Abstract
Specification of the anteroposterior (AP) axis in Drosophila oocytes requires proper organization of the microtubule and actin cytoskeleton. The establishment and regulation of cytoskeletal polarity remain poorly understood, however. Here, we show important roles for the tumor suppressor Lethal (2) giant larvae (Lgl) and atypical protein kinase C (aPKC) in regulating microtubule polarity and setting up the AP axis of the oocyte. Lgl in the germline cells regulates the localization of axis-specifying morphogens. aPKC phosphorylation of Lgl restricts Lgl activity to the oocyte posterior, thereby dividing the cortex into different domains along the AP axis. Active Lgl promotes the formation of actin-rich projections at the oocyte cortex and the posterior enrichment of the serine/threonine kinase Par-1, a key step for oocyte polarization. Our studies suggest that Lgl and its phosphorylation by aPKC may form a conserved regulatory circuitry in polarization of various cell types.
Keywords: Lethal (2) giant larvae (Lgl), Atypical protein kinase C (aPKC), Oocyte polarity, Par-1, Microtubule, Oogenesis, Drosophila
Introduction
Cell polarity, the asymmetry in distribution of cellular constituents within a single cell, is fundamental to cellular life and essential for generating cell diversity. For example, epithelial cells have a characteristic apicobasal polarity that is necessary for their function as barriers between different extracellular environments; this polarity facilitates directed trafficking of molecules between those environments. The polarity established in neuroblasts is the basis for their asymmetrical division, which produces distinctive cell types. Loss of polarity in cells is frequently related to the disruption of cellular function and can sometimes result in tumorigenesis.
The Drosophila oocyte exhibits an anteroposterior (AP) asymmetry with respect to its cytoskeletal organization and the distribution of some mRNAs and proteins (Riechmann and Ephrussi, 2001; van Eeden and St Johnston, 1999). This asymmetry provides the basis for the formation of the major body axes in the subsequent embryo. A key step for oocyte polarity formation is the reorganization of the microtubules in the germline during mid-oogenesis. Previous studies show that the microtubule (MT) nucleating activity is associated with the centrosome-nucleus complex (Januschke et al., 2006). After stage 6, MT reorganization in the oocyte occurs at the onset of anterior migration of the nucleus (Januschke et al., 2006), presumably as a consequence of the polarizing signal from the follicle cells (Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995; Ruohola et al., 1991). Reorganization of the posterior microtubule organizing center is believed to allow formation of a gradient of MT from high density at the anterior to low density at the posterior (Cha et al., 2001; Januschke et al., 2006; Theurkauf et al., 1992). When the reorganization of MT within the oocyte takes place at this stage, the MT plus ends, as visualized by the localization of a protein fusion of the plus-end MT motor kinesin (Kin:β-Gal) (Clark et al., 1994), accumulate in the posterior. This new microtubule orientation is necessary for localization of maternal determinants such as oskar (osk), bicoid (bcd), and gurken (grk) mRNAs, which are the foundation for specification of the AP and dorsoventral axes (Neuman-Silberberg and Schupbach, 1993; Nilson and Schupbach, 1999; van Eeden and St Johnston, 1999).
Lethal (2) giant larvae (Lgl), a WD40 domain-containing protein, is implicated in cellular asymmetry formation in a number of cell types (Vasioukhin, 2006; Wirtz-Peitz and Knoblich, 2006), but its role in the oocyte is unclear. Lgl is evolutionarily conserved across the eukaryotes; orthologs range from budding yeast (Sro7/Sro77) to human (Lgl1 and Lgl2) (Vasioukhin, 2006). In Drosophila, lgl mutations result in loss of apicobasal polarity in epithelial cells and produce tumors of the brain, the imaginal disc and the follicular epithelium (Bilder et al., 2000; Ohshiro et al., 2000; Peng et al., 2000). In neuroblasts, Lgl is phosphorylated by aPKC in the apical region to direct localization of basal components involved in asymmetric cell divisions (Betschinger et al., 2003). The precise role of Lgl in cell polarity formation in Drosophila and other multicellular organisms remains largely unknown. Here, we report that Lgl and its phosphorylation by aPKC are required for oocyte polarity formation. aPKC phosphorylation of Lgl restricts Lgl activity to the oocyte posterior and regulates posterior enrichment of Par-1 and organization of microtubule polarity that is required for morphogen localization and axis specification.
Materials and Methods
Fly strains and genetics
The strains were raised at 25°C on standard media. We used lgl4 and lgl4w3 as null alleles for lgl (Bilder et al., 2000; Manfruelli et al., 1996; Ohshiro et al., 2000). lgl and apkc germline clones were generated by FLP-FRT-induced mitotic recombination (Xu and Rubin, 1993), and lgl germline clones were also generated by the DFS FRT/FLP-ovoD1 system (Chou and Perrimon, 1996) from the following strains: lgl4P[neoFRT]40A/Cyo (strong LOF allele), lgl4w3P[neoFRT]40A/Cyo (strong LOF allele), P[ry+, FRT]42D apkcK06403/Cyo (strong LOF allele), yw P[mini-w+, hsFLP]1; P[Ubi-GFP]33 P[Ubi-GFP]38 P[neoFRT]40A/CyO, yw P[mini-w+, hsFLP]1; P[ovoD1-18]2La P[ovoD1-18]2Lb P[neoFRT]40A/CyO, yw P[mini-w+, hsFLP]1; P{arm-lacZ.V}36BC P[neoFRT]40A/CyO, w[1118]; P[neoFRT]42D P{w[+mC]=Ubi-GFP(S65T)nls}2R/Cyo. We induced germline clones by administering 2 hour 37°C heat shocks on two consecutive days during the third instar. For aPKC and GFP-Par-1(N1S) overexpression, we obtained UAS:aPKC and UAS:aPKCΔN transgenic fly stocks from C. D. Doe (Lee et al., 2006), transgenic fly stocks with pUASP: GFP-Par-1(N1S) on the 2nd chromosome from D. St Johnston (Huynh et al., 2001), and transgenic fly stocks with pUASP:GFP-Par-1(N1S) K* and pUASP:GFP-Par-1(N1S) on the 3rd chromosome from A. Ephrussi (Vaccari et al., 2005). Matα4-tubulin-Gal4-VP16 (Mat-Gal4) was used for the overexpression in the germline.
Molecular biology and biochemical analysis
The cDNA of lgl and lgl-3A from J. A. Knoblich (Betschinger et al., 2003) and apkc from DGRC were amplified, sequenced and subcloned into the pUASP (Rorth, 1998) or pUASP:GFP vectors for Drosophila transgenes. Transgenic lines of (1) pUASP:Lgl, (2) pUASp:Lgl-3A, pUASP:Lgl-GFP, (3) pUASP-Lgl-3A-GFP, (4) pUASP:aPKC-GFP and (5) pUASP:aPKCΔN-GFP constructs were generated by standard methods and overexpressed by means of the germline Gal4 drivers (Mat-Gal4). One of each of these transgenic lines were used for phenotypic and biochemical analyses.
