Abstract
Background
Blockade of the CD28 costimulatory molecule by recombinant human (h)CTLA4-Ig or CD40-CD154 interaction with the monoclonal antibody (mAb) 5C8 together with donor-specific-transfusion led to enhanced engraftment in the canine model of DLA-identical marrow transplantation after 1 Gy total body irradiation (TBI). In order to reduce or eliminate TBI conditioning regimens, we have sought to develop canine specific reagents.
Methods
We have created a fusion protein of the extracellular domain of canine (c)CTLA-4 linked to the hinge-CH2-CH3 domains of canine IgG1 in a pcDNA3.1+ vector. CHO cells were co-transfected with CTLA4-Ig vector and a dhfr-containing vector. Stable, high producing clones were generated.
Results
Cell binding and mixed leukocyte reactions (MLR) indicated no significant differences in activity between cCTLA4-Ig and hCTLA4-Ig. MLR data indicated that combinations of cCTLA4-Ig and the mAb 5C8 together was superior in blocking 3H-thymidine uptake compared to either reagent alone. In dogs, the circulating half-life of cCTLA4-Ig was approximately 7 days with no immune response against the fusion protein. Finally, two injections of cCTLA4-Ig effectively tolerized two dogs against eight consecutive challenges with sheep red blood cells (SRBC), given over 330 days as indicated by a complete block of IgG antibody production. Tolerance was broken in one of the two dogs when a ninth injection of SRBC was given subcutaneously in incomplete Freund's adjuvant.
Conclusion
cCTLA4-Ig is an effective non-immunogenic blocking reagent of the CD28 costimulatory pathway in dogs and is a promising reagent for studies of tolerance induction in hematopoietic cell transplantation in the canine model.
Keywords: CTLA4-Ig, fusion protein, tolerance, canine, T-cell dependent antibody response
Introduction
The process of mature T-cell activation involves both recognition of antigen in association with major histocompatibility complex (MHC) by the T-cell receptor complex (signal 1) and costimulatory signals provided by a variety of cell surface determinants of the CD28:B7 or TNF:TNFR families (signal 2) (1). For example, without CD28 costimulation, T-cells fail to upregulate IL-2 expression and progress through the cell cycle (2). Inhibition of CD28 signaling in a variety of model systems can be achieved through soluble or recombinant human CTLA-4 linked to human IgG1 heavy chain (hCTLA4-Ig) (3-5).
We have used hCTLA4-Ig in a canine hematopoietic cell transplantation (HCT) model both to prevent graft-vs-host disease and enhance engraftment after low-dose total body irradiation (TBI) (6,7). As for the latter, sustained DLA-identical marrow grafts have been uniformly accomplished after 2 Gy TBI when this was combined with at short course of post-grafting immunosuppression consisting of mycophenolate mofetil and cyclosporin (8). In contrast grafts were rejected when the TBI dose was lowered to 1 Gy. When 1 Gy TBI was preceded by infusions of donor peripheral blood mononuclear cells (PBMC) and hCTLA4-Ig, two thirds of the dogs showed sustained marrow engraftment (7). This encouraging but not uniformly successful observation prompted us to generate a completely canine CTLA4-Ig (cCTLA4-Ig).
Recently, two reports described the cloning of recombinant cCTLA4-Ig. Yasunaga et al. (9) produced a cCTLA4-IgE fusion construct in COS cells designed to bridge antigen-presenting cells and IgE-allergen complexes for treating allergen-induced immune responses in dogs. Shin et al. (10) produced cCTLA4-IgA in E. coli that suppressed unidirectional mixed lymphocyte reactions (MLR). Neither of the two cCTLA4-Ig constructs has been tested in vivo.
Here we describe a novel cCTLA4-Ig comprised of the extracellular domain of cCTLA-4 and the hinge-CH2-CH3 domains of canine IgG1, linked by a five amino acid antigenically inert peptide. Both in vitro and in vivo studies suggested that cCTLA4-Ig was a potent blocker of the CD28:B7.1/B7.2 costimulatory pathway in the canine model as evidenced by suppression of MLR and indefinite prevention of T-cell dependent antibody responses to a strong antigen, sheep red blood cells (SRBC).
