Abstract
Background
Advanced glycation end-products (AGEs) are believed to increase ventricular (LV) and vascular stiffness, in part via cross-linking proteins. We determined if and where AGEs were increased in elderly hypertensive non-diabetic dogs and whether an AGE cross-link breaker (ALT-711) improved vascular or ventricular function.
Methods and Results
Elderly dogs with experimental hypertension were randomized to receive ALT-711 (OH+ALT, n=11; 1 mg/kg PO) or not (OH, n=11) for 8 weeks. Conscious blood pressure (BP) measurements (weekly), echo (week 8) and anesthetized study (week 8) with LV pressure-volume analysis and aortic pressure-dimension and pressure-flow assessment over a range of preloads and afterloads was performed. In LV and aorta from OH, OH+ALT and young normal (YN) dogs, AGE content (immunohistochemistry and Western analysis for Nε-(carboxymethyl)lysine (CML)) was assessed. Aortic CML content was markedly increased in OH and OH+ALT dogs as compared to YN dogs. CML was localized to aortic and aortic vaso vasorum smooth muscle but not to collagen or elastin. CML was essentially undetectable in YN, OH, or OH+ALT myocardium but was visible in large vessels in the LV. ALT-711 therapy was associated with lower BP and pulse pressure, decreased systemic vascular resistance, increased aortic distensibility and arterial compliance and notably, significant aortic dilatation. Neither LV systolic nor diastolic function was different in OH+ALT vs OH dogs.
Conclusions
In elderly hypertensive canines, AGE accumulation and AGE cross-link breaker effects were confined to the vasculature without evidence of myocardial accumulation or effects. The lack of AGE accumulation in collagen rich areas suggests that the striking vascular effects may be mediated by mechanisms other than collagen cross-linking.
Keywords: Hypertension, Heart Failure, Aorta, Diastole, Aging
INTRODUCTION
The aorta dilates with age due to degenerative changes in elastin, the long-lived protein which comprises 60% of the thoracic aorta. These degenerative changes lead to transfer of stress to less extensible collagenous elements of the aorta and increased aortic stiffness1. However, increases in the intrinsic stiffness of the aorta due to processes other than elastin degeneration may also contribute to age-associated vascular dysfunction and development of systolic hypertension, hypertensive heart disease, and heart failure in the elderly2,3. One mechanism postulated to increase both aortic and LV diastolic stiffness is protein modification by advanced glycation end-products (AGEs).
AGEs are a diverse group of compounds formed by the reaction of the amino groups on proteins with reducing sugars with subsequent rearrangement and sometimes oxidation, to form stable structures which accumulate on long-lived proteins. AGE formation is accelerated in hyperglycemia, renal failure, and inflammatory conditions4. Some AGEs lead to cross-linking of proteins. It is believed that AGE cross-linking of collagen and elastin increases the tensile strength of these proteins, deters collagen degradation, and increases aortic and LV diastolic stiffness5-7. Other AGEs (ie, Nε-(carboxymethyl)lysine; CML) do not cross-link proteins but may produce effects by interacting with receptors, leading to activation of a number of kinases and growth factors involved in inflammation, oxidative stress, and atherosclerosis4-8.
Thiazolium derivatives such as ALT-711 (3-phenacyl-4,5-dimethylthiazolium chloride; ALT) can break the protein cross-links formed by those AGEs which contain an α-dicarbonyl moiety7,9. Studies have reported improvements in arterial stiffness indices with ALT therapy in diabetic or aged animals and in elderly humans10-13. However, ALT also reduces systemic vascular resistance11,13,14. Concomitant vasodilation complicates interpretation of the effects of ALT on indices of aortic stiffness as most are highly dependent on prevailing arterial tone. Further, few studies have assessed the effect of ALT on arterial size. This is important as a therapy which alters collagen or elastin may promote arterial dilatation11 and further complicate assessment of arterial properties.
The objectives of this study were first to determine if AGEs were increased in elderly canines with experimental hypertension and second to examine the effect of chronic ALT therapy on vascular and ventricular function in this model. To avoid confounding effects of changes in resistance and/or artery dimension, we performed comprehensive assessment of arterial function using both pressure-dimension and pressure-flow based indices over a range of cardiac outputs and levels of peripheral resistance.
METHODS
All animal experiments were approved by the Mayo Institutional Animal Care and Use Committee. Euthanasia methods conformed to recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Elderly mongrel dogs (n=22, age 8 to 12 years) underwent bilateral renal wrapping and placement of an abdominal aortic fluid filled catheter for weekly blood pressure (BP) monitoring as described previously15-17. At 72 hours post-op, dogs were randomized to receive ALT-711 (1 mg/kg orally once daily) for 8 weeks (n=11, OH+ALT) or not (n=11, OH)14. At week 8, dogs underwent conscious echocardiography, invasive hemodynamic study under anesthesia, and tissue harvest.
Previously harvested tissue from young (age ≈ 1 year), normal (no interventions) dogs (YN, n=6) was used as controls for immunohistochemistry and western blot analysis of CML.
