Abstract
Reproductive function involves an interaction of three regulatory levels: hypothalamus, pituitary, and gonad. The primary drive upon this system comes from hypothalamic gonadotropin-releasing hormone (GnRH) neurosecretory cells, which receive afferent inputs from other neurotransmitter systems in the central nervous system to result in the proper coordination of reproduction and the environment. Here, we hypothesized that the recreational drug ±-3,4-Methylenedioxymethamphetamine (MDMA; “ecstasy”), which acts through several of the neurotransmitter systems that affect GnRH neurons, suppresses the hypothalamic-pituitary-gonadal (HPG) reproductive axis of male rats. Adult male Sprague-Dawley rats self-administered saline or MDMA or saline either once (acute) or for 20 days (chronic), and were euthanized 7 days following last administration. We quantified hypothalamic GnRH mRNA, serum luteinizing hormone (LH) concentrations, and serum testosterone levels, as indices of hypothalamic, pituitary, and gonadal functions, respectively. The results indicate that the hypothalamic and gonadal levels of the HPG axis are significantly altered by MDMA, with GnRH mRNA and serum testosterone levels suppressed in rats administered MDMA compared to saline. Furthermore, our finding that hypothalamic GnRH mRNA levels are suppressed in the context of low testosterone concentrations suggests that the central GnRH neurosecretory system may be a primary target of inhibitory regulation by MDMA usage.
Keywords: MDMA, ecstasy, neuroendocrinology, GnRH, testosterone, endocrine disruption, male reproduction
Recreational use of 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) is particularly popular among college students and those involved in the dance culture [1, 2]. MDMA abusers often consume the drug at all-night dance parties because it induces a state of euphoria, increased energy, insomnia, and enhanced sensory perception. MDMA causes neurocognitive deficits in attention, verbal and non-verbal learning and memory, psychomotor speed and executive systems functioning [reviewed in 3]. Other central nervous system effects of MDMA have been reported for cognitive function [4, 5], circadian rhythms [6, 7], and thermoregulation [8, 9].
The widespread and diverse actions of MDMA on brain and behavior are explained at least in part by its numerous target neurotransmitter systems [reviewed in 10]. The serotonergic and dopaminergic systems are most strongly implicated as primary targets of MDMA action, as shown by in vivo microdialysis studies [11–14] and by in vitro and ex vivo studies [15, 16]. In addition, MDMA stimulates norepinephrine [17–20], acetylcholine [21–23], and GABA release [24]. Thus, MDMA has potential to alter disparate nervous system functions through its multiple targets.
The impact of MDMA on reproductive neuroendocrine function and the reproductive axis has not, to our knowledge, been rigorously explored. Nevertheless, this question is biologically relevant, because the same neurotransmitter systems that control these aforementioned central nervous system actions of MDMA, including (but not limited to) serotonergic and dopaminergic pathways, also regulate reproduction [25–27; for review see 28]. Reproductive function in mammals is driven by about 1000 gonadatropin-releasing hormone (GnRH) neurons, localized in the preoptic area and hypothalamus of rodents [28], which in turn are regulated by afferent inputs from other brain regions. This neural circuitry enables the hypothalamic GnRH cells to coordinate reproduction with other environmental and homeostatic cues. Thus, GnRH neurons, through these central afferent inputs, are a potential target of MDMA.
The objective of the present study was to assess the neuroendocrine disrupting effects of MDMA in adult male Sprague-Dawley rats as a model for reproductive effects of ecstasy usage in humans. Although our primary interest was actions of MDMA on hypothalamic GnRH neurons, we also assessed actions of MDMA on the other two levels of the hypothalamic-pituitary-gonadal (HPG) axis: serum luteinizing hormone (LH) concentrations as an index of pituitary output, and sex steroid hormones as an indicator of testicular function.
METHODS
MDMA
(±)3,4-Methylenedioxymethamphetamine HCl (MDMA) was obtained through NIDA Drug Inventory Supply and Control, and was dissolved in 0.9% saline.
