Abstract
High resolution 13C NMR field cycling (covering 11.7 down to 0.002 T) relaxation studies of the sn-2 carbonyl of phosphatidylcholines in vesicles provide a detailed look at the dynamics of this position of the phospholipid in vesicles. The spin-lattice relaxation rate, R1, observed down to 0.05 T is the result of dipolar and CSA relaxation components characterized by a single correlation time τc, with a small contribution from a faster motion contributing CSA relaxation. At lower fields, R1 increases further with a correlation time consistent with vesicle tumbling. The τc is particularly interesting since it is 2-3 times slower than what is observed for 31P of the same phospholipid. However, cholesterol increases the τc for both 31P and 13C sites to the same value, ~25 ns. These observations suggest faster local motion dominates the dipolar relaxation of the 31P while a slower rotation or wobble dominates the relaxation of the carbonyl carbon by the α-CH2 group. The faster motion must be damped with the sterol present. As a general methodology, high resolution 13C field cycling may be useful for quantifying dynamics in other complex systems as long as a 13C label (without attached protons) can be introduced.
Motions of phospholipids in membranes have been the subject of intensive research for several decades. While chain motions are understood in a quantitative fashion as a result of diverse 2H and 13C NMR studies,1 the dynamic behavior of the interfacial region, including the fatty acyl carbonyls and the phosphorus moiety, is much less well characterized. Spin-lattice relaxation rates (R1) measured over a range of magnetic fields can in principle be very useful for determining time scales for different motions in the lipid bilayer. However, for phospholipid aggregates only a few studies have measured R1 (2H and 13C) at three or four fields, and those above 1 T, to try and assess lipid motions.2,3 Recent high-resolution 31P field cycling spin-lattice relaxation studies have yielded information on the motions of the phosphorus of different phospholipids in diverse aggregates.4-6 In this technique, the magnetic field is cycled by mechanically shuttling the sample from the center of the probe to a substantially lower magnetic field above the probe for the delay times normally used in conventional NMR relaxation sequences, then shuttling the sample back to the probe for readout of the relaxation at the lower field. This method, which allows access to a very wide field range (0.002 to 11.7T) for measuring R1 and interpreting it in terms of spectral density functions,4,5 is particularly useful for spin ½ nuclei without a directly bonded proton where the relaxation at the high fields of modern spectrometers is moderately long (R1 ~1 s-1) and dominated by mechanisms other than dipole-dipole interactions (e.g., chemical shift anisotropy, CSA). For multicomponent phospholipid vesicles, the analysis of the 31P R1 profile as a function of field for each phospholipid molecule yields several correlation times ranging from ps to μs for the motion of this segment of the phospholipid.4,5
The carbonyl region of phospholipids is likely to be critical for interactions with some peripheral proteins and other membrane components and might sense different motions than the phosphate portion of the molecule. In this report we present the first high-resolution 13C field cycling relaxation studies of sn-2 carbonyl 13C-labeled phosphatidylcholines (prepared by acylation of 1-palmitoyl-phosphatidyl-choline with either [1-13C]-oleic acid or [1-13C]-palmitic acid7) in small unilamellar vesicles (SUVs) as a direct probe of this interfacial region of phospholipids.