Echocardiography
Two-dimensional guided M-mode echocardiography along with BP measurement was performed to assess LV volumes (Teichholz formula), stroke volume (SV), cardiac output (CO), LV mass (ASE criteria), and ejection fraction (EF) as previously described15,17. Effective arterial elastance (Ea, 0.9*systolic BP/SV), systemic vascular resistance (mean arterial pressure (MAP)/CO), and systemic arterial compliance (SAC, SV/pulse pressure (PP)) were calculated15,17.
Acute Hemodynamic Study and Analysis
Dogs were anesthetized with fentanyl (0.25 mg/kg bolus followed by 0.18 mg/kg/h) and midazolam (0.75 mg/kg bolus followed by 0.59 mg/kg/h), given autonomic blockade (propranolol 2m/Kg and atropine, 1 mg) and instrumented with LV and ascending aortic micromanometer catheters, piezoelectric crystals for LV volume and aortic dimensions, ascending aortic volumetric flow probe, atrial pacemaker, and vena cava (IVC) occluder. Dogs were atrial paced 10-20 bpm above sinus rate after autonomic blockade. Data were collected at baseline, following volume expansion with dextran (250 ml over 15 minutes) and during phenylephrine infusion to increase peripheral resistance with dose titrated to achieve systolic aortic pressure ≈ 200 mmHg.
At each data collection period, acute IVC occlusion was used to define the end-systolic pressure (ESP) volume (ESV) relationship (ESPVR; ESP=Ees(ESV-V0)) where Ees is endsystolic elastance and the end-diastolic pressure (EDP) volume (EDV) relationship (EDPVR; EDP=αe(β*EDV)) where β represents the diastolic stiffness coefficient and α, the curve fitting constant18. The time constant of LV relaxation (tau) was calculated as previously described15,16.
MAP, CO, and SVR were calculated from invasive aortic pressure and flow. Phasic aortic area change (Ao Area Δ) was calculated as (π/4*DS2- π/4* DD2) where DS and DD are aortic systolic and diastolic dimensions. Aortic distensibility was calculated as (Ao Area Δ /(pulse pressure). Characteristic aortic impedance (Zo-Volume, dyne sec cm-5) was calculated from the water-hammer equation as the slope of the relationship between aortic pressure and flow from end diastole to 50% peak flow19. Zo-Velocity (dyne sec cm-3) was similarly calculated using linear flow velocity (volumetric flow/aortic cross sectional area). SAC was calculated by the method of Liu et al20.
Histological and Biochemical Analysis
Total collagen and collagen solubility (pepsin digestion) were measured by the hydroxyproline assay as previously described15,17. Myocardial brain natriuretic peptide (BNP) concentration was measured by radioimmunoassay to assess LV hypertrophy15. Aortic wall thickness was measured in fixed specimen and averaged from five measurements.
Immunohistochemistry
Five um sections from canine LV or Aorta were deparaffinized and rinsed with PBS. Three percent hydrogen peroxide in methanol was used for endogenous blocking. Antigen retrieval was performed by incubating slides with pepsin/HCL for 30 min at 37°C. Tissues were blocked for exogenous proteins using normal rabbit serum 1:10 in PBS. Tissues were incubated in primary antibody CML26 1:1000 21 for 60 minutes at room temperature in a humidity chamber. After rinsing with PBS, tissues were incubated with secondary rabbit-anti-mouse-biotin-F(ab')2 for 30 minutes at room temperature (Jackson Immunoresearch, Westgrove, PA) followed by incubation with ABComplex/HRP for 1 hour. Visualization was achieved by a 5 minute diaminobenzidine (Sigma-Aldrich) incubation and counterstained with Gill's hematoxylin.
Aortic sections were scored by an investigator blinded to study group on a 0 (no staining) to 4 (dense staining) scale. In LV, staining was only seen in epicardial and occasional intramyocardial muscular arteries and thus a score was not used. Sequential cuts of aortic and LV tissue was stained with picros-sirius red and Lawson's Elastic Van Gieson stains to illustrate CML distribution in relation to collagen or elastin respectively.
Western Analysis
Five hundred mg tissue was polytron homogenized in 1ml Triton lysis buffer (20mM Tris HCL pH 7.5,150mM NaCl, 1mM EDTA, % Triton) +1 μl DTT, and 4 μl of protease inhibitor cocktail (Calbiochem, Gibbstown, NJ). Protein concentration was determined by Bradford method (Bio-Rad, Hercules, CA), and 40 μg protein was loaded on a 10% SDS-PAGE gel and electrophoretically transferred to a 0.45-μm PDVF membrane (Millipore, Bedford, MA). The membrane was incubated in 2ug/ml α-CML antibody (R&D systems, Minneapolis, MN), 2.5 ug/ml α-CML antibody (Cosmo Biotech, Tokyo, Japan) or 1:1000 α-CML26 21, or α-GAPDH 1:5000 (Abcam, Cambridge, MA) at 4°C overnight. Membranes were probed with secondary peroxidase conjugated anti-F (ab')2 fragment goat anti-mouse IgG (Jackson ImmunoResearch, Westgrove, PA) for 1 hour. Probed proteins were detected using SuperSignal Chemiluminescent Substrate (Pierce, Rockford, IL). Densitometry was completed using Bio-Rad Chemidoc software.
