Abstract
Calorie restriction extends lifespan and produces a metabolic profile desirable for treating diseases of ageing such as type 2 diabetes1,2. SIRT1, an NAD+-dependent deacetylase, is a principal modulator of pathways downstream of calorie restriction that produce beneficial effects on glucose homeostasis and insulin sensitivity3–9. Resveratrol, a polyphenolic SIRT1 activator, mimics the anti-ageing effects of calorie restriction in lower organisms and in mice fed a high-fat diet ameliorates insulin resistance, increases mitochondrial content, and prolongs survival10–14. Here we describe the identification and characterization of small molecule activators of SIRT1 that are structurally unrelated to, and 1,000-fold more potent than, resveratrol. These compounds bind to the SIRT1 enzyme—peptide substrate complex at an allosteric site amino-terminal to the catalytic domain and lower the Michaelis constant for acetylated substrates. In diet-induced obese and genetically obese mice, these compounds improve insulin sensitivity, lower plasma glucose, and increase mitochondrial capacity. In Zucker fa/fa rats, hyperinsulinaemic-euglycaemic clamp studies demonstrate that SIRT1 activators improve whole-body glucose homeostasis and insulin sensitivity in adipose tissue, skeletal muscle and liver. Thus, SIRT1 activation is a promising new therapeutic approach for treating diseases of ageing such as type 2 diabetes.
To identify activators of human SIRT1 a high-throughput in vitro fluorescence polarization assay was developed and used to screen a large collection of small molecules. The hits identified were structurally distinct from resveratrol11 and subsequently optimized for in vitro enzyme activity using a high-throughput mass spectrometry assay. Potency was tracked by determining the concentration of compound required to increase enzyme activity by 50% (EC1.5) and the percentage maximum activation achieved at the highest doses of compound tested (resveratrol EC1.5 = 46.2 μM and maximum activation = 201%; SRT1460 EC1.5 = 2.9 μM and maximum activation = 447%; SRT2183 EC1.5 = 0.36 μM and maximum activation = 296%; and SRT1720 EC1.5 = 0.16 μM and maximum activation = 781%) (Fig. 1a, b). The variation in maximum activation is reminiscent of agonist activity observed with receptors, where compounds can vary in their ability to mediate full receptor activation as compared to an endogenous ligand15. These compounds are selective for activation of SIRT1 versus the closest sirtuin homologues, SIRT2 and SIRT3 (SIRT2: resveratrol, SRT1460 and SRT2183 EC1.5 > 300 μM, SRT1720 EC1.5 = 37 μM; SIRT3: resveratrol, SRT1460, SRT1720 and SRT2183 EC1.5 > 300 μM).
Figure 1. Identification of potent SIRT1 activators unrelated to resveratrol.
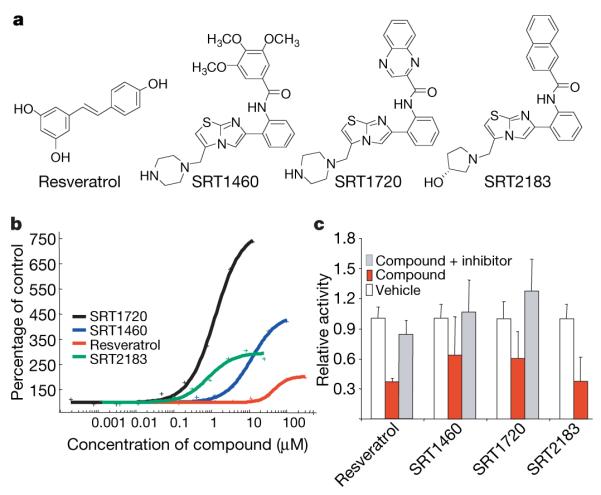
a, Chemical structures of SIRT1 activators, resveratrol, SRT1460, SRT2183, and SRT1720. b, The effect of activators on human SIRT1 enzyme activity measured by mass spectrometry. c, Cellular activity was measured using an ICW that monitors the degree of p53 deacetylation in U2OS cells using β-tubulin as a normalization control. Compounds were tested at concentrations of: resveratrol (100 μM), SRT2183 (10 μM), SRT1460 (10 μM), SRT1720 (0.10 μM). Each concentration represents the approximate EC50 for each compound. n = 6 for all compounds tested except for resveratrol, where n = 3. Data are expressed as mean ± s.d. The activation of SIRT1 resulting in p53 deacetylation could be blocked by a SIRT1 inhibitor, 6-chloro-2,3,4,9-tetrahydro-1-H-carbazole-1-carboxamide (10 μM). n = 3 for all compounds tested.