Animals and Procedures
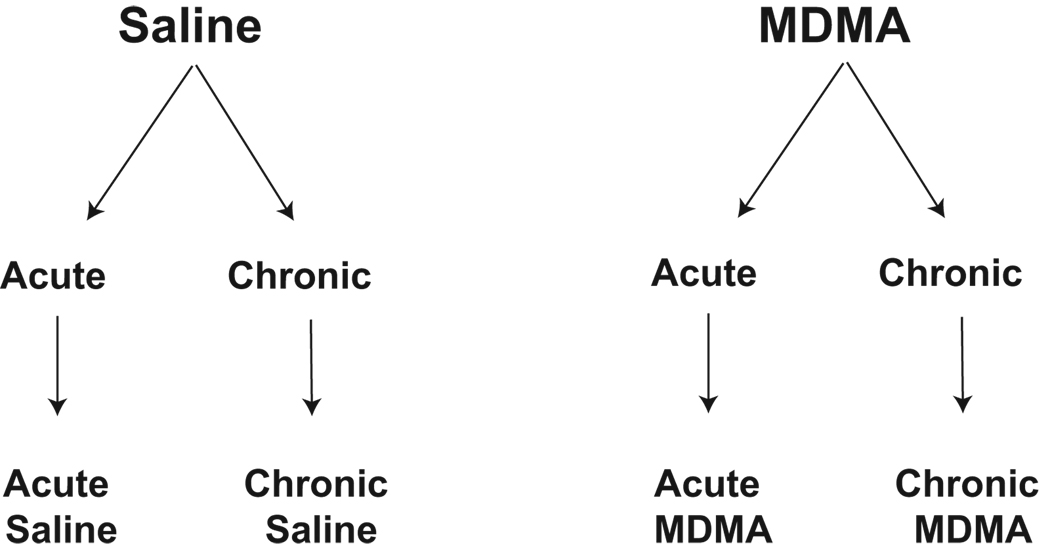
All experimental procedures were carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals, and performed following protocols approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Adult male (250 – 300 g) Sprague-Dawley rats Charles River Laboratories, Inc. Wilmington, MA) were used in this study. The experimental model used herein was to train rats to level press for 45 mg sugar pellets (Bio-Serv, Frenchtown, NJ) on a fixed ratio 1 schedule for a minimum of 8 days, as part of a separate study seeking to enable rats to self-administer either MDMA or vehicle [published in 8]. Additional details on animals, surgeries, and procedures, are reported in that paper. In brief, rats self-administered saline or MDMA either once (acute) or for 20 days (chronic), and were euthanized 7 days following last administration, for a total of 4 treatment groups (Figure 1). All administration occurred intravenously, via a chronically implanted jugular catheter (see [8] for details). The acute MDMA experimental group administered a single 3 mg/kg dose of MDMA, chosen to mimic a recreational MDMA “binging” experience with the drug, when a large dose of the drug is ingested in one session. This dose was selected upon results of preliminary microdialysis experiments in one of our labs (C.L.D.; data not shown), which demonstrated significant increases in serotonin and dopamine in the nucleus accumbens at this dose. The chronic MDMA experimental group was designed to mimic long-term heavy use of MDMA among young people at dance clubs or “raves,” where repeated doses are taken intermittently in the same evening. Male rats administered unit doses of MDMA (or saline control) ad libitum for five consecutive days for a period of 4 weeks (2 hour sessions, 5 days on, 2 days off each week for the 4 week period). During Days 1–10 the unit dose was 1 mg/kg per injection, and during Days 11–20 the unit dose was 0.5 mg/kg per injection [8] to optimize self-administration behavior [29]. Average daily MDMA intake for chronic MDMA animals was found to be 4 mg/kg body weight. The number of animals per experimental group were as follows: acute saline: 5, chronic saline: 6, acute MDMA: 5, chronic MDMA: 9. In the results section, any deviation from the number of animals per group noted here represents a lost data point due to experimental error or tissue loss.
Figure 1.
Experimental model. Adult male Sprague-Dawley rats self-administered saline or MDMA either once (acute) or ad libidum for a period of 5 days over a 4-week time period, resulting in a total of 20 days (chronic). They were then euthanized 7 days following last administration, for a total of 4 treatment groups.
Blood & Tissue Collection
Acute- and chronic-treated MDMA and saline rats were euthanized by carbon dioxide asphyxiation 7 days following the final treatment. Brains were removed and one half of the preoptic area - anterior hypothalamus (POA-AH), which contains the majority of GnRH cell bodies, was dissected out on wet ice, and snap frozen within 2 minutes of removal on dry ice and stored at −80°C until RNA extraction. Terminal blood samples were taken, and serum was separated by centrifugation at 6000 g and stored at −80°C until further processing.
Serum Hormone Assays
The LH level in serum samples was measured in the laboratory of Dr. Michael Woller, University of Wisconsin-Whitewater, by double antibody competitive binding RIA as previously described [30, 31], using reagents provided by Dr. A. F. Parlow of the National Hormone and Pituitary Program at NIDDK. The reference standard used was rat LH-RP-3. Duplicate volumes of 100 µl serum were used for each sample, and three separate assays were performed. The assay sensitivity was 0.2 ng/ml, the intra-assay coefficient of variability (CV) based on duplicate samples for each assay was 2.36%, 4.39%, and 4.35%, respectively, and the inter-assay CV was 7.23%.
Total serum testosterone was determined in two assays using the Active® Testosterone coated well EIA kit (Catalog # DSL-10-4000, Lot # 08035-B, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), according to the manufacture instructions. Duplicate volumes of 50 µl serum were used for each sample. The assay limit of detection was 0.04 ng/ml, and the intra-asay CV based on duplicate samples for each assay was 3.11% and 5.76%, and the inter-assay CV was 8.07%.
Progesterone concentrations were determined in a single assay using the ACTIVE®. Progesterone Coated-Tube Radioimmunoassay Kit (Catalog # DSL-3900, Lot # 07076, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), according to the manufacturer’s instructions. Duplicate volumes of 25 µl serum were used for each sample. The sensitivity of the assay was 0.12 ng/ml, and the intra-assay CV was 2.3%. For all assays, samples for which the CV between duplicates was 10% or greater were excluded from analysis.
Estradiol concentrations were determined in a single assay using an ultrasensitive double-antibody RIA kit (Catalog # DSL-4800, Lot # 07076, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), according to the manufacture instructions. Duplicate volumes of 200µL serum were used for each sample. The assay limit of detection was 2.2 pg/mL, and the intra-assay coefficient of variability based on duplicate samples for each assay was 2.83%.