For the co-immunoprecipitation (co-IP) analysis, ovarian extracts were prepared from wild-type flies or transgenic flies expressing pUASP:GFP-Par-1 (N1S) in homogenization buffer [150 mM NaCl, 50 mM HEPES (pH 7.2), 0.5 mM DTT, PMSF, Complete Protease Inhibitor cocktail (Roche)]. Lysate was incubated with primary antibody (rabbit anti-Lgl antibody) (Betschinger et al., 2003) or mouse anti-GFP antibody (Santa Cruz Biotechnology) for 10 hours at 4°C. Protein A/G plus-agarose (Santa Cruz) was then added, and the reaction was allowed to proceed for 4 hours at 4°C. Analysis was conducted with SDS-PAGE followed by western blots. In anti-Lgl immunoprecipitates, controls were only beads (without added antibody) and peptide blocks; in anti-GFP immunoprecipitates, controls were beads (without added antibody) and the ovarian extract from wild-type flies. Anti-Cut antibody was used as a negative control in the co-IP experiment, which did not detect the Cut band in the western blot containing proteins pulled down by anti-Lgl antibody (data not shown).
Antibody staining, imaging and analysis
Antibody staining was performed according to standard procedures (Sun and Deng, 2005). Primary antibodies were diluted as follows: rabbit anti-Lgl and anti-pLgl, 1:200 (Betschinger et al., 2003); rabbit anti-aPKC, 1:1000 (Santa Cruz Biotechnology); rabbit anti-Stau, 1:5000 (St Johnston et al., 1991); mouse anti-Gurken, 1:20 (Developmental Studies Hybridoma Bank (DSHB)); rabbit anti-β-galactosidase, 1:5000 (Sigma); Alexa Fluor 546 Phalloidin, 1:50 (Invitrogen), mouse anti-Cut, 1:50 (DSHB); mouse anti-Hindsight (Hnt), 1:20 (DSHB); mouse anti-Eyes absent (Eya), 1:50 (DSHB). For microtubule staining, samples were fixed for 10 minutes in 8% paraformaldehyde (Doerflinger et al., 2006) and stained with a FITC-conjugated monoclonal anti-α-tubulin antibody DM1A (1:400; Sigma). Secondary antibodies conjugated to Alexa Fluor 546 goat anti-mouse and goat anti-rabbit (Molecular Probes) were used at 1:400. Fluorescently labeled samples were counterstained with DAPI for visualization of DNA. In situ hybridizations were carried out with RNA probes labeled with Digoxigenin-UTP (Roche). Immunohistochemical detection was performed with alkaline phosphatase-conjugated anti-DIG (1:5000; Roche). Images were captured with a Zeiss LSM 510 confocal microscope and assembled in Adobe Photoshop.
Results
Lgl is involved in the germline cells for oocyte polarity formation
In a study designed to determine whether genes regulating epithelial polarity can regulate oocyte polarization, we analyzed the staining patterns of antibodies against Staufen (Stau) and Gurken (Grk) in lgl4 germline clones using the FLP/FRT system (Xu and Rubin, 1993). Stau, an RNA-binding protein that colocalizes with osk mRNA to the oocyte posterior (St Johnston et al., 1991) (Fig. 1A,E), showed abnormal localization in lgl4 germline clones at stages 9/10 (58%, n=81; 17% mislocalized to the center (hereafter called ‘complete mislocalization’), 31% had both ectopic and posterior localization (‘partial mislocalization’) and 10% had diffuse mislocalization (‘diffuse mislocalization’); Fig. 1B and data not shown). The TGF-α homolog Grk, which normally moves with the oocyte nucleus from the posterior to an anterior corner to induce dorsoventral patterning (Neuman-Silberberg and Schupbach, 1993) (Fig. 1C), failed to reach its correct destination in some lgl4 germline clones (16%, n=144; Fig. 1D). In these cases, Grk and the oocyte nucleus were either mislocalized to a lateral position in the oocyte (Fig. 1D) or remained at the posterior (data not shown). To confirm the involvement of Lgl in localizing oocyte polarity markers, we generated germline clones of a different lgl mutant allele, lgl4w3, and similar defects of Stau and Grk localization were found (data not shown). When RNA in situ hybridization was used to stain the lgl4 germline clones generated by a dominant female sterile (DFS) technique, the posterior determinant osk mRNA (Kim-Ha et al., 1991) (Fig. 1E) was mislocalized in the oocyte at stages 9/10 (68%, n=143; 13% complete, 38% partial and 17% diffuse; Fig. 1F and data not shown). bcd mRNA, which is normally localized to the extreme anterior cortex of the oocyte in a disc-shaped pattern (Driever and Nusslein-Volhard, 1988) (Fig. 1G), accumulated as dots in the anterior of lgl germline clones (38%, n=128; Fig. 1H), also indicating a defect in localizing the anterior determinant.
Fig. 1. lgl germline clones affect oocyte polarity.
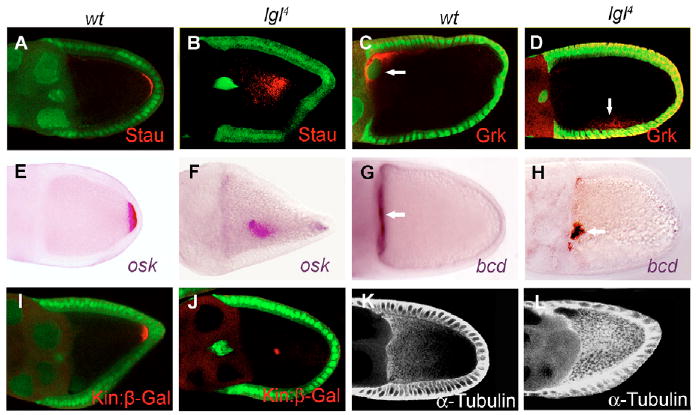
(A,B) Stau was localized at the posterior in the wild type (A) but is mislocalized in lgl4 mutant germline clones (B). (C,D) Grk and the oocyte nucleus (arrow) were localized to the anterior corner (C) but were mislocalized to a lateral position in some lgl4 mutant germline clones (D). (E,F) osk mRNA in wild-type egg chambers showed a posterior localization at stage 10 (E), whereas lgl4 germline clones displayed clear mislocalization of osk mRNA to the center of the oocyte (F). (G,H) bcd mRNA in wild-type egg chambers showed disc-shaped localization in the oocyte anterior (G, arrow), but in lgl4 germline clones bcd sometimes accumulated as dots at the anterior of the oocyte (H, arrow). (I,J) Kin:β-Gal showed posterior localization in the wild type (I) but was mislocalized to the center of the oocyte in lgl4 germline clones (J). (K,L) α-Tubulin showed an anterior-to-posterior gradient in the wild-type oocyte (K) but was uniformly distributed in lgl4 germline clones (l). Mutant germline clones were marked by the absence of GFP (green) in the nuclei of the nurse cells (B,D,J) or were generated by the dominant female sterile method (F,H,L). Anterior is towards the left and posterior is towards the right in all panels.
