stiffening and diastolic dysfunction contribute to changes in pulmonary artery pressure. Importantly, increasing pulmonary artery systolic pressure was associated with increased all-cause mortality, independent of both age and the presence of clinically-evident cardiopulmonary disease. These findings need to be confirmed in other populations, and if proven, suggest that pulmonary artery systolic pressure may be a novel cardiovascular risk factor. The observation of increasing mortality provides rationale for future investigations into treatments targeting factors which lead to increased pulmonary artery pressure.
References
- 1.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–7. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 2.Perry HM, Jr, Davis BR, Price TR, Applegate WB, Fields WS, Guralnik JM, Kuller L, Pressel S, Stamler J, Probstfield JL. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the Systolic Hypertension in the Elderly Program (SHEP) Jama. 2000;284:465–71. doi: 10.1001/jama.284.4.465. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 5.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 6.Nichols WW, O'Rourke M. McDonald's Blood Flow in Arteries. Third. Philadelphia, PA: Lea & Febiger; 1990. [Google Scholar]
- 7.Lansing AI, Rosenthal TB, Alex M. Significance of medial age changes in the human pulmonary artery. J Gerontol. 1950;5:211–5. doi: 10.1093/geronj/5.3.211. [DOI] [PubMed] [Google Scholar]
- 8.Castillo Y, Kruger H, Arias-Stella J, Hurtado A, Harris P, Heath D. Histology, extensibility, and chemical composition of pulmonary trunk in persons living at sea-level and at high altitude in Peru. Br Heart J. 1967;29:120–8. doi: 10.1136/hrt.29.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984;35:615–21. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- 10.Plank L, James J, Wagenvoort CA. Caliber and elastin content of the pulmonary trunk. Arch Pathol Lab Med. 1980;104:238–41. [PubMed] [Google Scholar]
- 11.Harris P, Heath D, Apostolopoulos A. Extensibility of the human pulmonary trunk. Br Heart J. 1965;27:651–9. doi: 10.1136/hrt.27.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrar JF, Blomfield J, Reye RD. The structure and composition of the maturing pulmonary circulation. J Pathol Bacteriol. 1965;90:83–96. doi: 10.1002/path.1700900109. [DOI] [PubMed] [Google Scholar]
- 13.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax. 1978;33:335–44. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 15.Granath A, Jonsson B, Strandell T. Circulation in Healthy Old Men, Studied by Right Heart Catheterization at Rest and During Exercise in Supine and Sitting Position. Acta Med Scand. 1964;176:425–46. doi: 10.1111/j.0954-6820.1964.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 16.Emirgil C, Sobol BJ, Campodonico S, Herbert WH, Mechkati R. Pulmonary circulation in the aged. J Appl Physiol. 1967;23:631–40. doi: 10.1152/jappl.1967.23.5.631. [DOI] [PubMed] [Google Scholar]
- 17.Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clin Sci (Lond) 1983;65:653–60. doi: 10.1042/cs0650653. [DOI] [PubMed] [Google Scholar]
- 18.Davidson WR, Jr, Fee EC. Influence of aging on pulmonary hemodynamics in a population free of coronary artery disease. Am J Cardiol. 1990;65:1454–8. doi: 10.1016/0002-9149(90)91354-9. [DOI] [PubMed] [Google Scholar]
- 19.Ghali JK, Liao Y, Cooper RS, Cao G. Changes in pulmonary hemodynamics with aging in a predominantly hypertensive population. Am J Cardiol. 1992;70:367–70. doi: 10.1016/0002-9149(92)90621-5. [DOI] [PubMed] [Google Scholar]
- 20.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 22.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Annals of Epidemiology. 2004;14:579–84. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 25.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–9. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 26.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–6. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 27.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9:549–54. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 28.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 29.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–65. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 30.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 31.Willens HJ, Chirinos JA, Gomez-Marin O, Fertel DP, Ghany RA, Alfonso CE, Hare JM. Noninvasive differentiation of pulmonary arterial and venous hypertension using conventional and Doppler tissue imaging echocardiography. J Am Soc Echocardiogr. 2008;21:715–9. doi: 10.1016/j.echo.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 33.Miller A, Thornton JC, Warshaw R, Bernstein J, Selikoff IJ, Teirstein AS. Mean and instantaneous expiratory flows, FVC and FEV1: prediction equations from a probability sample of Michigan, a large industrial state. Bull Eur Physiopathol Respir. 1986;22:589–97. [PubMed] [Google Scholar]
- 34.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 35.Mehta S, Charbonneau F, Fitchett DH, Marpole DG, Patton R, Sniderman AD. The clinical consequences of a stiff left atrium. Am Heart J. 1991;122:1184–91. doi: 10.1016/0002-8703(91)90498-7. [DOI] [PubMed] [Google Scholar]
- 36.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–28. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 37.Khattar RS, Acharya DU, Kinsey C, Senior R, Lahiri A. Longitudinal association of ambulatory pulse pressure with left ventricular mass and vascular hypertrophy in essential hypertension. J Hypertens. 1997;15:737–43. doi: 10.1097/00004872-199715070-00005. [DOI] [PubMed] [Google Scholar]
- 38.Brecker SJ, Gibbs JS, Fox KM, Yacoub MH, Gibson DG. Comparison of Doppler derived haemodynamic variables and simultaneous high fidelity pressure measurements in severe pulmonary hypertension. Br Heart J. 1994;72:384–9. doi: 10.1136/hrt.72.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]