GnRH and Cyclophilin Gene Expression Analysis by Real-time PCR
GnRH and cyclophilin (internal control: [32, 33]) gene expression were measured using real-time PCR with the Stratagene Brilliant® qPCR kit and the Stratagene MX3000 detection system. Messenger RNA from frozen POA-AH dissections of individual rats was extracted using a double-detergent lysis buffer system [32]. In brief, frozen tissues were homogenized in cold lysis buffer through a 22-gauge needle, and cytoplasmic RNA was separated from nuclear RNA using a sucrose gradient. Samples were treated with proteinase K to remove proteins, and cytoplasmic RNA was extracted with chloroform and isopropanol, followed by precipitation at −20°C. The RNA was pelleted by centrifugation, washed, and resuspended in 10 µl nuclease-free water (Catalog # AM9937, Applied Biosystems, Foster City, CA, USA). Genomic contamination was removed using the DNA-free™ Kit (Catalog #AM1906, Applied Biosystems) according to the manufacture instructions. RNA concentration was quantified using a Nanodrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and its RNA purity was assessed using the 260:280 and 260:230 nm ratios. All samples had 260:280 ratios between 1.8 and 2.1, and 260:230 ratios above 1.7. RNA integrity was assessed by examining representative samples loaded onto a 1.5% agarose gel stained with ethidium bromide. Two μg of RNA were then converted to cDNA via a reverse-transcriptase (RT) PCR reaction using the SuperScript™ First-Strand Synthesis System for RT-PCR (Catalog # 11904-018, Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions. All RT reactions contained a negative control, which consisted of nuclease-free water instead of reverse transcriptase enzyme, to confirm absence of genomic contamination in each sample. cDNA reactions were diluted 1:5 and a 2 µl aliquot of cDNA was used as template for amplification in PCR. Primers used were 5'-TGTGCCAGGGTGGTGACTT-3' (sense) and 5'-TCAAATTTCTCTCCGTAGATGGACTT-3' (anti-sense) and probe 5’-CCACCAGTGCCATTATGGCGTGT-3’ for cyclophilin [34] and 5'-CCCTTTGGCTTTCACATCCA-3' (sense) and 5'-AACAGCGGCCATCAGTTTG-3' (anti-sense) and probe 5'-ACAGAATGGAAACGATCC-3’ for GnRH. PCR conditions were 95°C for 10 min, followed by 50 cycles of denaturing at 95°C for 30 sec, annealing at 55°C for 60 sec, and extension at 72°C for 30 sec. For each sample, GnRH gene expression was normalized to cyclophilin and relative expression was determined using the Comparative Ct Method [35]. RNA extracted from the POA-AH of an intact untreated male rat was used as a positive control, and was included as a standard on each assay plate. Samples were run in triplicate and analyzed in duplicate to account for pipetting errors. The mean positive control value was used as a calibrator for inter-plate variability. The interassay CV was 6%.
Statistical Analysis
The effects of MDMA on serum hormone levels and GnRH gene expression were analyzed using two-way ANOVAs [variables: drug (saline, MDMA) X duration (acute, chronic)] with Statview 5.0 software for Macintosh computer. Significant interactions and main effects were probed further with Fisher’s PLSD post-hoc tests. Differences were considered significant at p < 0.05. All data are reported as mean ± S.E.M. unless otherwise noted.
RESULTS
Effects of MDMA on Hypothalamic GnRH Gene Expression
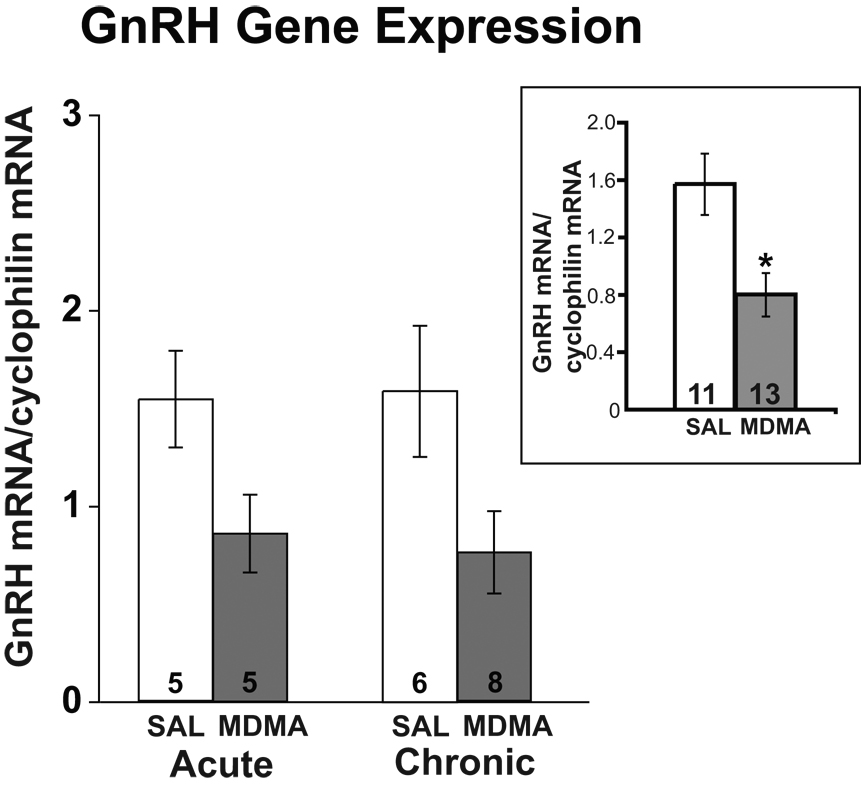
GnRH gene expression was measured using real-time PCR in POA dissections. Two-way ANOVA revealed a significant main effect of drug treatment upon GnRH mRNA levels, with overall lower levels of GnRH mRNA expressed in MDMA compared to saline rats (F = 8.584, p < 0.01; Figure 2). No significant main effect was detected for duration of treatment (chronic vs. acute), nor were any significant interactions between the two variables observed.