To gain more insight into the oocyte polarity defects of lgl germline clones, we analyzed the localization of a microtubule plus-end marker, Kinesin:β-galactosidase (Kin:β-gal) (Clark et al., 1994), which is normally localized at the posterior end of the oocyte during stages 8-9 (Fig. 1I). In lgl germline clones, Kin:β-gal was not concentrated at the posterior and instead mislocalized as ectopic dots in the center or partially mislocalized or diffused from the posterior of the oocyte (57%, n=97; 14% complete, 36% partial and 7% diffuse; Fig. 1J and data not shown). In oocytes of lgl germline mutants, the anterior-to-posterior gradient of microtubules at stages 7-8 (Theurkauf et al., 1992) (Fig. 1K), as visualized by direct staining with a FITC-conjugated antibody against α-Tubulin, was also disrupted. Instead of an AP gradient, a high density of microtubules was detected throughout the oocyte of lgl germline clones (Fig. 1L). These defects in microtubule organization could contribute to the defects in Stau/osk and Grk mislocalization.
Follicle-cell differentiation is normal in lgl germline clones
Because the reorientation of microtubule polarity in the oocyte depends on a polarizing signal from the posterior follicle cells (PFC), we examined whether these follicle cells were specified correctly in egg chambers bearing lgl germline clones. First, we stained the lgl mosaics with antibodies against Cut and Fasciclin 3 (Fas3), which are both downregulated by Notch after stage 6 (Lopez-Schier and St Johnston, 2001; Sun and Deng, 2005) (Fig. 2A,A′ and data not shown). In the PFC covering the lgl germline clones, we found no change in the expression of these two immature follicle cell-fate markers (Fig. 2B,B′ and data not shown). Hindsight (Hnt), a Notch target in follicle cells during mid-oogenesis (Sun and Deng, 2007), maintained its expression correctly in the PFC after stage 6 (Fig. 2C,C′), suggesting Notch signaling is unaffected in lgl germline clones (Fig. 2D,D′). We then examined the expression of Eyes absent (Eya), which is normally expressed in both immature follicle cells and the anterior follicle cells after stage 7 (Bai and Montell, 2002) (Fig. 2E,E′), and found no expression in the PFC covering the lgl germline clone (Fig. 2F,F′), suggesting that the PFC do not take either the immature or anterior follicle cell fate. In addition, a PFC fate marker, Pointed-lacZ (Pnt-lacZ) (Gonzalez-Reyes and St Johnston, 1998; Poulton and Deng, 2006), continued to be expressed in the PFC covering the lgl germline clones (Fig. 2H,H′). The correct expression of the follicle cell-fate markers in follicle cells over the lgl germline clone suggests that the activation of the Notch and EGFR pathways is unaffected. Therefore, the possibility that Lgl is involved in the germline signaling that induces follicle-cell differentiation can be excluded. Together, these data indicate that Lgl acts downstream of the polarizing signal from the PFC to specify the oocyte AP axis.
Fig. 2. Follicle-cell fate markers are unaffected in lgl germline clones.
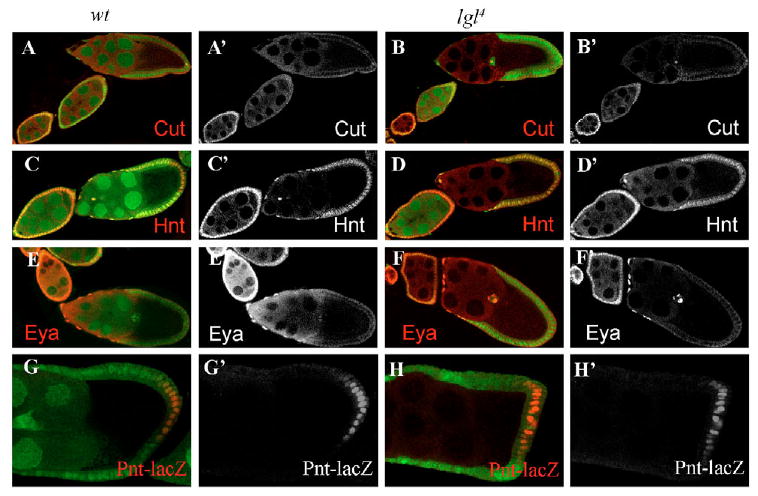
(A-H′) Cut is an immature cell fate marker that is downregulated by Notch signaling at stage 6. Hindsight (Hnt) is expressed after stage 6 and Eyes absent (Eya) is normally expressed in anterior follicle cells and immature follicle cells. Pointed-lacZ is a posterior follicle-cell fate marker. No differences in expression of these markers between lgl4 germline clones (B,D,F,H) and the wild type (A,C,E,G) were detected. Mutant germline clones were marked by the absence of GFP (green) in the nuclei of the nurse cells.
Phosphorylation of Lgl by aPKC is critical for oocyte polarity
Lgl can be phosphorylated by aPKC at three conserved serine residues in the central region of the protein, a process required for inactivation of Lgl (Betschinger et al., 2005; Betschinger et al., 2003). Using the co-IP technique, we detected aPKC in the protein complex pulled down by a polyclonal antibody against Lgl from the ovarian lysate (Fig. 6E), suggesting that Lgl and aPKC interact during oogenesis.
Fig. 6. Lgl interacts with Par-1 and is required for Par-1 localization.