In small unilamellar vesicles (prepared by sonication) at 25°C, 1-palmitoyl-2-[1-13C]oleoylphosphatidylchoine (PO[1-13C]PC) mixed 1:1 with dioleoylphosphatidylmethanol (DOPMe) exhibits the field dependence profile shown in Figure 1A. Qualitatively, the profile between 0.04 and 11.7 T has features similar to that for 31P R1 of the POPC in these same bilayers.4,5 The simplest analysis treats this field-dependent profile as the result of dipolar and CSA relaxation components characterized by a single correlation time τc, with a small contribution from a faster motion contributing CSA relaxation.4 The balance of each of these terms for PO[1-13C]PC in the POPC/DOPMe vesicles is shown in Figure 1B. Above 2 T, R1 is dominated by CSA relaxation, while dipolar relaxation is the major mechanism below 1 T. The small rise in 13C R1 at high fields, due to faster, sub-nanosecond motions that contribute to CSA relaxation of the carbonyl, is much smaller for the 13C site compared to the 31P interaction.4,5 More interestingly, the correlation time, τc, associated with the dipolar relaxation is 16±2 ns. This value is about 2-3 times the value obtained for the 31P nucleus of POPC in POPC/DOPMe vesicles.4,6 Thus, the 5-7 ns motion that effectively relaxes the phosphate group either does not alter the orientation of the sn-2 carbonyl site or contributes much less to relaxation of the 13C-carbonyl compared to a slower motion. The value for Rc(0), the relaxation rate extrapolated to zero field, from this region of the field dependence of R1 provides an estimate of rCH, the distance of the 13C-labeled carbon to the major proton(s) that relax it,4 in this case the sn-2 α-CH2.8 The value obtained, 2.4 Å, is similar to what one would expect for the 13C-C-H distance involved.
Figure 1.
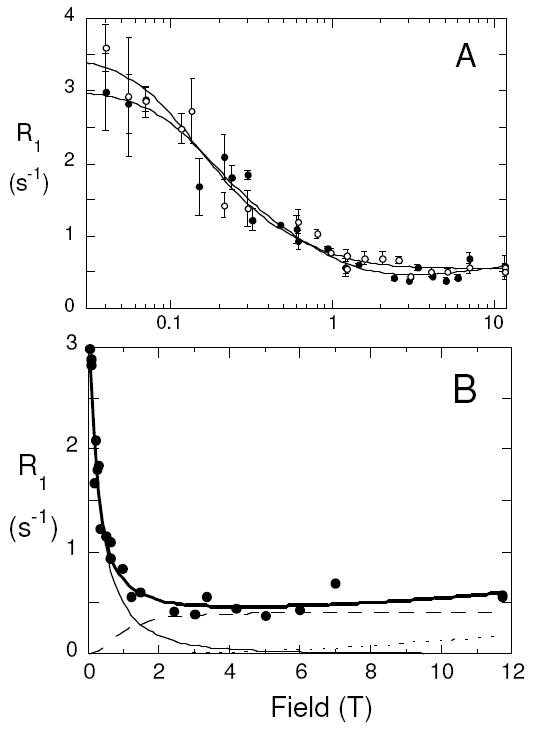
(A) Field dependence of 13C spin lattice relaxation rates, R1, for PO[1-13C]PC in SUVs composed of POPC (5 mM)/DOPMe (5 mM) in the absence (●) and presence (○) of 5 mM cholesterol; the semi-log plot emphasizes the behavior below 1T). (B) Deconvolution of R1 in the absence of cholesterol into a dipolar (—) and CSA (- - -) component with correlation time τc, and a faster CSA motion (dotted line visible above 6T).
We also examined the 13C relaxation profile for an sn-2 13C-labeled saturated chain lipid, dipalmitoyl-PC (PP[1-13C]PC), in this case mixed with unlabeled POPC to stabilize the small vesicles for extended observation at 40°C. The temperature was chosen to keep the fluid bilayer for the many acquisitions covering a course of a few days and to avoid getting too close to the maximum temperature of the shuttling system (50°C). As seen in Figure 2A, the PP[1-13C]PC profile was very similar to that for the PO[1-13C]PC and could be fit with a single τc of 16.2±2.5 ns; the average rCH was 2.51 Å.
Figure 2.