Figure 2.
Effects of acute and chronic MDMA or saline (SAL) on POA-AH GnRH mRNA levels, normalized relative to cyclophilin mRNA levels. Group data (mean ± SEM) are shown here and in subsequent figures. A significant main effect of drug was detected, with MDMA rats having decreased GnRH mRNA levels compared to saline rats. Because there were no differences in results between the acute and chronic groups, the inset shows the comparison between all MDMA and SAL rats combined for both durations of treatment. Numbers of rats per group are shown within each bar in this and all other figures. *p < 0.01 compared to SAL.
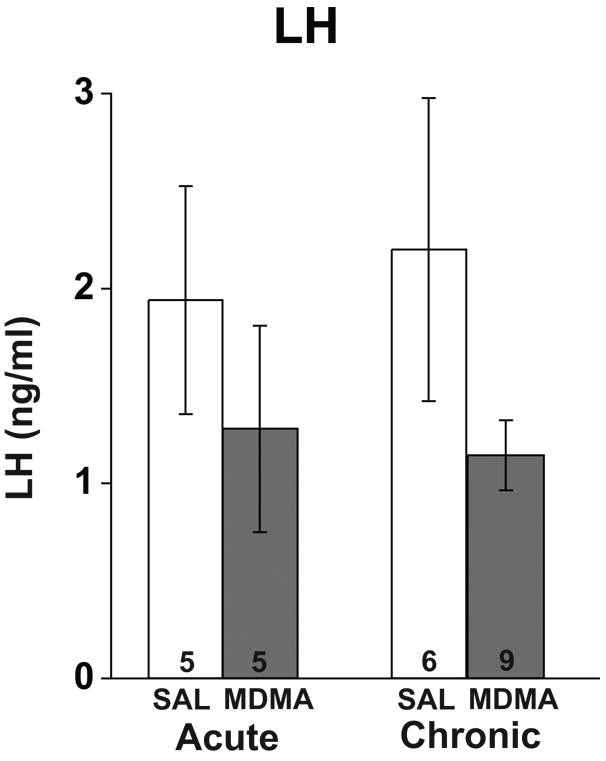
Effects of MDMA on Serum LH Levels
Serum LH levels varied considerably among rats, probably due to the pulsatile nature of LH release [31]. Two-way ANOVA showed no significant main effect of drug (p = 0.11) or duration (p = 0.91) upon LH levels, nor were any significant interactions between the two variables observed (p = 0.71; Figure 3).
Figure 3.
Effects of acute and chronic MDMA or saline (SAL) on serum luteinizing hormone (LH) concentrations. No significant differences in serum LH were detected for treatment or duration, with no significant interactions of the two variables.
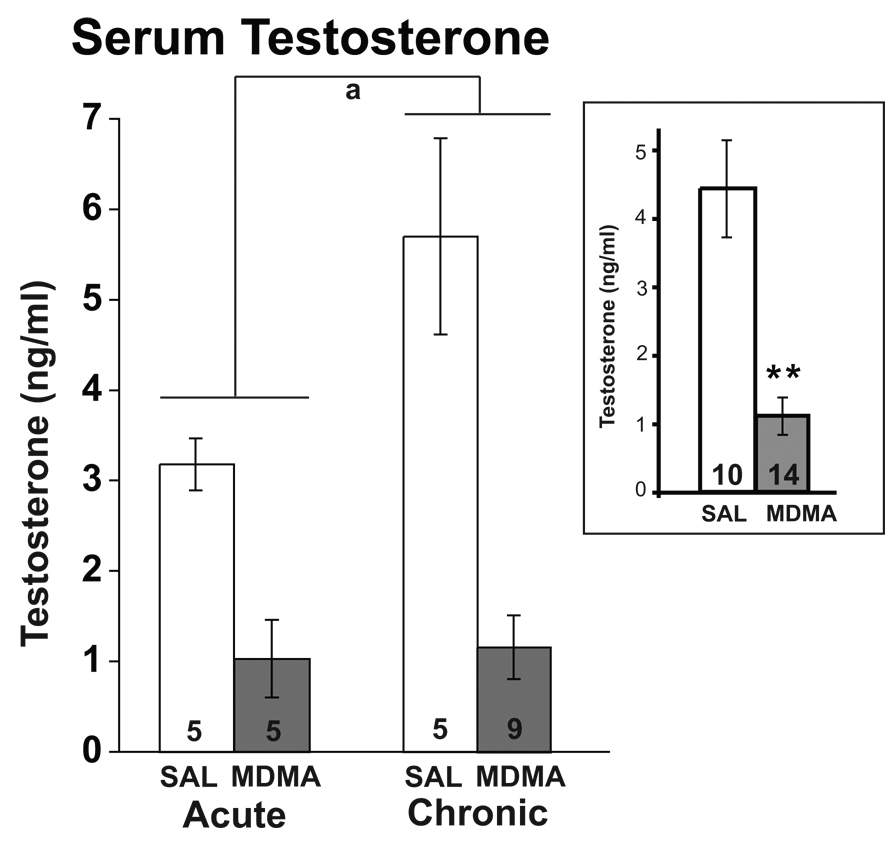
Effects of MDMA on Serum Testosterone Levels
A significant main effect of drug treatment was found for serum testosterone concentrations, with decreased levels in the MDMA compared to the saline group (F = 33.23, p < 0.0001; Figure 4). A significant main effect of duration (F = 5.15, p < 0.05) on serum testosterone levels was also found, with chronic rats having higher testosterone levels than acute (Figure 4). There was a non-significant trend for an interaction of treatment with duration (p = 0.054), and results suggest that effect of duration is largely due to differences among the saline treated animals. Nevertheless, MDMA significantly suppressed serum testosterone levels at both durations compared to respective saline controls.