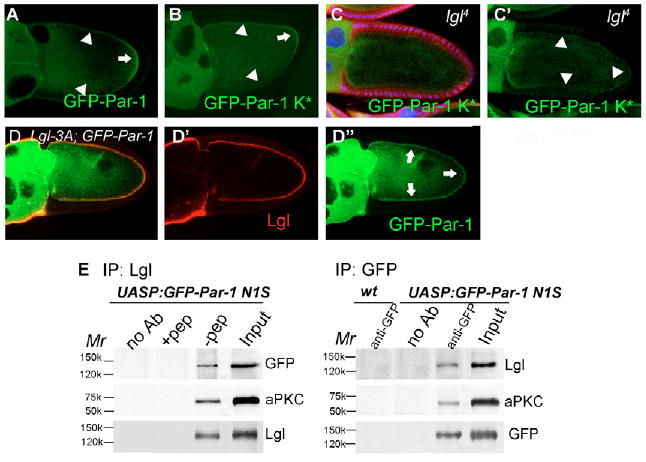
(A,B) Both the GFP-Par-1(N1S) (A) and GFP-Par-1(N1S) K* (B) were enriched at the posterior at stage 10. (C,C′) The GFP-Par-1(N1S) K* lost the posterior enrichment in lgl4 germline cells. Mutant germline clones were marked by the absence of β-gal staining (red) in the nuclei of the nurse cells. (D-D″) GFP-Par-1(N1S) colocalized with Lgl and was detected throughout the oocyte cortex in egg chambers with co-overexpression of Lgl-3A and GFP-Par-1(N1S). Arrows indicate the strong GFP-Par-1 and arrowheads indicate the weak GFP-Par-1. (E) Immunoblots of anti-Lgl or anti-GFP immunoprecipitation from ovaries with GFP-Par-1 (N1S) overexpression (mat-Gal4 driver) or the wild-type control (mat-Gal4 driver only). aPKC and GFP-Par-1 (N1S) proteins were precipitated by the anti-Lgl antibody from GFP-Par-1 (N1S) overexpression, and aPKC and Lgl proteins were precipitated by an anti-GFP antibody from GFP-Par-1 (N1S) overexpression. Input was one-fifth of the total ovarian extract. In anti-Lgl immunoprecipitates, controls were beads (without added antibody) and peptide blocks (+pep); in anti-GFP immunoprecipitates, controls were beads (without added antibody) and ovarian extract from wild-type flies.
To determine whether the phosphorylation of Lgl by aPKC is required for oocyte polarization, we generated UASP:Lgl and UASP:Lgl-3A (a mutated form of Lgl in which all three aPKC phosphorylation sites were mutated to alanine) (Betschinger et al., 2003) transgenes, overexpressed them using a germline Gal4 driver Matα4-Gal4-VP16 (Mat-Gal4), and examined oocyte polarity markers in these egg chambers. To confirm the expression of Lgl and Lgl-3A in the germline, we performed a western blot using the anti-Lgl and anti-phosphorylated-Lgl (pLgl) (Betschinger et al., 2003). We found that levels of pLgl in ovaries with germline overexpression of the wild-type Lgl transgene were more than twice as high as endogenous levels, whereas only endogenous levels of pLgl and a similar level of Lgl were detected in Lgl-3A-overexpressing egg chambers (see Fig. S1A,B in the supplementary material). Therefore, levels of expression of Lgl in Lgl and Lgl-3A overexpressing chambers were similar, as were levels of phosphorylatable Lgl in wild-type and Lgl-3A overexpressing chambers. Stau and osk mRNA were mislocalized in the majority of the Lgl-3A-overexpressing egg chambers at stages 9/10 (Stau: 74%, n=149; 44% complete, 10% partial and 20% diffuse, Fig. 3C,I; see Fig. S2C in the supplementary material; and data not shown; osk: 80%, n=130; 46% complete, 15% partial and 19% diffuse; Fig. 3F,I; and data not shown), Grk mRNA in the oocyte nucleus was mislocalized in 8% (n=150) of these egg chambers (Fig. 3L), and bcd mRNA was mislocalized to the posterior in 5% (n=120) of these egg chambers (see Fig. S3B in the supplementary material). In addition, Kin:β-gal was mislocalized in the oocyte (77%, n=121; 54% complete, 13% partial and 10% diffuse; Fig. 3O and data not shown), and a high density of microtubules was detected around the cortex (Fig. 3R) or in the central or lateral part of the oocyte (data not shown), indicating severe defects in microtubule organization in the oocyte. By contrast, although wild-type Lgl-overexpression also caused some Stau or osk mislocalization, the percentage was much lower (Stau: 31%, n=123; 10% complete, 17% partial and 4% diffuse, Fig. 3B,H, and see Fig. S2B in the supplementary material; and data not shown; osk: 32%, n=149; 9% complete, 20% partial and 3% diffuse; Fig. 3E,H; and data not shown), diffuse localization of bcd mRNA was detected at the anterior (3%, n=125; data not shown), and no defect was apparent in Grk localization (n=123; Fig. 3K). Microtubules appeared to be only mildly affected in these egg chambers (Fig. 3Q), and Kin:β-gal was modestly disrupted at the posterior (36%, n=117; 10% complete, 18% partial, and 8% diffuse; Fig. 3N). Because the differences between Lgl-3A and Lgl lie in whether they can be phosphorylated by aPKC, the stronger oocyte polarity defects in Lgl-3A-overexpressing egg chambers suggest that Lgl phosphorylation by aPKC is important for oocyte polarization.
Fig. 3. Lgl phosphorylation can regulate oocyte polarity.
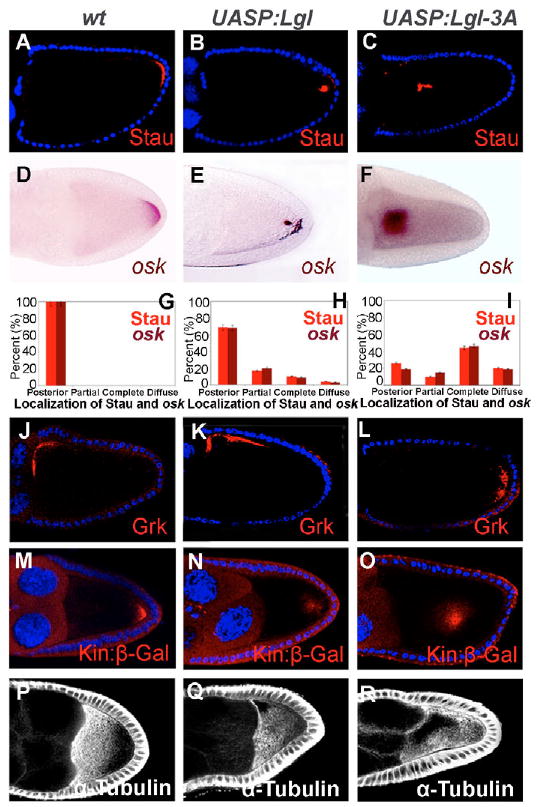
Stau (A-C), osk (D-F), the quantification of Stau and osk mRNA localization (G-I), Grk (J-L), Kin:β-Gal (M-O), α-Tubulin (P-R) in the wild type (driver only) (A,D,G,J,M,P), Lgl-overexpressing (B,E,H,K,N,Q) and Lgl-3A-overexpressing (C,F,I,L,O,R) egg chambers are shown. In egg chambers with Lgl overexpression, Stau (B,H), osk (E,H) and Kin:β-gal (N) were partially mislocalized, but Grk (K) showed normal localization, and α-Tubulin (Q) displayed a weaker anterior-posterior gradient than in the wild type (P). In egg chambers with Lgl-3A overexpression, Stau (C,I), osk (F,I) and Kin:β-gal (O) were frequently mislocalized to the center of the oocyte, Grk and the oocyte nucleus (L) were mislocalized in some egg chambers, and α-Tubulin showed higher concentrations along the cortex and lower concentration at the center of the oocyte (R).