Statistics
The student t-test was used to assess between-group comparisons. Simple and multiple linear regression was performed to assess associations between variables with log transformation of skewed variables as needed to satisfy modeling assumptions. Interaction between group and the other tested covariate were tested by introducing an interaction term. Two-way, repeated measures ANOVA was used to compare variables between groups over time (BP) or experimental period. Data are expressed as mean ± SD; p value <0.05 was considered significant and all comparisons were two sided.
RESULTS
Evolution of blood pressure after renal wrapping
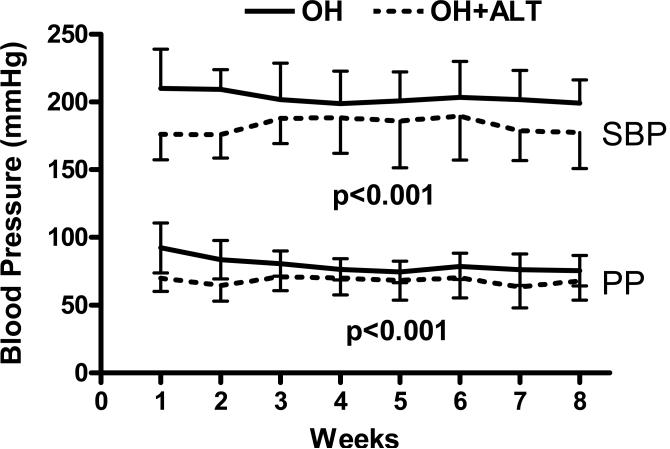
ALT treated dogs had lower systolic BP and PP after renal wrapping (Figure 1).
Figure 1. Conscious blood pressure.
In elderly dogs subjected to renal wrapping, those treated with ALT-711 (OH+ALT) had lower systolic (SBP) and pulse (PP) pressure than untreated (OH) dogs.
Conscious assessment of LV and vascular function
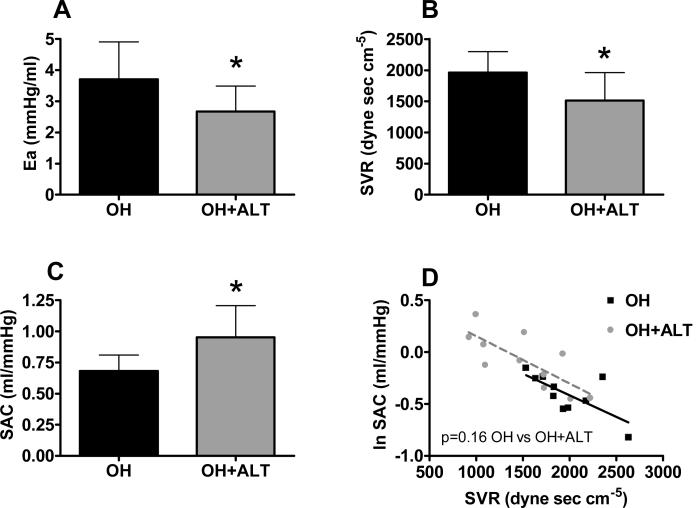
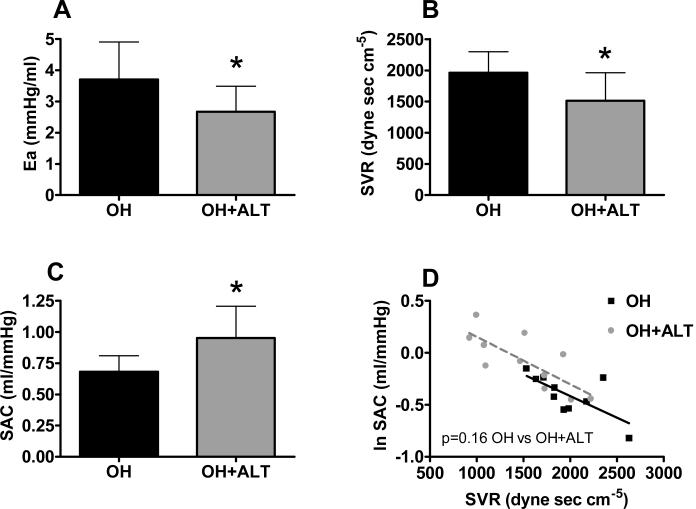
Heart rate and LV end-diastolic volume were similar between groups (Table 1). Ejection fraction and stroke volume tended to be higher, and systolic BP was lower in the OH+ALT dogs. Ea was lower in treated dogs with reductions in both the fixed (lower SVR) and pulsatile (higher SAC) components of arterial load (Figure 2 A-C). SAC was related to SVR (p<0.001) but the relationship between SVR and SAC was not different between groups (Figure 2 D). However, there were few points with overlapping SVR between groups.
Table 1.