Figure 4.
Effects of acute and chronic MDMA or saline (SAL) on serum testosterone concentrations. Testosterone concentrations were significantly lower in the MDMA compared to the saline group, as indicated in the inset (p < 0.0001). Chronic rats also had higher testosterone levels than acute rats (p < 0.05). **, p < 0.0001 compared to SAL; a, p < 0.05 for acute vs. chronic treatments.
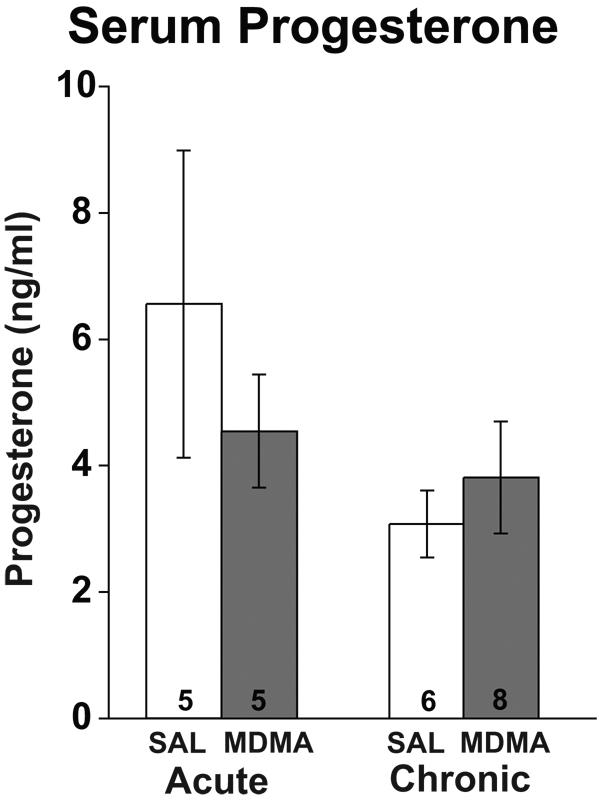
Effects of MDMA on Serum Progesterone and Estradiol Levels
Serum estradiol and progesterone concentrations were measured to further assess the effect of systemic MDMA on steroid hormones. For progesterone, two-way ANOVA revealed no significant main effects of drug (p = 0.62) or duration (p = 0.11), nor were any interactions between drug and duration observed (p = 0.29; Figure 5). For the estradiol assay, a high percentage of samples had a large assay CV, and a lack of serum for repeating the assay resulted in a very small sample size that did not provide adequate statistical power for analysis. For those animals with detectable estradiol concentrations, preliminary results suggested that there were no differences among any of the groups. Estradiol concentrations (pg/ml) for the Acute-Saline, Chronic-Saline, Acute-MDMA and Chronic-MDMA groups were 10.0 ± 4.1, 7.1 ± 4.5, 9.3 ± 1.9 and 10.3 ± 4.1, respectively (data are presented as mean ± standard deviation due to small N’s).
Figure 5.
Effects of acute and chronic MDMA or saline (SAL) on serum progesterone concentrations. No significant differences in serum progesterone were found for drug or duration of treatment.
Effects of MDMA on Paired Testes Weights
Paired testes were removed and weighed, with Acute-Saline, Chronic-Saline, Acute-MDMA and Chronic-MDMA groups having weights (g) of 2.66 ± 0.72, 3.37 ± 0.25, 3.79 ± 0.96 and 3.25 ± 0.46, respectively (mean ± SEM). Although there was no effect of treatment or duration, there was a significant interaction of these two variables (p < 0.05), attributable to differences between Acute-MDMA and Acute-Saline.
DISCUSSION
The present study demonstrates that MDMA administered at dosages relevant to human intake [36–38] causes a significant disruption of hypothalamic and gonadal function. Specifically, and as discussed in more detail below, rats taking MDMA had significantly lower GnRH gene expression and lower serum testosterone concentrations compared to their saline control counterparts. Because rats were euthanized seven days after the last MDMA administration, these findings further suggest that both acute and chronic MDMA use has lasting endocrine disrupting actions on the HPG axis. Although we recognize that metabolism and secretion of MDMA varies between species [10], these results showing that MDMA disrupts reproductive neuroendocrine function in healthy adult male rats has potential relevance to humans who use MDMA even once.
The results show that MDMA administered either acutely or chronically resulted in a significant, approximately 50% decrease in GnRH mRNA levels seven days later compared to saline administered rats. Pituitary LH levels were slightly lower in these same MDMA animals but this latter result did not attain significance [e.g. 31]. Because we do not know when during the LH pulse a rat was euthanized, it is possible that more careful analyses of effects of MDMA on LH pulsatility would reveal differences between the treatment groups. In addition, our current observation that serum testosterone was profoundly suppressed in MDMA rats is consistent with decreased drive upon the testes by the serum gonadotropins. Therefore, our results showing significant decreases in GnRH mRNA and serum testosterone concentrations, together with the non-significant decrease in serum LH, is consistent with diminished drive from hypothalamic GnRH neurons upon the rest of the HPG axis. Moreover, we speculate that the GnRH neurosecretory system is the primary target for HPG axis disruption by MDMA. If the gonad were the primary target of the MDMA suppression, thereby resulting in decreased testosterone concentrations, we would predict that negative feedback upon the hypothalamus would be reduced, resulting in an increase in GnRH gene expression, as reported in other experimental models [39]. As this was not the case, the suppressed testosterone levels are likely due to decreased feed-forward input from the hypothalamus, and subsequently the pituitary, upon the testes.