The severe oocyte polarity defects caused by the nonphosphorylatable form of Lgl prompted us to study the requirement for aPKC in oocyte polarization. Previous clonal analysis revealed a requirement for aPKC in the maintenance of the oocyte cell fate (Cox et al., 2001). Because overexpression of Lgl-3A in the germarium using the nanos-Gal4 drivers (Van Doren et al., 1998; Wang and Lin, 2004) did not cause any oocyte differentiation defect (data not shown), other aPKC targets may be involved in the maintenance of the oocyte fate during early oogenesis. Although 80% (n=101) of the apkck06403 germline clones were arrested at stage 5 or 6, we found that some germline clones developed until stages 9 and 10. In oocytes of these clones, the posterior localization of Stau was disrupted (47%, n=114; 35% complete, 7% partial and 5% diffuse; Fig. 4B and data not shown), suggesting a previously unidentified role for aPKC in polarizing the AP axis of the oocyte during mid-oogenesis. In these apkc germline clones, follicle-cell fate markers (Cut, Eya, and Hnt) showed normal expression patterns (data not shown). These data suggest that aPKC and Lgl may function in the same pathway to regulate the oocyte polarity.
Fig. 4. The genetic interaction between Lgl and aPKC shows that Lgl phosphorylation by aPKC can regulate oocyte polarity.
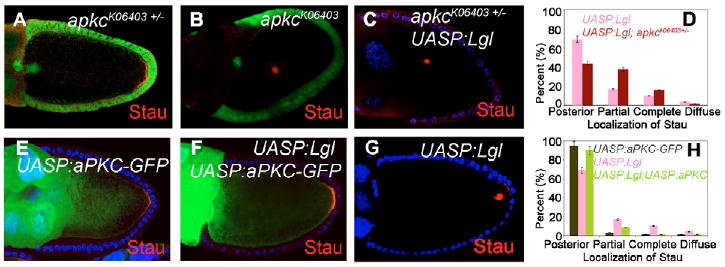
(A, B) Stau was normally localized in the apkck06403 heterozygous mutant (A), but mislocalized in apkck06403 mutant germline clones (B); mutant clones were marked by the absence of nuclear GFP (green). (C, D) Loss of one copy of apkc exaggerated the Stau mislocalization phenotype stemming from Lgl overexpression. Stau localization in these backgrounds was quantified (D). (E) Stau is normally localized in aPKC-GFP overexpression. (F) Overexpression of aPKC-GFP can rescue the Stau mislocalization phenotype caused by the Lgl overexpression. (G) Stau is mislocalized in the Lgl-overexpressing egg chambers. (H) Stau localization in these backgrounds was quantified.
To determine whether apkc and lgl interact genetically in germline cells, we overexpressed Lgl in a heterozygous apkc mutant background. These egg chambers demonstrated a greater penetrance of oocyte polarity defects (Stau: 56%, n=128; 16% complete, 38% partial and 2% diffuse; Fig. 4C,D) than did those with Lgl overexpression alone, suggesting that Lgl and aPKC are functionally related in oocyte polarization. Moreover, co-overexpression of Lgl with aPKC alleviated the oocyte polarity defects (Stau: 10%, n=111; 1% complete, 8% partial and 1% diffuse; Fig. 4F,H) caused by Lgl overexpression alone (Fig. 4G and Fig. 3B), whereas overexpression of aPKC alone did not result in any obvious oocyte polarity defects (Stau: 96%, n=117; Fig. 4E). By contrast, co-overexpression of Lgl-3A with aPKC produced oocyte polarity defects similar to those of Lgl-3A overexpression alone (data not shown), suggesting that aPKC cannot modify the phenotypes caused by overexpression of the nonphosphorylatable form of Lgl. Together, these results strongly suggest that the phosphorylation of Lgl by aPKC plays a crucial role in regulating oocyte polarity formation.
aPKC restricts Lgl localization to the posterior of the oocyte
To examine the localization of Lgl in the oocyte, we stained egg chambers with anti-Lgl antibody, but endogenous Lgl was only detected at a minimal level in the germline cells (data not shown). We therefore used the UASP:Lgl transgene and stained for Lgl in egg chambers with Lgl overexpression driven by Mat-Gal4. Lgl was enriched at the posterior oocyte cortex in the majority of these egg chambers starting at stage 7 (stage 7, posterior: 79%; the entire cortex: 21%, n=115; Fig. 5A and data not shown; stage 9/10, posterior: 35%; posterior and half of the lateral: 42%; the entire cortex: 23%, n=106; Fig. 5B and data not shown). To confirm these localization patterns, we generated GFP-tagged Lgl (UASP:Lgl-GFP) and expressed it in the germline using the same driver, Mat-Gal4. Consistent with the Lgl pattern, Lgl-GFP was enriched at the posterior cortex in the majority of the egg chambers also starting at stage 7 (Fig. 5C,D).
Fig. 5. aPKC phosphorylation of Lgl restricts Lgl localization to the oocyte posterior.
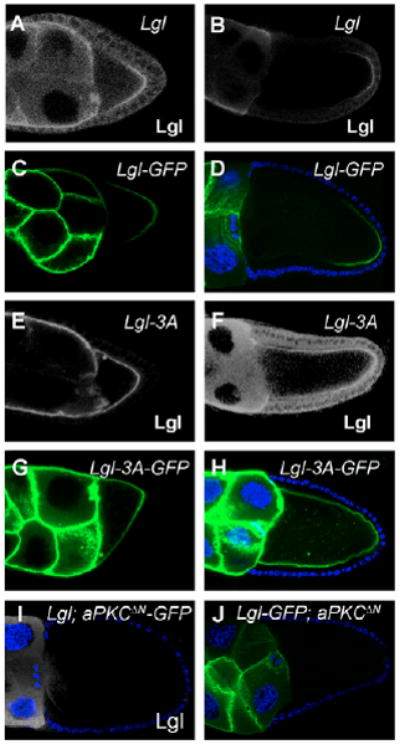
(A,B) Lgl was localized at the oocyte posterior in Lgl-overexpressing egg chambers at stage 7 (A) and stage 9/10 (B). (C,D) Lgl was localized at the oocyte posterior in Lgl-GFP-overexpressing egg chambers at stage 7 (C) and stage 9/10 (D). (E,F) Lgl was localized throughout the cortex in Lgl-3A-overexpressing egg chambers at stage 7 (E) and stage 9/10 (F). (G,H) Lgl was localized throughout the cortex in Lgl-3A-GFP-overexpressing egg chambers at stage 7 (G) and stage 9/10 (H). (I,J) When Lgl or Lgl-GFP was co-overexpressed with aPKCΔN-GFP (GFP is not shown here) or aPKCΔN, Lgl or Lgl-GFP was excluded from the cortex.