The compounds were tested for functional activity in a SIRT1 cell-based (U2OS cells) deacetylation assay (Fig. 1c)16,17. SRT2183 was used as a positive control because it is well tolerated in cellular assays. The SIRT1 activators decreased the acetylation state of p53, a known SIRT1 substrate. A selective SIRT1 inhibitor, 6-chloro-2,3,4,9-tetra-hydro-1-H-carbazole-1-carboxamide18, abrogated the deacetylation activity, indicating that the effect was dependent on SIRT1.
The Sir2 gene in Saccharomyces cerevisiae and Drosophila melanogaster extends lifespan when overexpressed19,20, and the ability of calorie restriction to extend lifespan is abrogated when the gene is deleted20–22. We have observed lifespan extension in yeast with analogues structurally related to SRT1460 that activate the yeast SIR2 enzyme (data not shown).
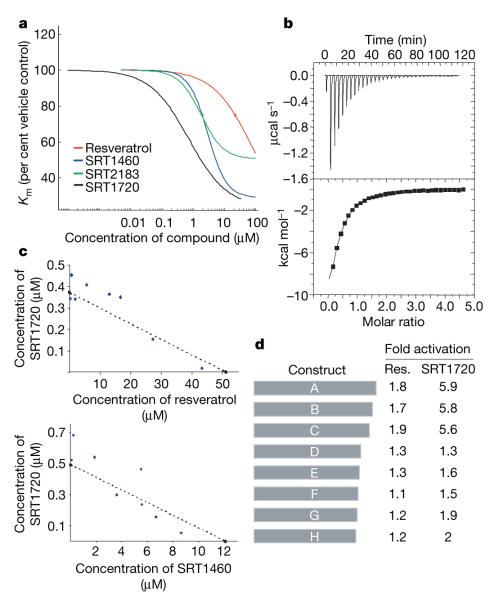
To address the mechanism of activation of the human SIRT1 enzyme, we determined the effect of compound on the Vmax (velocity of enzyme-catalysed reaction at infinite concentration of substrate) and the Km (Michaelis constant) of SIRT1 for its two substrates, NAD+ and acetylated peptide. None of the compounds affected the Km for NAD+ or the Vmax (data not shown); however, all compounds decreased the Km of SIRT1 for acetylated peptide substrate (Fig. 2a). The magnitude of the Km effect positively correlated with the EC1.5 values for the compounds. A similar Km-type activation mechanism has been proposed for small molecule activators that bind to an allosteric site of glucokinase23.
Figure 2. In vitro characterization of activators of human SIRT1.
a, The effect of SIRT1 activators on peptide substrate Km. b, Calorimetric titrations of SIRT1-C—peptide substrate complex with the activator SRT1460. Top panel: heat of binding SRT1460 to enzyme—peptide complex. Bottom panel: integrated fit with a one-site binding model. c, Isobologram analysis of resveratrol versus SRT1720 and SRT1720 versus SRT1460. The experimental data are best fit to the theoretical line of additivity (dashed line). d, SIRT1 N-terminal truncations define the allosteric compound binding site. The ability of resveratrol and SRT1720 to activate SIRT1 was examined against a series of N-terminal deletions in the mass spectrometry assay.
The energetics of binding of SRT1460 to purified SIRT1 enzyme were studied by isothermal titration calorimetry. The titration of SRT1460 against purified human SIRT1-C did not result in detectable binding (data not shown). In the presence of acetylated peptide substrate SRT1460 exhibited signs of protein-binding-site saturation best fitting a one site binding model (Fig. 2b). The dissociation constant (Kd) for SRT1460 was 16.2 μM and the enthalpy (ΔH) was −6.1 kcal mol−1. The data suggest that SRT1460, and other related SIRT1 activators, bind to a SIRT1—peptide substrate complex and promote a more productive conformation that enhances catalytic activity. Binding of substrate may induce a conformational change that leads to the exposure of an allosteric binding site. In support of this hypothesis, it has been demonstrated that NAD+ does not bind in the absence of acetylated peptide substrate to a closely related sirtuin, SIRT2 (ref. 24). A Km-type mechanism is supported by isothermal titration calorimetry experiments that show a roughly twofold increase in the affinity for peptide substrate binding to SIRT1-C in the presence of SRT1460 (Kd(-SRT1460) = 25.7 μM and Kd(+SRT1460) = 14.1 μM; data not shown).