Materials and Methods
Dogs
Beagles and beagle/mini-mongrel mixes were raised at the Fred Hutchinson Caner Research Center (FHCRC), assessed for disease, and enrolled in a preventive veterinary medicine for helminths and a standard immunization series (11). The study was approved by the Institutional Animal Care and Use Committee at the FHCRC (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International).
Cloning and Assembly of Canine CTLA4-Ig
Cloning and construction of cCTLA4-Ig was done according to published methods (12). Briefly, peripheral blood mononuclear cells (PBMC) from dogs were isolated by Ficoll-Hypaque gradient (density = 1.074). Total RNA was isolated from 24-hour phorbol myristate acetate (PMA) and ionomycin activated PBMC and cDNA was synthesized with M-MLV reverse transcriptase and oligo dT primer (Invitrogen, Carlsbad, CA). The forward (5′-GGACAACTTAAGGCCATGGCTGGGTTTGGATTC) and reverse primers (5′-GGACCAAAGCTTGCAAGGTTCAGGATCGATGAC) were used with Platinum PCR Supermix (Invitrogen Carlsbad, CA) to amplify leader sequence and extracellular domain of cCTLA-4 (GenBank accession number AF143204) and introduce AFlII and HindIII restriction sites. Sequencing was done with the above primers. The translated sequence was compared to the extracellular domain of hCTLA-4 and identity noted as 82.7 % (data not shown).
The cDNA of canine IgG1 was generated from dog PBMC by RT-PCR using Platinum PCR Supermix and a forward primer (ACCCAGCCAGCAACACTAAA) and a reverse primer (TTTCATGATGGGTGCCTACC) based on the GenBank sequence (AF354264) of Canis familiaris immunoglobulin gamma heavy chain A mRNA. The PCR product was isolated and ligated into the pGEM-T Easy vector (Promega, Madison, WI) for sequencing as above. For assembly of cCTLA4-Ig, a Gly4Ser linker was added at the 5′ end of the hinge region using the forward (ATAATTAAGCTTGGAGGTGGAGGTAGTTTCAATGAATGCAGATGC ACT) and reverse (GAATTGTATGCGGCCGCTCATTTACCCGGAGAATGGGA) primers, respectively. The cCTLA-4 leader and extracellular domain sequences were digested with AflII and HindIII and ligated into a similarly digested canine IgG1 vector. Following gel purification, the PCR products were digested and ligated into AflII and NotI digested pcDNA3.1+ creating a cCTLA4/canine IgG1/pcDNA3.1 construct. Verification of the denatured, alkylated, and reduced cCTLA4-Ig sequence was determined using standard LC MS/MS techniques (13), and yielded 165 peptides that matched the extracellular domain of cCTLA4 and canine IgG1; of these 19 peptides were unique to the fusion protein (data not shown). Cell culture and expression were done according to reported methods (12) and serum-free expression levels (extinction cultures) of cCTLA4-Ig from CHO cells were monitored by ELISA and ranged between 122 and 164 mg/liter.
Immunoreactivity of cCTLA4-Ig
Immunoreactivities of cCTLA4-Ig and hCTLA4-Ig (Abatacept, Bristol Meyers Squibb, Princeton, NJ) were determined in a competitive assay by flow cytometry (FACScan2, Becton Dickinson, Franklin Lakes, NJ) on the human cell line RAJI (CCL-86, American Type Culture Collection, Manassas, VA) or canine dendritic cells and monocytes generated from CD34+ bone marrow cells that were cultured for 7 days (14). Both cCTLA4-Ig and hCTLA4-Ig were labeled with fluorescein isothiocyanate (FITC) using standard methods. CTLA4-Ig-FITC (10 μg/ml), either canine or human, was mixed with dilutions of unlabeled cCTLA4-Ig or hCTLA4-Ig, added to cells, and allowed to compete for binding at 4°C for 45 minutes. The cells were washed and analyzed for fluorescence intensity by flow cytometry. The geometric mean of fluorescence intensity was determined from a histogram plot.