Characteristics of untreated (OH) and treated (OH+ALT) elderly hypertensive dogs
| OH | OH+ALT | p | |
|---|---|---|---|
| Echocardiography | |||
| Heart rate (bpm) | 126 ± 31 | 119 ± 21 | 0.58 |
| Systolic blood pressure (mmHg) | 199 ± 17 | 177 ± 26 | 0.04 |
| LV end-diastolic volume (ml) | 95 ± 18 | 100 ± 20 | 0.56 |
| Ejection fraction (%) | 56 ± 12 | 63 ± 9 | 0.18 |
| Stroke volume | 53 ± 14 | 63 ± 15 | 0.11 |
| LV mass / body weight | 5.84 ± 1.12 | 4.96 ± 1.02 | 0.07 |
| Aortic Structure | |||
| Aortic Collagen (μg/mg) | 68.2±19.3 | 56.2±10.4 | 0.10 |
| Aortic Collagen Solubility (% Insoluble) | 11.4±6.5 | 12.9±4.7 | 0.92 |
| LV Function | |||
| Heart rate | 102 ± 13 | 88 ± 6 | 0.001 |
| End-diastolic volume (ml) | 60 ± 18 | 55 ± 12 | 0.6 |
| End-diastolic pressure (mmHg) | 10 ± 5 | 13 ± 5 | 0.33 |
| Ees (mmHg/ml) | 4.82 ± 2.70 | 4.99 ± 2.54 | 0.88 |
| Vo (ml) | 4 ± 15 | 4 ± 9 | 0.98 |
| β (mmHg/ml) | 0.053 ± 0.032 | 0.042 ± 0.026 | 0.38 |
| α | 1.31 ± 1.39 | 2.20 ± 1.34 | 0.14 |
| Tau (ms) | 47 ± 9 | 50 ± 11 | 0.49 |
| LV Structure | |||
| LV Mass/Body Weight (g/kg) | 5.6±1.2 | 5.0±0.7 | 0.20 |
| LV Brain Natriuretic Peptide (pg/mg protein) | 0.42±0.38 | 0.19±0.18 | 0.08 |
| LV Collagen (μg/mg tissue) | 2.6±0.7 | 2.9±0.7 | 0.33 |
| LV Collagen Solubility (% Insoluble) | 67.9±4.6 | 70.4±3.6 | 0.24 |
Figure 2. Conscious assessment of arterial properties.
Effective arterial elastance (Ea, A) was lower in ALT-711 treated dogs (OH+ALT) with decreases in systemic vascular resistance (SVR, B) and increases in systemic arterial compliance (SAC, C) as compared to untreated (OH) dogs. SAC was inversely related to SVR (p<0.001) and this relationship was similar in treated and untreated animals (D).
Invasive assessment of vascular function
After anesthesia, instrumentation, and autonomic blockade, heart rate was lower in OH-ALT dogs (Table 1).
Pressure dimension based indices of aortic stiffness
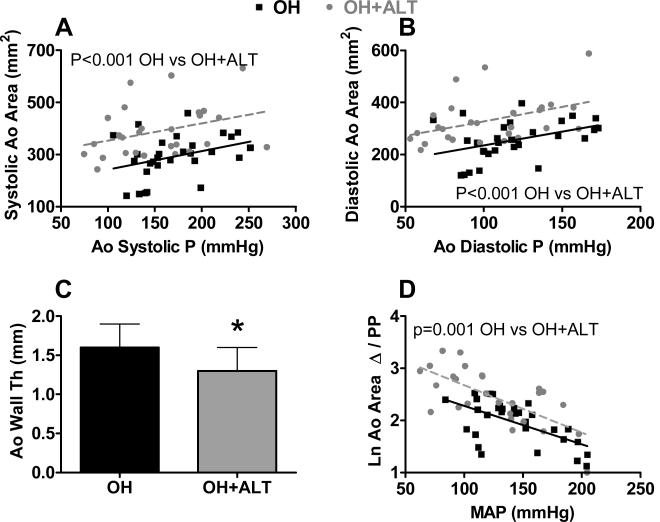
Systolic and diastolic aortic area increased with increasing systolic and diastolic aortic pressure respectively (p<0.001 for both) but were larger at any corresponding aortic pressure in the OH+ALT group. The slope of the relationship between aortic area and pressure was similar between groups. (Figure 3 A-B). Adjusting for dog weight or heart rate did not alter this finding. Aortic wall thickness was decreased in the OH+ALT group (Figure 3 C). Phasic aortic area Δ was greater in ALT dogs (57±20 mm2 vs 39±13 mm2, p<0.0002) and this difference persisted after adjusting for MAP. Phasic aortic distensibility decreased with increasing MAP (P<0.001). The relationship between phasic aortic distensibility and MAP was shifted upward in ALT treated dogs (but with similar slope) suggesting greater distensibility in ALT treated dogs at any given distending pressure (Figure 3 D).
Figure 3. Pressure - dimension based assessment of arterial properties in anesthetized animals.
Both systolic (A) and diastolic (B) aortic (Ao) area increased with increases in distending pressure (Ao systolic and diastolic pressure (P) respectively) (p<0.001 for both) but were higher in ALT-711 treated dogs (OH+ALT) than untreated dogs at any distending pressure. Aortic wall thickness was lower in OH+ALT dogs (C). The logarithm (Ln) of phasic aortic distensibility (Ao area change (Δ) /pulse pressure (PP); multiplied * 10) decreased with increasing mean Ao P (MAP) (p<0.001) but was higher in OH+ALT than OH dogs at any distending pressure (D).