To determine whether SRT1460 and SRT1720 bind and activate the enzyme at the same molecular site as resveratrol, an isobologram analysis was performed. A concentration matrix of two compounds, resveratrol versus SRT1720 and SRT1720 versus SRT1460, was examined to determine whether the combination was antagonistic, additive or synergistic. In both cases the compound combination resulted in additivity consistent with the hypothesis that a single allosteric site exists on the SIRT1—substrate complex to which structurally diverse compounds can bind (Fig. 2c).
To define the compound binding site a series of SIRT1 truncation mutants was generated (Fig. 2d). SIRT1-A (156–664) and SIRT1-B (172–664) retain similar activity and activation to the parent construct (SIRT1-C), whereas SIRT1-D (219–664) exhibits a marked decrease in enzyme activity. SIRT1-D expresses poorly in Escherichia coli and may lose activity simply due to misfolding. The SIRT1-E (225–664), SIRT1-F (230–664), SIRT1-G (235–664), and SIRT1-H (240–664) constructs exhibit enzymatic activity comparable to SIRT1-C, but the ability to be activated by SRT1720 and resveratrol is significantly decreased. The SIRT1 truncation data suggest that amino acids 240–664 comprise the core catalytic domain, and that the amino acids 183–225 N-terminal to the core domain are important in defining the compound binding site.
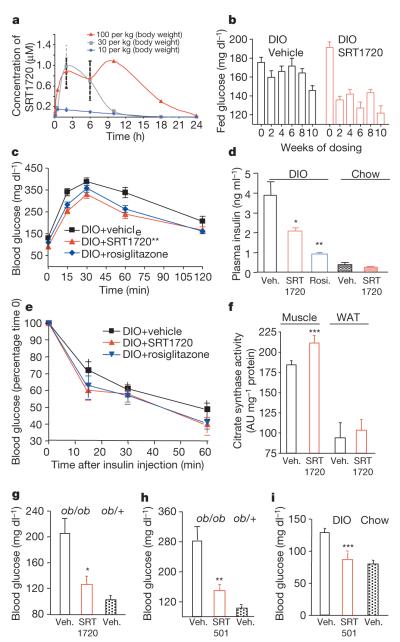
The therapeutic potential of SIRT1 activators to treat insulin resistance and diabetes was tested in three in vivo models of type 2 diabetes. SRT1720 exhibited a pharmacokinetic profile (Fig. 3a) suitable for in vivo evaluation in both mouse (bioavailability = 50%, terminal t1/2 = ~5 h, Area Under the Curve (AUC) = 7,892 ng h−1 ml−1) and rat (bioavailability = 25%, terminal t1/2 = ~8.4 h, AUC = 3,714 ng h−1 ml−1). SRT501, a reformulated version of resveratrol with improved bioavailability (11% bioavailability, terminal t1/2 of ~ 1 h and an AUC of 10,524 ng h−1 ml−1), was also examined in genetically obese mice (Lepob/ob) and diet-induced obesity (DIO) mice. SRT501 (500 or 1,000 mg per kg (body weight)) and SRT1720 (100 mg per kg (body weight)) were dosed in all efficacy studies once daily by oral gavage. During these studies we did not measure food intake or metabolic rates so there may be uncertainty about how the compound is working. We did measure body weight during these studies and this did not change.
Figure 3. SIRT1 activators in mouse models of type 2 diabetes.
a, Plasma levels of SRT1720 after administration by oral gavage. b, Effect of SRT1720 treatment over 10 weeks on fed plasma glucose levels in DIO mice. c, Glucose excursion during an intraperitoneal glucose tolerance test (5 weeks) in DIO mice treated with the indicated compounds. d, Plasma insulin levels after 10 weeks treatment with indicated compounds. e, Glucose response in DIO mice during an insulin tolerance test after 10 weeks treatment with the indicated compounds. f, Skeletal muscle citrate synthase activity (Vmaxmg−1 protein, 11 weeks, n = 5). g, Effect of SRT1720 treatment in diabetic Lepob/ob mice (1 week). h, Effect of SRT501 treatment (1,000 mg per kg (body weight)) in diabetic Lepob/ob mice after 2 weeks. i, Effect of SRT501 treatment (500 mg per kg (body weight), 4 weeks) in DIO mice. All studies consisted of ten mice per group unless noted. Statistics were conducted as an ANOVA; asterisk P < 0.05, double asterisk P < 0.01 and triple asterisk P < 0.001. Data are expressed as mean ± s.e.m.