Functional Assays
The immunosuppressive activities of cCTLA4-Ig and hCTLA4-Ig were tested in 7-day, unidirectional MLR as described (15). Cells from DLA-non-identical dog pairs were used (16,17). Purified cCTLA4-Ig, hCTLA4-Ig, or anti-human monoclonal antibody (mAb) 5C8, specific to CD154 (18), was added in a dose escalation manner at the beginning of the assay.
Pharmacokinetics
Two dogs had pharmacokinetic sampling after IV administration of cCTLA4-Ig, 4 mg/kg, on days 0 and 14. Blood samples (2 ml) were collected before and at 10 and 30 minutes, 1, 2, 4, 6, 9, 24 hours after administration, then daily for 10 days, and then every 5 days thereafter. Sera were isolated and frozen for later analysis. Quantitation of circulating levels of cCTLA4-Ig was determined by ELISA using recombinant human B7-1/Fc chimera (R & D Systems, Minneapolis, MN) and peroxidase-labeled goat anti-dog IgG1 (Bethyl, Montgomery, TX) as capture and detection reagents, respectively. Calculations of serum concentrations of cCTLA4-Ig were done by regression analysis standardized with cCTLA4-Ig. Noncompartmental analysis was conducted using WinNonlin (Pharsight Mountain View, CA) to calculate the terminal elimination rate constant (kel), half-life (0.693/kel), and area under the plasma concentration time curve (AUC) from time 0 to infinity (AUC0-∞).
Anti-SRBC and Anti-Hemocyanin Antibodies
Two random-bred dogs from our colony, naïve to SRBC (Innovative Research Inc., Southfield, MI) and mollusk (C. concholepas) hemocyanin (Blue Carrier Immunogenic Protein, Pierce, Rockford, IL) were injected IV with 1.5 mg/kg diphenhydramine as a preventative for immediate hypersensitivity reactions and 30 minutes later injected IV with cCTLA4Ig (4 mg/kg, day 0). A 10% suspension of 1 ml of SRBC in phosphate buffered saline was given within an hour of injection of cCTLA4-Ig. The treatment was repeated on day 14. Additional injections of SRBC without cCTLA4-Ig were given IV on days 28, 106, 137, 167, 271, and 300. To test the response against a non-tolerized antigen, 10 μg/kg of hemocyanin was injected simultaneously with SRBC on days 106, 137 and 167. Eventually, in an effort to break tolerance to the immunogen, SRBC were suspended in Incomplete Freund's Adjuvant (IFA) (Pierce Protein Research Products, Pierce, Rockford, IL) and injected SC into both flanks of each dog on day 333. Serum samples were collected for antibody responses to either SRBC or hemocyanin at multiple time points, frozen at -20°C, and collectively tested for IgM and IgG antibody titers by standard ELISA methods. SRBC membrane antigen was prepared according to previously established methods (19). SRBC and hemocyanin antigens were coated on ELISA plates at 1 μg/ml in phosphate buffered saline and the assays conducted as previously described (20).