To determine the effect of phosphorylation of Lgl on its localization, we stained for Lgl protein in Lgl-3A-overexpressing egg chambers and found Lgl localization throughout the oocyte cortex (100%, n=153; Fig. 5E,F). This localization pattern was confirmed in another transgenic line with overexpression of GFP-tagged Lgl-3A (Lgl-3A-GFP) in the germline (Fig. 5G,H). The expansion of nonphosphorylatable and active Lgl along the oocyte cortex suggests that Lgl phosphorylation by aPKC plays an important role in restricting Lgl to the posterior of the oocyte.
To analyze further the relationship between Lgl phosphorylation and the subcellular localization of Lgl in the oocyte, we monitored the distribution of Lgl in egg chambers with co-overexpression of Lgl (or Lgl-GFP) and aPKCΔN-GFP (or aPKCΔN) (a dominant active form of aPKC) (Betschinger et al., 2003). Most of these egg chambers failed to show Lgl association with the oocyte cortex, and the rest showed only weak posterior or weak entire cortex Lgl localization (no cortex localization: 59%; weak posterior: 23%; weak entire cortex: 18%, n=87; Fig. 5I,J and data not shown), indicating Lgl is excluded from the cellular cortex by aPKC phosphorylation. By contrast, the localization of Lgl-3A along the oocyte cortex was not changed in egg chambers with co-expression of Lgl-3A and aPKCΔN-GFP when compared with Lgl-3A overexpression alone (see Fig. S4 in the supplementary material). These results suggest that phosphorylation of Lgl by aPKC is crucial for Lgl localization in the oocyte.
Active Lgl is involved in posterior enrichment of Par-1
The GFP tagged Par-1 (N1S) showed enrichment of the serine/threonine kinase Par-1 in the posterior (Fig. 6A), starting at stage 7, which was the earliest step of oocyte polarization after the PFC-oocyte signaling (Doerflinger et al., 2006; Vaccari et al., 2005). Because Lgl is also localized at the posterior cortex beginning at stage 7, we performed co-IP experiments to determine whether Par-1 (N1S) and Lgl interact biochemically in the germline cells. In the lysate from GFP-Par-1(N1S)-overexpressing egg chambers (Benton and St Johnston, 2003; Huynh et al., 2001), Lgl and GFP-Par-1 (N1S) immunoprecipitated together (Fig. 6E), suggesting that Lgl and Par-1 could form a complex in the germline cells during oogenesis.
To determine the relationship of Lgl and Par-1 localization in the oocyte, we used both UASP:GFP-Par-1(N1S) and UASP:GFP-Par-1 (N1S) K*, a kinase-dead form of the Par-1(N1S) isoform that has exactly the same localization pattern as the normal Par-1 (N1S) protein (Vaccari et al., 2005) (Fig. 6B), to monitor any change in Par-1 localization in lgl germline clones. We found that both GFP tagged forms of Par-1 [(N1S) K* and (N1S)] were no longer enriched at the posterior but became uniformly distributed in the cytoplasm and cortical region of the oocyte (55%, n=31; Fig. 6C,C′ and data not shown), suggesting that Lgl is required for Par-1 (N1S) enrichment at the oocyte posterior. In egg chambers with Lgl-3A overexpression, active Lgl expanded towards the lateral domains of the oocyte cortex. We asked whether mislocalized active Lgl would disrupt Par-1 localization as well. In egg chambers with co-expression of Lgl-3A and GFP-Par-1 (N1S) in the germline, we found that GFP-Par-1 (N1S) expanded throughout the oocyte cortex, colocalizing with Lgl-3A (Fig. 6D-D″), indicating that active Lgl may play a role in directing Par-1 (N1S) to the oocyte cortex.
Lgl regulates actin organization in the oocyte
Par-1 (N1S) localization depends on actin but not microtubule organization in the oocyte (Doerflinger et al., 2006), and GFP-Par-1 (N1S) showed tight association with the cortical actin at the posterior cortex (Fig. 7A). In egg chambers with Lgl-GFP overexpression, Lgl-GFP also colocalized with the cortical actin cytoskeleton (Fig. 7B). Interestingly, under high magnification, the cortical actin in Lgl-GFP-overexpressing egg chambers exhibited projections mainly at the oocyte posterior (Fig. 7B). Careful observation of wild-type oocytes also identified such actin-rich structures at the posterior (Vanzo et al., 2007) (84%, n=45; Fig. 7C), albeit weaker than those in the Lgl-overexpressing oocytes, indicating that cortically localized Lgl may promote the formation of these actin projections. To test this hypothesis, we monitored actin organization in egg chambers with Lgl-3A overexpression or containing lgl4 germline clones. If Lgl function is involved in the formation of the actin projections, the lateral oocyte cortex should have actin projections in egg chambers with Lgl-3A overexpression, which have active Lgl localized along the entire cortex. Indeed, in these egg chambers we observed long and thick actin projections throughout the cortex of the oocyte (91%, n=55; Fig. 7D). When treated with an actin depolymerizing drug, latrunculin A, the actin projections were dramatically compromised and mislocalized into the cytoplasm (data not shown). By contrast, actin projections were undetectable in lgl4 germline clones (74%, n=35; Fig. 7E). These data suggest that active Lgl in the oocyte regulates the organization of the cortical actin cytoskeleton.
Fig. 7. Lgl regulates actin organization in the oocyte.