In DIO mice, fasting blood glucose levels are elevated (120–150 mg dl−1 range) after being placed on a high-fat diet. Administration of SRT1720 reduced fed glucose levels after 1 week of treatment with further reduction after 3 weeks of treatment (Fig. 3b) that continued through 10 weeks of dosing. Glucose excursion during an intraperitoneal glucose tolerance test was also significantly reduced in the SRT1720 group, and comparable to rosiglitazone, a PPARγ activator that has been used to treat type 2 diabetes (Fig. 3c; glucose AUC: control, 603 ± 32 mg h−1 dl−1; SRT1720, 462 ± 25 mg h−1 dl−1; rosiglitazone, 496 ± 20 mg h−1 dl−1). SRT1720 did not have an effect on fasting glucose in chow-fed mice (data not shown), indicating that pharmacological SIRT1 activation is unlikely to induce hypoglycaemia.
DIO mice are insulin resistant and hyperinsulinaemic (3.9 ± 0.7 ng dl−1) compared to normal chow fed controls (0.4 ± 0.1 ng dl−1). SRT1720 significantly reduced the hyperinsulinaemia after 4 weeks, partially normalizing elevated insulin levels similar to rosiglitazone treatment (Fig. 3d). Consistent with the effects on improved glucose tolerance and fasting insulin levels, a reduction in glucose levels during an insulin tolerance test was greater in SRT1720 or rosiglitazone treated animals (Fig. 3e). SRT1720 did not reduce insulin levels in mice fed a normal chow diet that are not insulin resistant (Fig. 3d). The effect of SRT1720 on blood glucose and insulin levels is not secondary to weight loss as there was no difference in the weight of mice treated with SRT1720 compared to vehicle-treated DIO mice (data not shown).
As observed in calorie restriction6,25, SRT1720 treatment (10 weeks) increases mitochondrial capacity by 15% in gastrocnemius muscle as measured by citrate synthase activity (Fig. 3f). In DIO mice SRT1720 mimics several of the effects observed after calorie restriction including improved insulin sensitivity, normalized glucose and insulin levels, and elevated mitochondrial capacity.
To study SRT1720 in a genetic model of type 2 diabetes, Lepob/ob mice were placed on a high-fat diet to induce a diabetic state and then dosed with SRT1720. SRT1720 treatment significantly reduced fasting blood glucose to near normal levels (Fig. 3g) after 1 week. SRT501, a much less potent SIRT1 activator, also lowered fasting blood glucose in Lepob/ob mice after 3 weeks (1,000 mg per kg (body weight)) and in DIO mice after 4 weeks (500 mg per kg (body-weight)) of oral dosing (Fig. 3h, i). SRT501 also reduces hyperinsulinaemia in DIO mice measured as fed insulin levels (data not shown). As observed with SRT1720, no difference in body weight was evident after treatment with SRT501 in Lepob/ob mice.
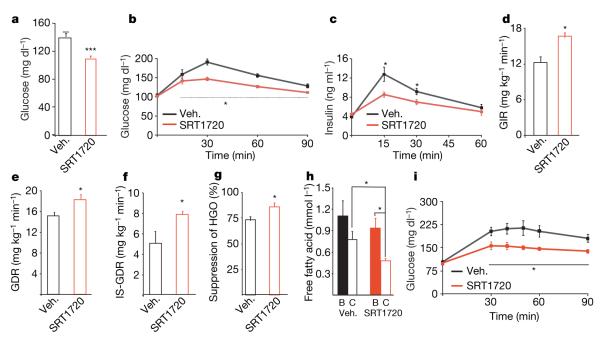
We also performed in vivo studies in the Zucker fa/fa rat, which is a genetically obese rodent model for studying insulin resistance. Rats were treated with vehicle or SRT1720 for 4 weeks. Body weight was not different between the two groups throughout the study. After 3 weeks treatment, fed blood glucose was significantly lower in fa/fa rats (Fig. 4a) treated with SRT1720 compared to vehicle-treated (140 ± 5 versus 110 ± 3mg dl−1, vehicle versus SRT1720, P < 0.001). In support of improved glucose metabolism after SIRT1 activation, both the glucose (AUC: 4,550 ± 463 versus 2,490 ± 236 mg min−1dl−1, vehicle versus SRT1720, P < 0.01) and insulin (AUC: 277 ± 32 vs. 127 ± 25 ng min−1dl−1, vehicle versus SRT1720, P < 0.01) responses during an oral glucose tolerance test were significantly improved in the SRT1720-treated group (Fig. 4b, c). The decrease in glucose in the face of reduced hyperinsulinaemia indicates improved insulin sensitivity. To assess more definitively insulin action after SRT1720 treatment, hyperinsulinaemiceuglycaemic clamps were performed on fa/fa rats after 4 weeks of treatment. Consistent with improved glucose tolerance, the glucose infusion rate required to maintain euglycaemia was ~35% higher in SRT1720-treated fa/fa rats (Fig. 4d, P < 0.01), and the total glucose disposal rate was increased by ~20% (Fig. 4e, P < 0.05). The insulin-stimulated glucose disposal rate, which primarily represents glucose disposal into skeletal muscle, was 55% higher in the SRT1720-treated group (Fig. 4f, P < 0.05). Along with the increased glucose disposal rate and glucose infusion rate, liver insulin sensitivity was significantly enhanced in the SRT1720 group, as evidenced by a greater suppression of hepatic glucose production during the clamp (Fig. 4g, P < 0.05). Adipose tissue insulin sensitivity was also improved after SIRT1 activation (Fig. 4h), as indicated by lower (~40%) plasma free fatty acid concentration during the clamp in the SRT1720-treated group (0.48 ± 0.03 versus 0.82 ± 0.10 mmol l−1, SRT1720 versus vehicle, P < 0.05).