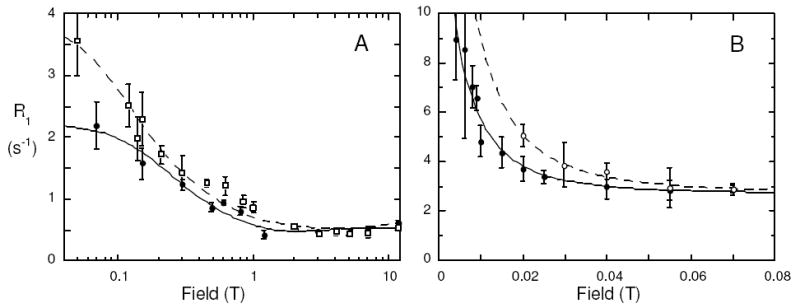
(A) Field dependence of PP[1-13C]PC in SUVs of DPPC (5 mM)/POPC (5 mM) at 40°C in the absence (●) and presence (□) of 5 mM cholesterol. (B) Very low field dependence of R1 on field from 0.07 down to 0.004 T for PO[1-13C]PC (mixed with DOPMe) in the absence (●) and presence (○) of 5 mM cholesterol.
If the 13C R1 is measured at much lower fields (down to 0.002 T), there is a further rise in the 13C relaxation rate that reflects the vesicle tumbling contribution to R1.5 For PO[1-13C]PC mixed with DOPMe, the correlation time is 1.4±0.5 μs (Figure 2B). This is very close to what one would expect as the correlation time for rotation of 250-300 Å vesicles (the average diameter measured by dynamic light scattering for this preparation is 254 Å with 91% of the sizes between 200 and 400Å).9 Geometric information can also be obtained from this very low field relaxation.5 The area under this dipolar portion of the R1 versus field curve compared to total dipolar relaxation yielded four values (±45.1° and ±65.7°) for the average θCH that the angle of the 13C-H vector makes with respect to the bilayer normal. While the orientation of the carbonyl to α-CH2 vectors in the crystal structure of dimyristoyl-PC might rule out the large negative angles,10 there may be sufficient segmental motion of the CH2 so that differentiating among these θCH is difficult. However, the ability to measure an averaged angle provides a novel way of characterizing changes induced by additives.
Cholesterol in a bilayer broadens the phase transition and makes membranes more gel-like. This physical change lengthens 31P τc values dramatically4,5; it would also be expected to alter the dynamics of the sn-2 carbonyl group of PC. With 33 mol% cholesterol in the PO[1-13C]PC/DOPMe vesicles (Figure 1A), the 13C-carbonyl of PC shows an increase in τc with cholesterol, but it is relatively small – from 16±2 ns to 23±3 ns. For the same amount of cholesterol in the PP[1-13C]PC/POPC vesicles, the τc increased to 28±4 ns (Figure 2A). Thus, the effect of cholesterol on the carbonyl motions of the two different vesicle systems is essentially the same – a small but significant increase in τc. For comparison, with cholesterol present, the 31P τc increased from 5-7 ns to ~25 ns (data not shown). In the presence of the sterol, the nanosecond correlation times for both the carbonyl and phosphate moieties are essentially the same, whereas in the absence of cholesterol, the two nuclei are sensitive to motions on slightly different time scales.
Another way of looking at differences in the 31P and 13C motions in the nanosecond regime is to examine the temperature dependence of R1 at a fixed low field where R1 is directly proportional to τc. Different motions are likely to have different energetics. For the 13C-labeled POPC, R1 was measured at 0.06 T. An Arrhenius plot of the 13C R1 (Figure 3) leads to a slope of 27±7 kJ/mol. For comparison the temperature dependence for the 31P of POPC at low field (0.032 T) yields a lower energy barrier, 12±3.8 kJ/mol.
Figure 3.