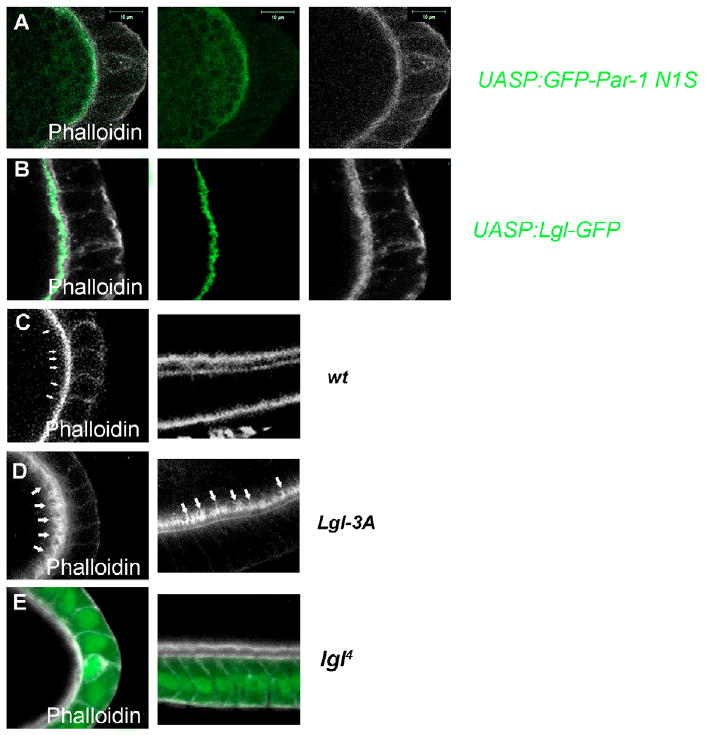
(A) GFP-Par-1 (N1S) was tightly associated with the cortical actin at the oocyte posterior. (B) In Lgl-GFP-overexpressing egg chambers, Lgl-GFP was enriched at the oocyte posterior and showed tight association with cortical actin, which had projections at the posterior of the oocyte. (C) Actin staining in the wild-type egg chamber. The weak long actin projections were indicated by arrows. (D) The long actin-rich projections (arrows) were very obvious along the oocyte cortex in Lgl-3A-overexpressing egg chambers. (E) In lgl4 germline clones, no cortical actin projections were detected. All of them are stage 10 egg chambers.
Discussion
The reorganization of the microtubule cytoskeleton during mid-oogenesis that leads to the formation of an AP gradient of microtubule density in the oocyte is essential for body-axis formation in Drosophila (Cha et al., 2001; Januschke et al., 2006; Theurkauf et al., 1992). Our findings show that genes such as aPKC and lgl that are important in polarizing other cell types play important roles in regulating the microtubule polarity in the oocyte. As in the neuroblast, aPKC regulates Lgl function by affecting its localization along the cellular cortex (Betschinger et al., 2003). Lgl-3A, the nonphosphorylatable and active form of Lgl, was localized along the oocyte cortex, whereas wild-type Lgl overexpression is mainly enriched at the posterior. Lgl was less localized to the posterior cortex when it was co-expressed with aPKCΔN. For establishment of the posterior-to-anterior gradient of cortical Lgl, anterior aPKC activity is probably stronger than at the posterior. Although preferential localization of aPKC to the anterior cortex of the oocyte is plausible, similar to the apical localization of aPKC in the cortex of the neuroblast or epithelial cells, we did not observe such a strict anterior localization of aPKC in oocytes with GFP-tagged aPKC overexpression or UAS-aPKC overexpression using anti-aPKC antibody (data not shown). Although the transgene may not localize exactly the same as the endogenous form, other mechanisms may also be involved in restricting aPKC activity in the anterior so that Lgl is enriched at the posterior oocyte cortex. For example, the mysterious signaling that is sent from the posterior follicle cells might be involved in inactivating aPKC in the posterior.
A previous report indicated that aPKC germline clones did not adversely affect oocyte polarity in stage 9 egg chambers (Doerflinger et al., 2006), but in our experiments we did observe defects in oocyte polarity in some apkc germline clones. Because no information was provided on the allele used or the number of clones analyzed in the report (Doerflinger et al., 2006), we do not know why the polarity defect we observed was not detected in this previous study. A possible reason is that not all apkc (apkcK06403) germline clones that develop to stage 9/10 possess oocyte polarity defects, so a significant number of escapers have to be analyzed before the phenotype can be observed.
The level or dose of Lgl and its phosphorylation by aPKC appear to be crucial for oocyte polarity formation. Both excessive and insufficient amounts of unphosphorylated Lgl cause oocyte polarity defects, probably explaining why overexpression of the wild-type Lgl also caused some defects in oocyte polarity, perhaps because of an upper limit on the amount of Lgl that can be phosphorylated by endogenous aPKC. When overexpression of Lgl is massive, only some of the overexpressed Lgl can be phosphorylated by aPKC, and the rest remains active and cortically localized. The alleviation of Lgl-overexpression-induced oocyte polarity defects by co-overexpression of Lgl and aPKC supports this hypothesis. A fine balance appears to be necessary between the amount of phosphorylated and unphosphorylated Lgl in the oocyte. Alteration of the levels of Lgl or aPKC can therefore cause varying severities of defects in oocyte polarity. Interestingly, lgl loss of function and Lgl-3A overexpression caused similar Stau/osk localization defects, which are probably results of disrupted actin and microtubule organization in these mutant backgrounds. The cytoskeletal defects could have been translated into defects in the Par-1 posterior enrichment that is crucial for Stau and osk localization.
Indeed, our data suggest that posterior enrichment of Par-1 is regulated by Lgl. Par-1 has also been shown to be a phosphorylation target of aPKC in HEK293 cells (Hurov et al., 2004), and Par-1 localization could be regulated by aPKC phosphorylation in the oocyte (Doerflinger et al., 2006). aPKC therefore probably has dual function in restricting Par-1 kinase activity to the oocyte posterior. First, it prevents Par-1 from localizing to the anterior cortex through direct phosphorylation, and second, it promotes Par-1 localization to the posterior by restricting active Lgl to the posterior. To explain the function of Lgl in localizing molecules in cell-polarity formation, two different, but not mutually exclusive, models have been proposed (Vasioukhin, 2006). The first model is based on findings in yeast and vertebrate cells, demonstrating that Lgl homologs regulate docking and fusion of post-Golgi vesicles with the plasma membrane through the SNARE complex (Lehman et al., 1999). In this scenario, Lgl could be involved in the exocytosis pathway and membrane trafficking. A sec5 mutant has been reported (Murthy et al., 2005) to show impaired membrane trafficking of Grk, but the cytoskeleton was correctly oriented. These phenotypes differ from the defects we found in lgl loss- and gain-of-function mutants. The potential involvement of Lgl in the exocytosis pathway in the oocyte is therefore unlikely to be the sole explanation of the serious polarity defects observed in lgl4- and Lgl-3A-overexpressing egg chambers. The second model is related to the role of Lgl in actomyosin cytoskeletal regulation, which is based on genetic interactions between Lgl and actin-binding proteins such as Myosin II [encoded by zipper (zip)] and Myosin VI (Ohshiro et al., 2000; Petritsch et al., 2003; Strand et al., 1994). Our finding that the cortical actin morphology was dramatically changed when the active Lgl was overexpressed supports this model. In neuroblasts, Lgl can promote the cortical localization of Miranda by restricting Myosin II localization to the apical cortex. Germline clones of a zip loss-of-function allele, however, caused arrest of oogenesis at an early stage, before the reorganization of the microtubules takes place (data not shown). By contrast, miranda loss-of-function germline clones showed no defects in oocyte AP polarity (Irion et al., 2006). A recent study demonstrated that actin projections at the oocyte cortex require Osk (Vanzo et al., 2007). Whether Lgl localization is normal in osk mutants is unknown. Because osk is mislocalized in the Lgl-3A overexpressing egg chambers, which still have long actin projections, the impact of Osk in actin organization is probably indirect. Lgl and Osk may affect some common targets that regulate cortical actin in the oocyte.