Figure 4. SIRT1 activator SRT1720 in the Zucker fa/fa rat model.
a, Post-absorptive blood glucose (3 weeks). b, c, Glucose and insulin responses during an oral glucose tolerance test (3.5 weeks, ANOVA). After 4 weeks a hyperinsulinaemic-euglycaemic clamp study was conducted. (d—f) Glucose infusion rate (GIR), glucose disposal rate (GDR) and insulin-stimulated glucose disposal rate (IS-GDR) were significantly enhanced. g, Hepatic glucose output (HGO) suppression (%). h, Plasma fatty acid concentration during the clamp was significantly lower in SRT1720-treated animals (ANOVA). B, basal conditions. C, clamp conditions. i, Glucose excursion during a PTT was reduced, indicating reduced gluconeogenic capacity. All studies consisted of n ≥ 5 rats per group. Statistics were conducted as student t-test unless noted otherwise; asterisk P < 0.05, double asterisk P < 0.01, and triple asterisk P < 0.001. Data are expressed as mean ± s.e.m.
Recent studies have suggested that SIRT1 might be important in regulating hepatic gluconeogenesis, through deacetylation and activation of PGC-1α26,27. However, transgenic mice that overexpress SIRT1 exhibit improved insulin sensitivity and are not hyperglycaemic28. To investigate if SRT1720 treatment alters hepatic gluconeogenesis, fa/fa rats were subjected to a pyruvate tolerance test (PTT) after 3 weeks of treatment. The glucose response during the PTT was markedly blunted in SRT1720-treated fa/fa rats relative to vehicle (Fig. 4i), demonstrating decreased hepatic gluconeogenesis. These results show that the overall in vivo effect of SIRT1 activation is to ameliorate the heightened gluconeogenic capacity of Zucker fa/fa rats and not to stimulate hepatic glucose production. In addition, because Zucker fa/fa rats exhibit hepatic insulin resistance, the decreased gluconeogenesis after SRT1720 treatment is further evidence of improved hepatic insulin sensitivity.
To address the therapeutic potential of SIRT1 activation for the improvement of age-related diseases, we have identified and characterized novel small molecule activators of SIRT1 both in vitro and in vivo. These compounds are 1,000-fold more potent activators than, and structurally unrelated to, resveratrol. Similar to resveratrol, these compounds bind directly to the SIRT1-acetylated peptide complex at the same site and lower the Km for peptide substrate resulting in a more productive catalytic complex. SIRT1 deletion experiments indicate that amino acids 183–225 are critical for maintaining activation of SIRT1 by these compounds and define the allosteric binding site. It is possible that acetylated peptide binding to SIRT1 induces a conformational change that exposes an allosteric site in this region of the enzyme. An endogenous regulator of SIRT1 has yet to be identified, and it is tempting to speculate that an endogenous activator of SIRT1 exists and may be increased after calorie restriction and other mild physiological stresses. Selective SIRT1 activators ablate insulin resistance and diabetes in DIO mice fed a high-fat diet and in diabetic Lepob/ob mice. In addition, these new SIRT1 activators ameliorate the metabolic disturbances in Zucker fa/fa rats. SIRT1 activators improve glucose homeostasis and insulin sensitivity in key metabolic tissues including liver, muscle and fat. These compounds seem to mimic the beneficial effects of calorie restriction on mitochondrial and metabolic function in mammals in vivo and hold promise for treating diseases of ageing such as type 2 diabetes.