Results
Immunoreactivity
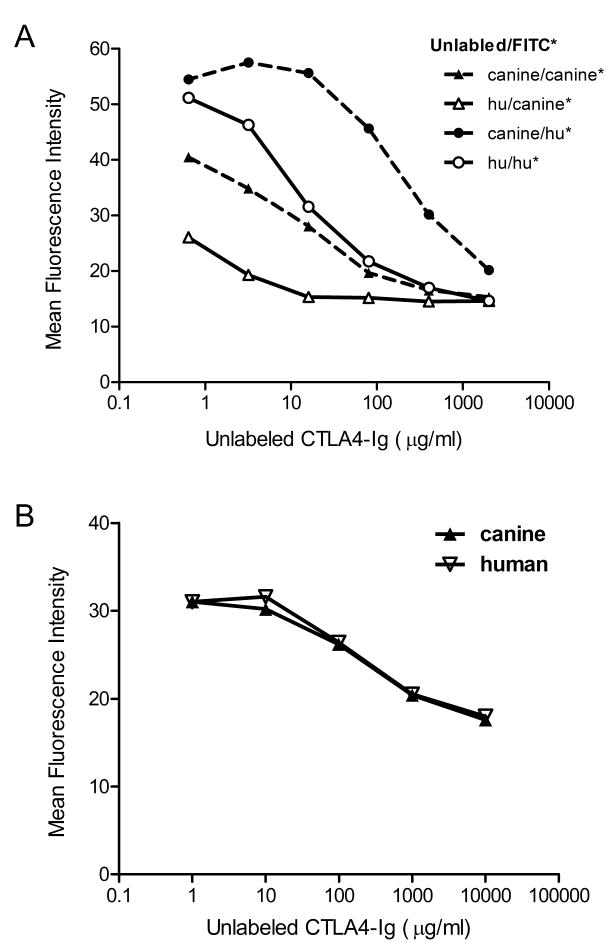
Competitive binding studies for cCTLA4-Ig and hCTLA4-Ig were first done using the human cell line Raji. Both fusion proteins were labeled with FITC, allowed to bind Raji cells in the presence or absence of the unlabeled proteins, and analyzed by flow cytometry. Of the two fusion proteins, hCTLA4-Ig out-competed cCTLA4-Ig for binding to the human Raji cell line (Figure 1a), while there was no significant difference between the two fusion proteins for binding to the canine cells (Figure 1b). Results indicated that the limited amino acid disparities between the two fusion proteins would likely not result in increased canine-specific efficacy.
Figure 1. Competitive immunoreactivity of cCTLA4-Ig and hCTLA4-Ig on Raji cells.
A) Either FITC-labeled cCTLA4Ig (triangles) or FITC-labeled hCTLAIg (circles) at 10 μg/ml were mixed with different concentrations of unlabeled cCTLA4Ig (closed symbols) or hCTLA4Ig (open symbols) and added to Raji cells. B) FITC-labeled cCTLA4Ig was mixed with either unlabeled cCTLA4Ig or hCTLA4Ig at different concentrations and the mixtures added to a 7-day mixed primary cell population of canine monocytes and dendritic cells. Cells were analyzed by flow cytometry. Data are presented in units of mean fluorescence intensity.
Evaluation of cCTLA4-Ig in MLR
In order to estimate the in vivo active concentration of cCTLA4-Ig and further compare the immunoreactivities of cCTLA4-Ig and hCTLA4-Ig, escalating doses of both fusion proteins were added to canine MLR. Four independent MLR indicated 10 μg/ml, cCTLA4Ig and hCTLA4Ig reduced the 3H thymidine uptake on average by 87.5% and 88.3%, respectively (data not shown). We failed to see significant differences between the two fusion proteins suggesting similar binding to canine antigen presenting cells.
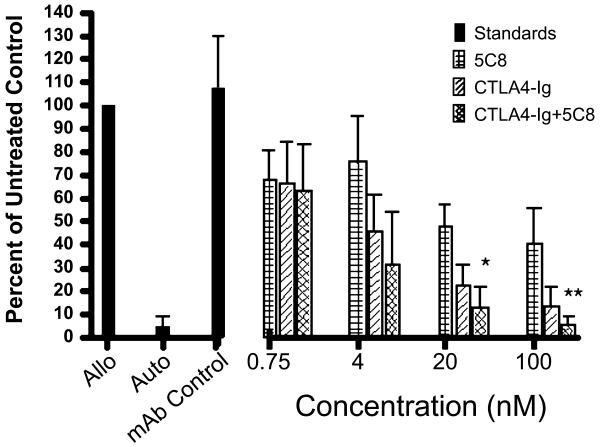
Previous studies showed that the mAb 5C8 directed against human CD154, when given along with donor PBMC, was effective in increasing stable engraftment of DLA-identical marrow after 1 Gy TBI (18). In order to determine the potential additive effects of blocking both the CD28:CD80/86 and CD40:CD154 pathways, we combined mAb 5C8 and cCTLA4-Ig at equal molar concentrations in a canine MLR. The combined data from four experiments indicated that cCTLA4-Ig was superior to mAb 5C8 alone in blocking 3H-thymidine uptake, but combining the two reagents was significantly better in blocking 3H-thymidine uptake at 100 and 20 nM concentrations than cCTLA4-Ig alone (Figure 2).