Pressure flow based indices of arterial properties
Zc-volume was independent of MAP (p>0.05) and was lower in OH+ALT dogs (Figure 4 A). Zc-velocity was independent of MAP (p>0.05) and was similar between groups (p=0.25, Figure 4 B). SAC was higher in OH+ALT dogs (Figure 4 C), decreased with increasing MAP or SVR (p<0.001 for both) but was higher in OH+ALT dogs at any SVR (Figure 4 D) indicating increased arterial compliance. The slope of the relationship between SAC and SVR was similar between groups.
Figure 4. Pressure - flow based assessment of arterial properties in anesthetized animals.
Characteristic aortic impedance calculated with volumetric flow (Zc-flow; A) was lower in ALT-711 treated dogs (OH+ALT) than untreated dogs. Zc calculated with velocity flow tended to be lower in OH+ALT dogs but this was not significant (B). Systemic arterial compliance (SAC) was higher in OH+ALT dogs (C) and the relationship between SAC and systemic vascular resistance (SVR) was shifted upward in OH+ALT dogs (D).
Aortic structure
The total collagen in the OH+ALT aortas tended to be lower than in OH but there was no difference in collagen solubility (Table 1).
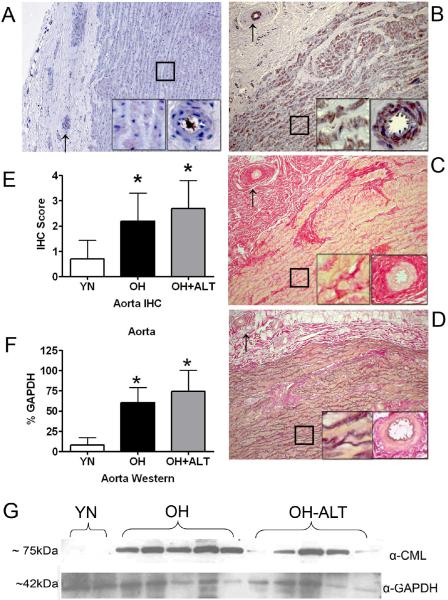
There was negligible staining for CML in YN aorta (Figure 5 A) but CML staining (α-CML26 21 antibody shown here) was prominent in OH dogs (Figure 5 B). Staining was not localized to areas of collagen (Figure 5 C) or elastin (Figure 5 D) but rather to smooth muscle and matrix proteins within the aortic wall and to the vaso vasorum where CML staining was prominent in the vascular smooth muscle. The aortic immunohistochemistry score was increased similarly in OH and OH+ALT as compared to YN dogs (Figure 5 E). On Western analysis, CML content in aorta was increased similarly in OH and OH+ALT dogs as compared to YN dogs (Figure 5 F and G). The representative blot is shown with R&D systems α-CML antibody, but findings were similar with the other antibodies.
Figure 5. CML content in Aorta.
In A, CML staining of aortic tissue (original magnification 10x) from a young normal (YN) dog showing lack of positive staining. In A-D, inserts show higher (20x) magnification of the aortic wall (box) and collagen rich adventitial layer including the vaso vasorum (arrow). In B, CML staining of aortic tissue (10x) from an elderly hypertensive (OH) dog showing dense CML staining throughout the aorta and in the vaso vasorum vascular smooth muscle cells. The CML staining is not associated with the elastin fibrils (pale serpentine appearance here) but rather in smooth muscle cells between the elastin fibrils. In C, picros-sirius staining of sequential section from B showing distribution of collagen (red). The CML staining in B is not in areas of collagen deposition in the media, adventitia or in the vaso vasorum. In D, elastin stain of sequential section from B showing distribution of elastin in relation to CML staining. The CML staining is not associated with elastin. In E, the results of immunohistochemistry CML scoring showing group data with increased score in OH and OH+ALT as compared to YN aorta. In F, group data from Western experiments showing increased CML proportional to GAPDH in OH and OH+ALT as compared to YN aortic tissue. In G, representative Western blot loaded with aortic protein from two YN, five OH and five OH+ALT dog showing staining for CML (≈ 75kDa) and GAPDH (≈ 42kDa).
Invasive assessment of ventricular function
At invasive study and before volume expansion or phenylephrine infusion, LV end-diastolic volume and pressure, Ees, Vo, β, α, and tau were all similar between groups (Table 1). By two-way repeated-measures ANOVA assessing changes in these parameters over the experimental periods and between groups, there was no difference in any of these variables between groups (group p > 0.05 for all) or any difference in the way in which the variables changed with volume expansion or phenylephrine infusion (interaction p > 0.05 for all).