METHODS SUMMARY
The full details of the materials and methods are presented in the Supplementary Information. The compounds, SRT1460, SRT1720, and SRT2183, were synthesized at Sirtris Pharmaceuticals. SIRT1 constructs were expressed in E. coli and purified by affinity, size exclusion, and/or ion exchange chromatography. The SIRT1 fluorescence polarization assay used a peptide based on p53 linked to biotin at the N-terminal end and to a fluorescent tag at the C-terminal end. The reaction was a coupled enzyme assay where the first reaction was the deacetylation reaction and the second was cleavage by trypsin at the exposed lysine. The mass spectrometry assay was used for all SAR studies and employs the same peptide as the HTS assay. For the in cell western assay (ICW) the acetylation state of p53 was determined using a polyclonal antibody against the acetylated lysine residue at position 382 in p53. The effect of test compounds on the Km of SIRT1 for peptide substrate was examined using the mass spectrometry assay. Isothermal titration calorimetry was performed using a VP-ITC (MicroCal, Inc.). For the isobologram analysis a concentration matrix of two compounds was created and tested against SIRT1 using the mass spectrometry assay. For pharmacokinetic studies compounds were administered by oral gavage or intravenously to C57BL/6 male mice at the doses indicated. For all in vivo efficacy studies vehicle or test compound was administered once daily by oral gavage. For the DIO studies, C57BL/6 male mice were fed a high-fat diet until their mean body weight reached approximately 40 g. Lepob/ob mice and heterozygous Lepob+ mice were placed on a high-fat diet for a minimum of 1 week before the start of a study and remained on the high-fat diet for the duration of the study. Male fatty (fa/fa) Zucker (ZF) rats (Harlan Sprague—Dawley, Inc.) were used for the studies.
Supplementary Material
Acknowledgements
We thank C. Ozbal and W. LaMarr from BioTrove, Inc. for running the mass spectrometry samples; S. Schaertl, D. Winkler, N. Fay and T. Hesterkamp for work on the SIRT1 fluorescence polarization assay development; M. Saberi, P. P. Li , M. Lu, and A. Hevener for assistance and advice with the Zucker fa/fa studies; P. Romero, K. Normington, and M. Dipp for experimental advice and comments on the manuscript; M. Inghilterra for help in data analysis and data mining. D.A.S. is supported by an Ellison Medical Foundation Senior Scholarship, and grants from NIH/NIA and the Paul F. Glenn Medical Foundation. J.M.O. is supported by a University of California Discovery Biostar grant and NIH. S.S. is supported by a Mentor-Based Postdoctoral Fellowship from the American Diabetes Association awarded to J.M.O.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare competing financial interests: details accompany the full text HTML version of the paper at www.nature.com/nature.
References
- 1.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 2.Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J. Clin. Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nature Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HY, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 5.Heilbronn LK, et al. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes. Res. 2005;13:574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 6.Nisoli E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 7.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 8.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 9.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 10.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 12.Jarolim S, et al. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–177. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 15.Brink CB, Harvey BH, Bodenstein J, Venter DP, Oliver DW. Recent advances in drug action and therapeutics: relevance of novel concepts in G-protein-coupled receptor and signal transduction pharmacology. Br. J. Clin. Pharmacol. 2004;57:373–387. doi: 10.1111/j.1365-2125.2003.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 18.Napper AD, et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J. Med. Chem. 2005;48:8045–8054. doi: 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- 19.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 23.Sarabu R, Grimsby J. Targeting glucokinase activation for the treatment of type 2 diabetes—a status review. Curr. Opin. Drug Discov. Dev. 2005;8:631–637. [PubMed] [Google Scholar]
- 24.Borra MT, Langer MR, Slama JT, Denu JM. Substrate specificity and kinetic mechanism of the Sir2 family of NAD+-dependent histone/protein deacetylases. Biochemistry. 2004;43:9877–9887. doi: 10.1021/bi049592e. [DOI] [PubMed] [Google Scholar]
- 25.Heilbronn LK, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. J. Am. Med. Assoc. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl Acad. Sci. USA. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banks A, et al. Overexpression of the Sirtuin SIRT1 increases insulin sensitivity in aging mice. Diabetes. 2007;56(S1):0234-OR. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.