Figure 2. Suppression of a 7-day MLR by cCTLA4Ig and anti-human CD40L (5C8).
The mAb 5C8 (checkered bars), cCTLA4Ig (diagonally-lined bars), or the combination of both inhibitors (cross-hatched bars) were added to the culture at the concentrations indicated. Solid bars indicate allogeneic response in the absence of costimulatory molecules, the autologous response, and allogeneic response with 100 nM of a mAb antibody isotype control for 5C8 (an IgG2a antibody specific for canine prostate esterase). Data are presented as mean (plus standard deviation) of 4 independent studies using DLA-nonidentical dog pairs and calculated as percent of control (response without inhibitors, range 10,021-48,052 CPM) as determined by cellular 3H-thymidine uptake. Addition of equal molar concentrations of 5C8 and cCTLA4-Ig showed significant (Student's two-tailed T-test) inhibition of 3H-thymidine uptake over cCTLA4-Ig alone at 20 nM (*, p> 0.02) and 100 nM (**, p> 0.01).
Pharmacokinetics of cCTLA4-Ig
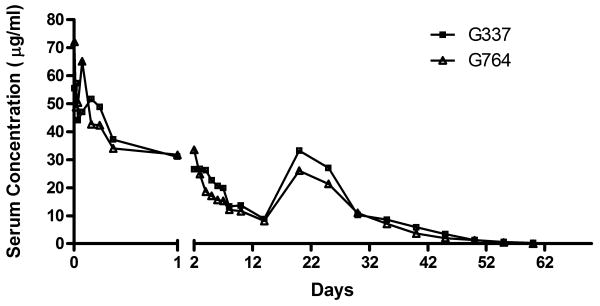
At various time points after cCTLA4-Ig injection, blood samples were taken for analysis of circulating serum levels of cCTLA4-Ig (Figure 3). The systemic exposure (i.e., AUC0-∞) was 775 and 658 μg/ml/day in the first and second dog, respectively (i.e., dogs G337 and G764). The cCTLA4-Ig concentrations 10 minutes after administration were 55.5 and 72.1 μg/ml, respectively. The elimination half-lives after doses 1 and 2 were 6.91 and 6.67 days, respectively, in the first dog and 8.51 and 6.14 days, respectively, in the second dog. No increased IgM or IgG antibody titers against cCTLA4-Ig over preinjection background titers were detected that might have affected pharmacokinetics (data not shown).
Figure 3. Pharmacokinetics of cCTLA4-Ig in two dogs.
Dogs G337 and G764 were injected with 4 mg cCTLA4Ig/kg on days 0 and 14. Blood samples (2 ml) were collected before injection and at 10 minutes, 1, 2, 4, 6, 8, 24 hours, then daily for 8 days and then weekly for 2 months. Circulating levels of cCTLA4-Ig were determined by ELISA.