Ventricular structure
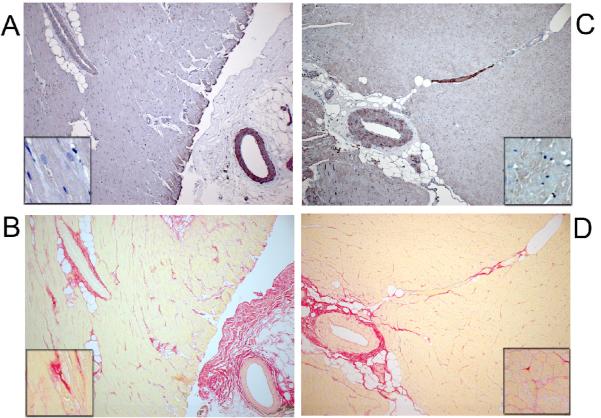
LV mass/body weight ratio at echo and autopsy and LV BNP all tended to be lower in the OH+ALT group suggesting less hypertrophy (Table 1). LV collagen content and solubility were similar. By immunohistochemistry, CML staining was not evident in the LV myocardium or areas of collagen deposition in YN (Figure 6 A and B) or OH (Figure 6 C and D) but was present in some muscular arteries in YN (Figure 6A) and OH (Figure 6C). Findings in OH+ALT were similar (not shown). Consistent with the immunohistochemistry, CML was undetectable in all three groups on Western analysis (regardless of antibody used).
Figure 6. CML content in Left Ventricle (LV).
CML (A) and picros-sirius (B) staining of sequential LV sections (original10x) in a representative young normal (YN) dog showing lack of CML staining in myocardium or myocardial or peri-vascular collagen. Insert in A-D show higher magnification of myocardium. There is staining in vascular smooth muscle cells. CML (C) and picros-sirius (D) staining of sequential LV sections (10x) in a representative elderly hypertensive (OH) dog showing lack of CML staining in myocardium or myocardial or peri-vascular collagen. There is staining in vascular smooth muscle cells.
DISCUSSION
These data demonstrate the presence of AGEs as assessed by CML content in an elderly hypertensive non-diabetic model and indicate that AGE deposition, at least as assessed by CML content, is confined to vascular smooth muscle cells in the aorta, the aortic vaso vasorum, and the LV vessels and is not apparent in areas of aortic collagen or elastin or LV collagen. Accordingly, an AGE cross-link breaker had clear effects on aortic dimension, phasic distensibility and stiffness and on vascular tone but not on LV diastolic properties. These data suggest that AGE accumulation and AGE protein cross-linking in elderly non-diabetic hypertensives may influence arterial properties via effects on proteins other than collagen and elastin.
CML content and localization in elderly, hypertensive dogs
Similar to previous studies in human or experimental diabetes, CML was localized to aortic smooth muscle22 and to the vaso vasorum but did not appear to be associated with collagen or elastin in the aorta. We cannot exclude presence of CML in vascular endothelial cells as well. CML was essentially undetectable in the LV in the OH dogs by both immunohistochemistry and Western analysis. Given the dramatic effects of ALT on LV diastolic properties in a previous study of elderly dogs14, we confirmed the absence of CML on Western analysis of LV tissue using three different α-CML antibodies. We did see some staining of arterial smooth muscle in vessels in the LV as previously described in experimental or human diabetes21,23 but this was evident in some YN samples as well.
While CML is commonly used as a marker of AGE accumulation, the relationship of CML content to that of other AGEs and differences in tissue deposition of CML and other AGEs throughout the body are poorly defined4. As CML does not form cross-links4,7,22, this AGE may not decrease with administration of a cross-link breaker as observed here. CML formation increases with oxidative stress, but can increase with hypertension in the absence of increases in oxidative stress24. Some studies have shown a decrease in CML with ALT therapy in diabetic models22,25, an effect postulated to be mediated by reduction in copper catalyzed glycoxidation22. The lack of reduction in CML with ALT here may suggest that the effects of ALT in this particular model are not mediated primarily by a reduction in oxidative stress.
Effect of ALT on arterial structure and function
ALT therapy attenuated development of hypertension and increases in PP in the renal wrapping model. The effect of ALT appeared apparent very early, consistent with in vitro studies with its precursor molecule which demonstrated a very rapid (within hours) breaking of protein cross-links 9. Whether the rapid effects were due to disruption of collagen cross-links or modification of other proteins is unclear given the lack of CML deposition in collagen rich areas of the aorta or aortic vaso vasorum noted above. Measured in the conscious state, SAC was increased in the ALT group, consistent with studies in diabetic rats and elderly humans 11,12 but whether this effect was independent of the effect on vascular tone is difficult to say given the lack of overlap in SVR in the two groups in the conscious state.
Effects of ALT on systolic BP or PP in humans with essential or age-related systolic hypertension have been also observed12,26. SVR was lower in ALT treated conscious dogs, a finding which has been observed with ALT therapy in elderly dogs and monkeys and in diabetic rats 11,13,14 but not in elderly humans 12. ALT may reduce SVR via effects on endothelial function27,28, although others have speculated that ALT may influence the biomechanical properties of the resistance vessels or the mechanical properties of the carotid sinus11,29. The consistent presence of CML in vascular smooth muscle cells in the aorta, the aortic vaso vasorum, and the LV arteries noted here provide support for an effect of CML or other AGEs on vascular smooth muscle function which goes beyond extracellular matrix collagen cross-linking.