In addition to androgens, the male adrenal and testes also produce substantial levels of progesterone [40, 41], a hormone that plays a role in the sexual behavior of male rodents [42]. This hormone was assayed in the current study, with no effects of drug treatment found. We also assayed serum estradiol, and although our sample sizes were too small to perform statistics, the preliminary results suggest that there was no apparent effect of any MDMA treatment. Therefore, effects of MDMA on steroid hormones measured thus far appear to be specific to testosterone.
Although it was beyond the scope of the current study to determine which neurotransmitter systems mediate the effects of MDMA on the hypothalamic GnRH system, these are likely to be similar to those that underlie MDMA’s other central nervous system actions. The two most plausible candidates are serotonin and dopamine, as they have been reported to be the primary targets of MDMA in other regions of the brain [8; reviewed in 10]. Serotonin and dopamine neurons innervate the hypothalamus, and receptors for both these neurotransmitters are expressed in the preoptic area, the location of the GnRH cell bodies [43, 44; reviewed in 28]. Serotonin agonists have been reported to be either stimulatory or inhibitory to GnRH neurons depending upon the experimental model [26, 45, 46]. A similar finding has been made for dopaminergic actions on GnRH cells [47, 25, 48]. Although it is difficult to reconcile both stimulatory and inhibitory effects of these neurotransmitter systems, they are probably best explained by there being a permissive window of normal functional activity of serotonin or dopamine, manipulations above or below which cause disruption of GnRH function. It is possible that through alterations in hypothalamic monoaminergic receptors, MDMA can influence these inputs into the GnRH neurosecretory system, either directly or indirectly.
This study was conducted to evaluate the impact of MDMA on the HPG axis of rats. However, we would like to note that the model of MDMA self-administration in rats is a topic of considerable controversy [49]. Several laboratories [50, 29, 51] including one of ours (C.L.D., [8]), but not others [reviewed in 49], have shown that rats and mice self-administer MDMA. Although acquisition occurs at a slower rate and supports fewer lever responses compared to other psychostimulants [49, 50; 52–55], the salient point is that our rats in the current study administered MDMA at levels comparable to voluntary human intake. Thus, we were able to make comparisons between animals given MDMA versus saline to determine specific effects on the reproductive axis.
The major novel finding of this study is that, to our knowledge, it provides the first evidence that MDMA disrupts the HPG axis of adult male rats, and even more specifically, that the mechanism for this effect involves the targeting of the hypothalamic-preoptic GnRH system. Our results are consistent with observations that environmental or pharmaceutical substances that disrupt monoaminergic neurotransmitter functions in the hypothalamus, including pesticides [56, 57] and PCBs [58, 59], affect GnRH and LH release. The popularity of MDMA among humans has increased in recent years [1], leading to concern over the potential health hazards associated with recreational drug use. The results of this study suggest that dosages approximating the recreational use of MDMA may impact the male reproductive axis.
Acknowledgements
The authors would like to acknowledge generous support from the NSF (NSF 04-615 to SMD), the NIH (T32 ES07247 to SMD), the NIEHS (ES012272 to ACG), and the NIDA (DA14640 to CLD). We extend our appreciation to Dr. A. F. Parlow of the National Hormone and Pituitary Program at NIDDK for providing LH assay reagents. We thank Dr. Michael J. Woller at the University of Wisconsin-Whitewater for performing the LH assays. We thank Esperanza Guevara for expert assistance with animal handling. A preliminary version of this work was presented at the 88th Annual Meeting of the Endocrine Society, June 2006.
References
- 1.Strote J, Lee JE, Wechsler H. Increasing MDMA use among college students: results of a national survey. J Adolesc Health. 2002;30:64–72. doi: 10.1016/s1054-139x(01)00315-9. [DOI] [PubMed] [Google Scholar]
- 2.Boyd C, McCabe S, d'Arcy H. Ecstasy use among college undergraduates: gender, race and sexual identity. J Subst Abuse Treat. 2003;24:209–215. doi: 10.1016/s0740-5472(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 3.Kalechstein AD, De La Garza R, 2nd, Mahoney JJ, 3rd, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- 4.Parrott AC, Lees A, Garnham NJ, Jones M, Wesnes K. Cognitive performance in recreational users of MDMA of ‘ecstasy’: evidence for memory deficits. J Psychopharmacol. 1998;12:79–83. doi: 10.1177/026988119801200110. [DOI] [PubMed] [Google Scholar]
- 5.Verkes RJ, Gijsman HJ, Pieters MS, Schoemaker RC, de Visser S, Kuijpers M, Pennings EJ, de Bruin D, Van de Wijngaart G, Van Gerven JM, Cohen AF. Cognitive performance and serotonergic function in users of ecstasy. Psychopharmacology (Berl) 2001;153:196–202. doi: 10.1007/s002130000563. [DOI] [PubMed] [Google Scholar]
- 6.Balogh B, Molnar E, Jakus R, Quate L, Olverman HJ, Kelly PA, Kantor S, Bagdy G. Effects of a single dose of 3,4-methylenedioxymethamphetamine on circadian patterns, motor activity and sleep in drug-naive rats and rats previously exposed to MDMA. Psychopharmacology (Berl) 2004;173:296–309. doi: 10.1007/s00213-004-1787-9. [DOI] [PubMed] [Google Scholar]