Inhibition of Anti-SRBC Responses
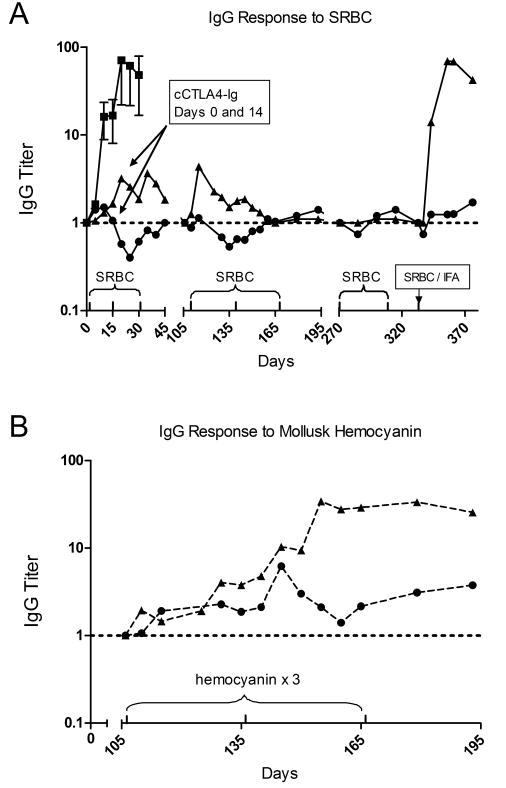
Prevention of class switch from IgM to IgG antibody responses against the T-dependent antigens of SRBC by cCTLA4-Ig is shown in Figure 4. In the first arm of the study, two dogs were injected with cCTLA4-Ig on days 0 and 14 and with SRBC on days 0, 14 and 28, while five control dogs were injected with SRBC on days 0 and 14 only (Figure 4A). The IgG responses in the cCTLA4-Ig treated dogs were blocked relative to the controls even after a third injection of SRBC on day 28. Anti-SRBC IgM titers of one of the two dogs were similar to controls while those of the second dog were lower (data not shown). In order to determine whether tolerance to SRBC could be broken, three additional immunizations of SRBC were begun in a second arm of the study 105 days after administration of cCTLA4-Ig. In addition, hemocyanin was co-administered to the two dogs to determine whether tolerance was specific to SRBC. To this end, the same pair of dogs were injected IV with one ml of a 10% SRBC suspension and 10 mg of hemocyanin subcutaneously on days 106, 137, and 167 (Figure 4B). Both dogs responded to the hemocyanin antigen as determined by ELISA; however, the IgG responses against SRBC remained at background but for the exception of a brief increase in titer in dog G337 at day 116. After a delay of 104 days, injections of SRBC were continued on days 271 and 300 without observing increases in IgG titers to SRBC (Figure 4A). In a final effort to break tolerance, SRBC were suspended in IFA and given subcutaneously on day 333. Four days after injection there was a rapid increase in anti-SRBC IgG titers in dog G337 that peaked at 17 days after injection to a level similar to the mean of 5 control animals injected with SRBC alone, while the response in the other dog remained flat.
Figure 4. Anti-SRBC and anti-hemocyanin IgG response in dogs treated with cCTLA4-Ig.
Dogs G337 (▲) and G764 (•) were injected iv on days 0 and 14 with 4 mg/kg cCTLA4-Ig and SRBC on days 0, 14 and 28, then SRBC plus hemocyanin on days 106, 137, 167, 271 and 300. A final injection of SRBC in IFA was given on day 333. Blood samples were collected during this period and sera were tested for titers of anti-SRBC (A), and anti-hemocyanin (B) IgG antibodies by ELISA. Results were compared to pre injection titers for each dog (titer = 1). Titers on the ordinate are in log10 units. Controls were comprised of 5 normal dogs injected with SRBC on days 0 and 14 only.
Discussion
CTLA4-Ig blockade of the CD28/B7 costimulatory pathway has led to suppression of immune responses in a number of different model systems with variable lengths of duration (21-23). In mice, a short course treatment of CTLA4-Ig was shown to temporarily block an immune response against the T-cell dependent antigen, SRBC (23). However the state of anergy lasted only 90 days and an IgG titer to SRBC was detected after a third injection of antigen. The salient finding of the present study was induction of long-term anergy to the highly immunogenic antigen SRBC by cCTLA4-Ig. Specifically, two dogs treated with two intravenous injections of SRBC concurrent with two cCTLA4-Ig injections 2 weeks apart failed to generate IgG antibody responses to SRBC. Six subsequent injections of SRBC over a period of nearly 300 days continued to result in anti-SRBC IgG production, consistent with a state of anergy. Coinjection of hemocyanin with SRBC on days 106, 137 and 167 failed to break tolerance to SRBC but resulted in normal immune responses to hemocyanin. When we eventually attempted to overcome the anergic state by immunizing subcutaneously with SRBC in IFA, only one of the two dogs generated IgG antibodies at a level similar to the five control dogs while the other remained unresponsive. The reason why the canine model demonstrated a more profound and long-lasting degree of hyporesponsiveness to SRBC compared to the mouse is not readily apparent; perhaps either basic differences between inbred mice and random-bred dogs or the need for a second injection of species-specific CTLA4-Ig played a role.