Data from the anesthetized study where peripheral tone and output were varied over a large range in both groups reveal more information regarding the effect of ALT on aortic function. A highly significant effect of ALT to increase aortic dimension was observed. This occurred despite lower BP over the course of the model and was independent of distending pressure or body size. While it was postulated that ALT could reduce the stiffness of the aorta, few studies have described the effect of ALT on vascular size. The careful study of Wolffenbuttel et al reported increases in carotid artery dimension in diabetic rats treated with ALT and consistent with our findings, demonstrated that this was present in vivo and in vitro and was independent of distending pressure or aortic smooth muscle tone11. Other studies of the effects of ALT on arterial properties did not assess effects on aortic size10,12,13,30.
Another major finding from this study was that phasic aortic area change and phasic aortic distensibility was increased in ALT treated dogs. Consistent with this finding, pressure flow indices (Zc-volume, SAC and the relationship between SAC and SVR) also suggested an improvement in aortic properties with ALT as previously described in diabetic rats and elderly monkeys 11,13, although the findings with Zc-velocity (which controls for differences in vessel size) were less dramatic. While age related elastin degradation leads to aortic dilatation and increases in Zc and pulse wave velocity1, here aortic dilatation was not associated with increased stiffness, a unique finding which underscores the fundamental difference in the mechanism of the aortic dilatation with ALT therapy.
These alterations in aortic function were associated with a trend towards decreases in aortic collagen and a decrease in aortic wall thickness but aortic collagen solubility was not higher in OH+ALT dogs. Collagen solubility in tissue with very high collagen content (i.e. rat tail skin) has been used as a marker of changes in AGE cross-links4, and we did not collect such tissue for analysis. While a trend towards reduction in total aortic collagen was observed and may suggest enhanced susceptibility to collagen degradation with ALT therapy 31, this was not statistically significant.
Effect of ALT on LV structure and function
There was a trend towards less hypertrophy in the ALT treated dogs observed with all indices used to assess hypertrophy (echo LV mass, autopsy weights and LV BNP concentration) but none reached statistical significance. We speculate that ALT did reduce hypertrophy but that small numbers, non-paired study design, and the modest magnitude of the reduction30 hindered our ability to unequivocally demonstrate it. Indeed, Little et al found a significant decrease in LV mass of approximately 4% in paired analysis (pre to post treatment) with ALT treatment in humans with diastolic heart failure 30.
In contrast to a previous study in elderly dogs14, we did not observe a difference in LVEDV or LV diastolic properties between the two groups. This may be due to the difference in the experimental model (aged dogs vs aged dogs with experimental hypertension) or to the marked differences in the methods used to assess LV diastolic properties. In the current study, pressure and volume were simultaneously and instantaneously assessed, the pericardium was open, and heart rate, respiration, and autonomic tone were controlled. Acute preload reduction was used to define the entire curvilinear EDPVR and this was repeated over a range of preloads and afterloads. The study of Asif et al used echo in conscious dogs with pericardium intact and collected only two pressure and volume data points, before and after marked acute volume expansion without autonomic blockade, heart rate or respiratory effort control or reduction in preload to define the EDPVR independent of extrinsic forces14. The effect on diastolic properties in the previous study was dramatic and difficult to reconcile with our findings. The more dramatic effects in the previous study could also reflect the paired study design. The dose and duration of therapy was similar to that used here. None-the-less, the difference in vascular and LV effects with ALT in this model is consistent with the differences in AGE accumulation as assessed by CML content. We speculate that effects on LV properties may be more dramatic in the presence of diabetes or renal dysfunction, where LV AGE content4,25and sequella32 may be more dramatic. However, it is of note that a recent study found CML deposition confined to the LV vessels in diabetic humans with heart failure23.
Limitations
We did not assess the presence of other AGE structures nor changes in the type of collagen with ALT therapy. A single dose and duration of therapy were used. Investigators were not blinded to treatment group. As blood pressure was not measured until one week after surgery, we can not exclude baseline differences in blood pressure between groups but assignment was random and the findings were consistent with previous studies and the presence of CML in the aorta. Due to the relatively small sample size and multiple comparisons, the chance of a false positive or negative result is acknowledged.
Conclusions
AGEs as assessed by CML content were increased in the aorta but not in the LV in an elderly hypertensive non-diabetic model, were localized to vascular smooth muscle cells, and not associated with areas of collagen deposition in the aorta or LV. Therapy with an AGE cross-link breaker had clear effects on aortic stiffness and on vascular tone but was associated with significant aortic dilatation and a lack of effect on LV diastolic properties. Based on these data, we speculate that AGE accumulation and AGE protein cross-linking in elderly hypertensive subjects without diabetes may influence arterial properties via effects on proteins other than collagen and elastin. Further, the potential for AGE modifying therapies to improve vascular or ventricular properties deserves further study but should include careful attention to potential changes in vascular size.
Acknowledgments
Funding Sources: Drug and partial financial support for the study was provided by Alteon, Inc. Drs. Redfield (HL 63281 and HL 76611), Mohammed (HL 76611) and Owan (HL07111) were funded in part by the National Institute of Health.