Our data indicate that genetic manipulations of either lgl or apkc produced incomplete penetrance of oocyte polarity defects. In fact, a number of previously reported mutations also had partial penetrance of Stau mislocalization (14-3-3, 62%; BAZS151A,S1085A misexpression, 49%; rab6, 64.47%), and the penetrance of mislocation of Grk/oocyte nucleus has not been reported in these mutants (Benton and St Johnston, 2003; Benton et al., 2002; Coutelis and Ephrussi, 2007). Because many genes and pathways are involved in the regulation of cytoskeletal organization in the oocyte, functional redundancy and crosstalk between different pathways are probably unavoidable in this complicated polarization process. Other genes may act in parallel with Lgl and aPKC to regulate actin organization and Par-1 localization, which in turn controls posterior localization of morphogens.
Supplementary Material
Acknowledgments
We thank J. A. Knoblich, H. S. Li, J. Poulton, A. B. Thistle, Y. Wang and F. Wirtz-Peitz for critically reading the manuscript. We thank C. Q. Doe, Y. N. Jan, J. A. Knoblich, D. St Johnston, A. Ephrussi, D. Bilder, the Bloomington Stock Center, DGRC and DSHB for sending us antibodies, cDNA clones and fly stocks, and the entire staff of the Deng and Wang laboratories for technical help. We also thank K. Riddle and the Biological Science Imaging Facility at FSU for help with confocal microscopy and the Duke University Non-Mammalian Model Systems Flyshop for generating the transgenic flies. A.T. was supported by a Florida/Puerto Rico Affiliate Postdoctoral Fellowship from the American Heart Association. W.M.D. was supported by a Scientist Development Grant from the American Heart Association, Florida/Puerto Rico affiliate, by the FSU College of Arts and Sciences set-up fund, and by NIH 1R01GM072562.
Footnotes
Supplementary material: Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/3/463/DC1
References
- Bai J, Montell D. Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development. 2002;129:5377–5388. doi: 10.1242/dev.00115. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Benton R, Palacios IM, St Johnston D. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev Cell. 2002;3:659–671. doi: 10.1016/s1534-5807(02)00320-9. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Eisenhaber F, Knoblich JA. Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr Biol. 2005;15:276–282. doi: 10.1016/j.cub.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Cha BJ, Koppetsch BS, Theurkauf WE. In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell. 2001;106:35–46. doi: 10.1016/s0092-8674(01)00419-6. [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN. Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol. 1994;4:289–300. doi: 10.1016/s0960-9822(00)00068-3. [DOI] [PubMed] [Google Scholar]
- Coutelis JB, Ephrussi A. Rab6 mediates membrane organization and determinant localization during Drosophila oogenesis. Development. 2007;134:1419–1430. doi: 10.1242/dev.02821. [DOI] [PubMed] [Google Scholar]
- Cox DN, Seyfried SA, Jan LY, Jan YN. Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proc Natl Acad Sci USA. 2001;98:14475–14480. doi: 10.1073/pnas.261565198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Torres IL, Zwart MF, St Johnston D. Drosophila anterior-posterior polarity requires actin-dependent PAR-1 recruitment to the oocyte posterior. Curr Biol. 2006;16:1090–1095. doi: 10.1016/j.cub.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, St Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development. 1998;125:2837–2846. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Huynh JR, Shulman JM, Benton R, St Johnston D. PAR-1 is required for the maintenance of oocyte fate in Drosophila. Development. 2001;128:1201–1209. doi: 10.1242/dev.128.7.1201. [DOI] [PubMed] [Google Scholar]
- Irion U, Adams J, Chang CW, St Johnston D. Miranda couples oskar mRNA/Staufen complexes to the bicoid mRNA localization pathway. Dev Biol. 2006;297:522–533. doi: 10.1016/j.ydbio.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Gillet L, Keryer G, Bornens M, Guichet A. The centrosome-nucleus complex and microtubule organization in the Drosophila oocyte. Development. 2006;133:129–139. doi: 10.1242/dev.02179. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Lehman K, Rossi G, Adamo JE, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfruelli P, Arquier N, Hanratty WP, Semeriva M. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development. 1996;122:2283–2294. doi: 10.1242/dev.122.7.2283. [DOI] [PubMed] [Google Scholar]
- Murthy M, Ranjan R, Denef N, Higashi ME, Schupbach T, Schwarz TL. Sec6 mutations and the Drosophila exocyst complex. J Cell Sci. 2005;118:1139–1150. doi: 10.1242/jcs.01644. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Nilson LA, Schupbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–596. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Petritsch C, Tavosanis G, Turck CW, Jan LY, Jan YN. The Drosophila myosin VI Jaguar is required for basal protein targeting and correct spindle orientation in mitotic neuroblasts. Dev Cell. 2003;4:273–281. doi: 10.1016/s1534-5807(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Poulton JS, Deng WM. Dystroglycan down-regulation links EGF receptor signaling and anterior-posterior polarity formation in the Drosophila oocyte. Proc Natl Acad Sci USA. 2006;103:12775–12780. doi: 10.1073/pnas.0603817103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001;11:374–383. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schupbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Beuchle D, Nusslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- Strand D, Raska I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J Cell Biol. 1994;127:1345–1360. doi: 10.1083/jcb.127.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Deng WM. Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development. 2005;132:4299–4308. doi: 10.1242/dev.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Deng WM. Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev Cell. 2007;12:431–442. doi: 10.1016/j.devcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992;115:923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Rabouille C, Ephrussi A. The Drosophila PAR-1 spacer domain is required for lateral membrane association and for polarization of follicular epithelial cells. Curr Biol. 2005;15:255–261. doi: 10.1016/j.cub.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- van Eeden F, St Johnston D. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- Vanzo N, Oprins A, Xanthakis D, Ephrussi A, Rabouille C. Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev Cell. 2007;12:543–555. doi: 10.1016/j.devcel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V. Lethal giant puzzle of Lgl. Dev Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F, Knoblich JA. Lethal giant larvae take on a life of their own. Trends Cell Biol. 2006;16:234–241. doi: 10.1016/j.tcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