- 7.Dafters RI, Biello SM. The effect of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) on serotonergic regulation of the mammalian circadian clock mechanism in rats: the role of dopamine and hyperthermia. Neurosci Lett. 2003;350:117–121. doi: 10.1016/s0304-3940(03)00855-3. [DOI] [PubMed] [Google Scholar]
- 8.Reveron ME, Maier EY, Duvauchelle CL. Experience-dependent changes in temperature and behavioral activity induced by MDMA. Physiol Behav. 2006;89:358–363. doi: 10.1016/j.physbeh.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35:1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- 10.Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 11.Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology. 1987;26(12):1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt CJ, Taylor VL. Depression of rat brain tryptophan hydroxylase following the acute administration of methylenedioxymethamphetamine. Biochem Pharmacol. 1987;36:4095–4102. doi: 10.1016/0006-2952(87)90566-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]
- 14.Colado MI, O'Shea E, Granados R, Esteban B, Martín AB, Green AR. Studies on the role of dopamine in the degeneration of 5-HT nerve endings in the brain of Dark Agouti rats following 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) administration. Br J Pharmacol. 1999;126:911–924. doi: 10.1038/sj.bjp.0702373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogen IL, Haug KH, Myhre O, Fonnum F. Short- and long-term effects of MDMA (“ecstasy”) on synaptosomal and vesicular uptake of neurotransmitters in vitro and ex vivo. Neurochem Int. 2003;43:393–400. doi: 10.1016/s0197-0186(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 16.Mlinar B, Mascalchi S, Morini R, Giachi F, Corradetti R. MDMA induces EPSP-spike potentiation in rat ventral hippocampus in vitro via serotonin and noradrenaline release and coactivation of 5-HT(4) and beta(1) receptors. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301512. EPub ahead of print; DOI: 10.1038/sj.npp.1301512. [DOI] [PubMed] [Google Scholar]
- 17.Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36:2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MP, Conarty PF, Nichols DE. [3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur J Pharmacol. 1991;200:9–16. doi: 10.1016/0014-2999(91)90659-e. [DOI] [PubMed] [Google Scholar]
- 19.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JL, Reid JJ. Interactions of methylenedioxymethamphetamine with monoamine transmitter release mechanisms in rat brain slices. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:313–323. doi: 10.1007/BF00167451. [DOI] [PubMed] [Google Scholar]
- 21.Fischer HS, Zernig G, Schatz DS, Humpel C, Saria A. MDMA (‘ecstasy’) enhances basal acetylcholine release in brain slices of the rat striatum. Eur J Neurosci. 2000;12:1385–1390. doi: 10.1046/j.1460-9568.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 22.Acquas E, Marrocu P, Pisanu A, Cadoni C, Zernig G, Saria A, DiChiara G. Intravenous administration of ecstasy (3,4-methylenedioxymethamphetamine) enhances cortical and striatal acetylcholine release in vivo. Eur J Pharmacol. 2001;418:207–211. doi: 10.1016/s0014-2999(01)00937-2. [DOI] [PubMed] [Google Scholar]
- 23.Nair SG, Gudelsky GA. 3,4-Methylenedioxymethamphetamine enhances the release of acetylcholine in the prefrontal cortex and dorsal hippocampus of the rat. Psychopharmacology. 2006;184:182–189. doi: 10.1007/s00213-005-0271-5. [DOI] [PubMed] [Google Scholar]
- 24.Bankson MG, Yamamoto BK. Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J Neurochem. 2004;91:852–859. doi: 10.1111/j.1471-4159.2004.02763.x. [DOI] [PubMed] [Google Scholar]
- 25.Drouva SV, Gallo RV. Further evidence for inhibition of episodic luteinizing hormone release in ovariectomized rats by stimulation of dopamine receptors. Endocrinology. 1977;100:792–798. doi: 10.1210/endo-100-3-792. [DOI] [PubMed] [Google Scholar]
- 26.Vitale ML, de las Nieves Parisi M, Chiocchio SR, Tramezzani JH. Serotonin stimulates gonadotrophin release by acting directly on the median eminence. Acta Physiol Pharmacol Latinoam. 1985;35:473–479. [PubMed] [Google Scholar]
- 27.Gore AC, Terasawa E. Neural circuits regulating pulsatile luteinizing hormone release in the female guinea-pig: opioid, adrenergic and serotonergic interactions. J Neuroendocrinol. 2001;13:239–248. doi: 10.1046/j.1365-2826.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 28.Gore AC. GnRH: The Master Molecule of Reproduction. Norwell, MA: Kluwer Academic Publishers; 2002. [Google Scholar]
- 29.Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology (Berl) 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- 30.Vella S, Gussick J, Woller M, Waechter-Brulla D. Modification of cell perifusion for extended study of hormone release in the rat pituitary. Methods Cell Sci. 2001;23:197–204. doi: 10.1023/a:1016339932088. [DOI] [PubMed] [Google Scholar]
- 31.Maffucci JA, Walker DM, Ikegami A, Woller MJ, Gore AC. The NMDA receptor subunit NR2b: Effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol. Neuroendocrinology. 2008 doi: 10.1159/000111136. In Press. DOI: 10.1159/000111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gore AC, Roberts JL, Gibson MJ. Mechanisms for the regulation of gonadotropin-releasing hormone gene expression in the developing mouse. Endocrinology. 1999;140:2280–2287. doi: 10.1210/endo.140.5.6711. [DOI] [PubMed] [Google Scholar]