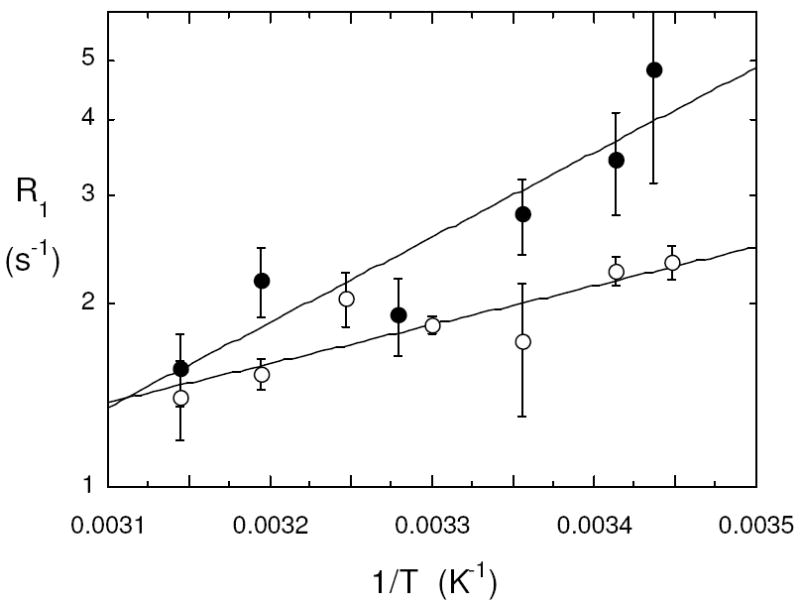
Fixed low field spin lattice relaxation rates for POPC in small vesicles with DOPMe as a function of temperature: (●) 13C R1 for PO[1-13C]PC measured at 0.06 T; (○) 31P R1 for POPC measured at 0.032 T.
These differences in the τc extracted from 31P and 13C in the same sample strongly indicate that the dipolar interactions of each group with its nearest protons do not reflect the same overall motion of the phospholipid. It has been suggested from molecular dynamics simulations that the ~10 ns 31P τc arises from motions that treat the phospholipid as a cylinder encompassing the 31P-glycerol-acyl chains that ‘wobbles’ around an axis perpendicular to the membrane surface.6 The shorter τc for 31P compared to 13C suggests that the phosphorus motion also contains some faster local motion along with the motion supplied by wobble (presumably what dominates the more rigid carbonyl relaxation). Interestingly, both 31P and 13C have the same correlation time when cholesterol is present, suggesting the faster motion of the phosphorus has been dampened by the presence of the sterol. High resolution 13C field cycling may also be useful for quantifying dynamics in other complex systems as long as the 13C label (without attached protons) can be introduced.
Acknowledgments
This research was supported by N.S.F. MCB-0517381 (to M.F.R.) and N.I.H GM077974 (to A.G.R).
References
- 1.For example, see: Brown MF. J Chem Phys. 1982;77:1576–1599.Huang TH, Lee CW, Das Gupta SK, Blume A, Griffin RG. Biochemistry. 1993;32:13277–13287. doi: 10.1021/bi00211a041.Sanders CR. Biophys J. 1993;64:171–181. doi: 10.1016/S0006-3495(93)81352-3.
- 2.Brown MF, Davis JH. Chem Phys Lett. 1981;79:431–435. [Google Scholar]
- 3.Lepore LS, Ellena JF, Cafiso DS. Biophys J. 1992;61:767–775. doi: 10.1016/S0006-3495(92)81881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts MF, Redfield AG. J Am Chem Soc. 2004a;126:13765–13777. doi: 10.1021/ja046658k. [DOI] [PubMed] [Google Scholar]
- 5.Roberts MF, Redfield AG. Proc Natl Acad Sci USA. 2004b;101:17066–17071. doi: 10.1073/pnas.0407565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klauda JB, Roberts MF, Redfield AG, Brooks BR, Pastor RW. Biophys J. 2008;94:3074–3083. doi: 10.1529/biophysj.107.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neises B, Steglich W. Angew Chem Int Ed. 1978;17:522–524. [Google Scholar]
- 8.Hong M, Schmidt-Rohr K, Zimmermann H. Biochemistry. 1996;35:8335–8341. doi: 10.1021/bi953083i. [DOI] [PubMed] [Google Scholar]
- 9.Pu M, Fang X, Gershenson A, Redfield AG, Roberts MF. J Biol Chem. 2009;284:16099–6107. doi: 10.1074/jbc.M809600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson RH, Pascher I. Nature. 1979;281:499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]