Financial Disclosures: Drug and partial financial support for the study was provided by Alteon, Inc. Drs. Redfield (HL 63281 and HL 76611), Mohammed (HL 76611) and Owan (HL07111) were funded in part by the National Institute of Health.
Footnotes
Conflict of Interest Disclosures: Drug and partial financial support provided by Alteon, Inc.
REFERENCES
- 1.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL, Jr., Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51:105–11. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 3.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–93. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 4.Smit AJ, Lutgers HL. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem. 2004;11:2767–84. doi: 10.2174/0929867043364342. [DOI] [PubMed] [Google Scholar]
- 5.Soldatos G, Cooper ME. Advanced glycation end products and vascular structure and function. Curr Hypertens Rep. 2006;8:472–8. doi: 10.1007/s11906-006-0025-8. [DOI] [PubMed] [Google Scholar]
- 6.Kass DA. Getting better without AGE: new insights into the diabetic heart. Circ Res. 2003;92:704–6. doi: 10.1161/01.RES.0000069362.52165.C9. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. The Endocrine Society. 2001:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasan S, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–8. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Masurekar MR, Vatner DE, Jyothirmayi GN, Regan TJ, Vatner SF, Meggs LG, Malhotra A. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am J Physiol Heart Circ Physiol. 2003;285:H2587–91. doi: 10.1152/ajpheart.00516.2003. [DOI] [PubMed] [Google Scholar]
- 11.Wolffenbuttel BHR, Boulanger CM, Crijns FRL, Huijberts MSP, Poitevin P, Sweennen GNM, Vasan S, Egan JJ, Ulrich P, Cerami A, Levy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial complaince by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 13.Vaitkevicius PV, Lane M, Spurgeon H, Ingram DK, Roth GS, Egan JJ, Vasan S, Wagle DR, Ulrich P, Brines M, Wuerth JP, Cerami A, Lakatta EG. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1171–1175. doi: 10.1073/pnas.98.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000;97:2809–13. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro BP, Lam CS, Patel JB, Mohammed SF, Kruger M, Meyer DM, Linke WA, Redfield MM. Acute and chronic ventricular-arterial coupling in systole and diastole: insights from an elderly hypertensive model. Hypertension. 2007;50:503–11. doi: 10.1161/HYPERTENSIONAHA.107.090092. [DOI] [PubMed] [Google Scholar]
- 16.Munagala VK, Hart CY, Burnett JC, Jr., Meyer DM, Redfield MM. Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation. 2005;111:1128–35. doi: 10.1161/01.CIR.0000157183.21404.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro BP, Owan TE, Mohammed S, Kruger M, Linke WA, Burnett JC, Jr., Redfield MM. Mineralocorticoid signaling in transition to heart failure with normal ejection fraction. Hypertension. 2008;51:289–95. doi: 10.1161/HYPERTENSIONAHA.107.099010. [DOI] [PubMed] [Google Scholar]
- 18.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–12. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 19.Nichols WW, O'Rourke M. McDonald's Blood Flow in Arteries. Third ed. Lea & Febiger; Philadelphia, PA: 1990. [Google Scholar]
- 20.Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986;251:H588–600. doi: 10.1152/ajpheart.1986.251.3.H588. [DOI] [PubMed] [Google Scholar]
- 21.Baidoshvili A, Krijnen PA, Kupreishvili K, Ciurana C, Bleeker W, Nijmeijer R, Visser CA, Visser FC, Meijer CJ, Stooker W, Eijsman L, van Hinsbergh VW, Hack CE, Niessen HW, Schalkwijk CG. N(varepsilon)-(carboxymethyl)lysine depositions in intramyocardial blood vessels in human and rat acute myocardial infarction: a predictor or reflection of infarction? Arterioscler Thromb Vasc Biol. 2006;26:2497–503. doi: 10.1161/01.ATV.0000245794.45804.ab. [DOI] [PubMed] [Google Scholar]
- 22.Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR, Cooper ME, Allen TJ. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–23. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 23.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 24.Baumann M, Stehouwer C, Scheijen J, Heemann U, Struijker Boudier H, Schalkwijk C. N{epsilon}-carboxymethyl-lysine is increased in plasma and kidney of spontaneous hypertensive rats during the early development of hypertension and is independent of renal function or oxidative stress. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1433.004. [DOI] [PubMed] [Google Scholar]
- 25.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–92. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- 26.Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers: a novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Kass DA. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens. 2007;25:577–83. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]
- 28.Schalkwijk CG, Baidoshvili A, Stehouwer CD, van Hinsbergh VW, Niessen HW. Increased accumulation of the glycoxidation product Nepsilon-(carboxymethyl)lysine in hearts of diabetic patients: generation and characterisation of a monoclonal anti-CML antibody. Biochim Biophys Acta. 2004;1636:82–9. doi: 10.1016/j.bbalip.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FCP, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–62. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 30.Little WC, Zile MR, Kitzman DW, Hundley WG, O'Brien TX, Degroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191–5. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Mott JD, Khalifah RG, Nagase H, Shield CF, 3rd, Hudson JK, Hudson BG. Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int. 1997;52:1302–12. doi: 10.1038/ki.1997.455. [DOI] [PubMed] [Google Scholar]
- 32.Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–12. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]