- 33.Schirman-Hildesheim TD, Bar T, Ben-Aroya N, Koch Y. Differential gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of the female rat across the estrous cycle. Endocrinology. 2005;146:3401–3408. doi: 10.1210/en.2005-0240. [DOI] [PubMed] [Google Scholar]
- 34.Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Peroutka SJ, Newman H, Harris H. Subjective effects of 3,4- methylenedioxymethamphetamine in recreational users. Neuropsychopharmacology. 1988;1:273–277. [PubMed] [Google Scholar]
- 37.Mas M, Farre M, De La Torre R, Roset PN, Ortuno J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- 38.Solowij N, Hall W, Lee N. Recreational MDMA use in Sydney: a profile of “Ecstasy” users and their experiences with the drug. Br J Addict. 1992;87:1161–1172. doi: 10.1111/j.1360-0443.1992.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 39.El Majdoubi M, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2000;12:167–176. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 40.Wagner CK. The many faces of progesterone: a role in adult and developing male brain. Front Neuroendocrinol. 2006;27:340–359. doi: 10.1016/j.yfrne.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Kalra PS, Kalra SP. Circadian periodicities of serum androgens, progesterone, gonadotropins and luteinizing hormone-releasing hormone in male rats: The effects of hypothalamic deafferentation, castration and adrenalectomy. Endocrinology. 1977;101:1821–1827. doi: 10.1210/endo-101-6-1821. [DOI] [PubMed] [Google Scholar]
- 42.Phelps SM, Lydon JP, O'Malley BW, Crews D. Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav. 1998;34:294–302. doi: 10.1006/hbeh.1998.1485. [DOI] [PubMed] [Google Scholar]
- 43.Tillet Y, Caldani M, Batailler M. Anatomical relationships of monoaminergic and neuropeptide Y-containing fibres with luteinizing hormone-releasing hormone systems in the preoptic area of the sheep brain: immunohistochemical studies. J Chem Neuroanat. 1989;2:319–326. [PubMed] [Google Scholar]
- 44.Chen WP, Witkin JW, Silverman AJ. Gonadotropin releasing hormone (GnRH) neurons are directly innervated by catecholamine terminals. Synapse. 1989;3:288–290. doi: 10.1002/syn.890030314. [DOI] [PubMed] [Google Scholar]
- 45.Arendash GW, Gallo RV. Serotonin involvement in the inhibition of episodic luteinizing hormone release during electrical stimulation of the midbrain dorsal raphe nucleus in ovariectomized rats. Endocrinology. 1978;102:1199–1206. doi: 10.1210/endo-102-4-1199. [DOI] [PubMed] [Google Scholar]
- 46.Wada K, Hu L, Mores N, Navarro CE, Fuda H, Krsmanovic LZ, Catt KJ. Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol Endocrinol. 2006;20:125–135. doi: 10.1210/me.2005-0109. [DOI] [PubMed] [Google Scholar]
- 47.Clemens JA, Tinsley FC, Fuller RW. Evidence for a dopaminergic component in the series of neural events that lead to the pro-oestrous surge of LH. Acta Endocrinol. 1977;85:18–24. doi: 10.1530/acta.0.0850018. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida H, Paruthiyil S, Butler P, Weiner RI. Role of cAMP signaling in the mediation of dopamine-induced stimulation of GnRH secretion via D1 dopamine receptors in GT1-7 cells. Neuroendocrinology. 2004;80:2–10. doi: 10.1159/000080519. [DOI] [PubMed] [Google Scholar]
- 49.De La Garza R, II, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology. 2006;10:1–10. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- 50.Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- 51.Trigo JM, Panayi F, Soria G, Maldonado R, Robledo P. A reliable model of intravenous MDMA self-administration in naïve mice. Psychopharmacology (Berl) 2006;184:212–220. doi: 10.1007/s00213-005-0229-7. [DOI] [PubMed] [Google Scholar]
- 52.Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Dependence. 1986;18:149–157. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- 53.Lamb RJ, Griffiths RR. Self-injection of 3,4-methylenedioxymethamphetamine (MDMA) in the baboon. Psychopharmacology. 1987;91:268–272. doi: 10.1007/BF00518175. [DOI] [PubMed] [Google Scholar]
- 54.Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78:135–140. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Ratzenboeck E, Saria A, Kriechbaum N, Zernig G. Reinforcing effects of MDMA (“ecstasy”) in drug-naïve and cocaine-trained rats. Pharmacology. 2001;62:138–144. doi: 10.1159/000056086. [DOI] [PubMed] [Google Scholar]
- 56.Gagnaire F, Micillino JC. Effects of triadimefon on extracellular dopamine, DOPAC, HVA and 5-HIAA in adult rat striatum. Toxicology. 2006;217:91–104. doi: 10.1016/j.tox.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Caudle WM, Richardson JR, Wang M, Miller GW. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology. 2005;26:721–728. doi: 10.1016/j.neuro.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan IA, Thomas P. Aroclor 1254 inhibits tryptophan hydroxylase activity in rat brain. Arch Toxicol. 2004;78:316–320. doi: 10.1007/s00204-003-0540-1. [DOI] [PubMed] [Google Scholar]
- 59.Khan IA, Thomas P. PCB congener-specific disruption of reproductive neuroendocrine function in Atlantic croaker. Mar Environ Res. 2006;62 Suppl:S25–S28. doi: 10.1016/j.marenvres.2006.04.029. [DOI] [PubMed] [Google Scholar]