Suppression of immune responses by costimulatory molecule blockade has been variably attributed to anergy (24), Th 1 cytokine suppression (4) apoptosis (25) and suppression (26). Following injection of SRBC in IFA, an increase in anti-SRBC IgG titers occurred within 4 days, suggesting deletion by apoptosis of Th clones recognizing SRBC was not a mechanism of tolerance in this model. However, elimination of a suppressed state may have resulted from a shift in cytokine production favoring the Th2 subset and antibody production. Studies on the prevention of diabetes using costimulatory molecule blockade with CD28−/− and CTLA4-Ig, transgenic mice showed diminished Th-2 cytokine Il-4 production by glutamic acid decarboxylase (GAD)-specific T cells (27). Thus the initial prevention of Th-2 induction by CTLA4-Ig and SRBC administered IV could have been reversed with Th type 2 cytokine production initiated by SRBC plus IFA injected subcutaneously.
The random-bred dog model has been extremely useful for developing strategies for improving the safety of human HCT. In order to properly evaluate the induction of tolerance to donor hematopoietic cells in the HCT recipient, it has been critical that species-specific antagonists of costimulatory molecule function are developed. Although we did not observe an in vitro functional difference between cCTLA4-Ig and hCTLA4-Ig, our studies demonstrated we have produced a cCTLA4-Ig fusion protein that provided us with a nonimmunogenic molecule for further investigations of tolerance in a canine model of allogeneic HCT.
Acknowledgments
We thank Michele Spector, DVM, Alix Joslyn, Brian Steinmetz, and the research technicians in the canine facilities of the Fred Hutchinson Cancer Research Center for assistance with animal care; and Bonnie Larson and Helen Crawford for help with manuscript preparation.
Abbreviations
- CHO
Chinese hamster ovarian
- CTLA-4
CTL-associated antigen 4
- dhfr
dihydrofolate reductase
- MLR
mixed leukocyte reactions
- TBI
total body irradiation
Footnotes
This work was supported by National Institutes of Health grants CA78902, CA15704, and AI067770. The laboratory also was supported by an award from the Joseph Steiner Krebsstifung, Bern Switzerland and a grant from the Lupin Foundation, Metairie, Louisiana (both to R.S.)
The authors have no conflicts of interest to disclose.
References
- 1.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance (Review) Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. erratum appears in. [DOI] [PubMed] [Google Scholar]; Immunol Rev. 2004 Feb;197:243. [Google Scholar]
- 2.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells [Review] Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 3.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 4.Sayegh MH, Akalin E, Hancock WW, et al. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayegh MH, Zheng XG, Magee C, Hancock WW, Turka LA. Donor antigen is necessary for the prevention of chronic rejection in CTLA4Ig-treated murine cardiac allograft recipients. Transplantation. 1997;64:1646–1650. doi: 10.1097/00007890-199712270-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Linsley P, Seidel K, et al. Cytotoxic T lymphocyte antigen 4-immunoglobulin fusion protein combined with methotrexate/cyclosporine as graft-versus-host disease prevention in a canine dog leukocyte antigen-nonidentical marrow transplant model. Transplantation. 2000;69:450–454. doi: 10.1097/00007890-200002150-00027. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 8.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 9.Yasunaga S, Tsukui T, Masuda K, Ohno K, Tsujimoto H. CTLA-4 recombinant protein genetically fused to canine Fc epsilon receptor Ialpha enhances allergen specific lymphocyte responses in experimentally sensitized dogs. J Vet Med Sci. 2004;66:611–617. doi: 10.1292/jvms.66.611. [DOI] [PubMed] [Google Scholar]
- 10.Shin IS, Choi EW, Chung JY, Hwang CY, Lee CW, Youn HY. Cloning, expression and bioassay of canine CTLA4Ig. Vet Immunol Immunopathol. 2007;118:12–18. doi: 10.1016/j.vetimm.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Schuening FG, Storb R, Goehle S, et al. Recombinant human granulocyte colony-stimulating factor accelerates hematopoietic recovery after DLA-identical littermate marrow transplants in dogs. Blood. 1990;76:636–640. [PubMed] [Google Scholar]
- 12.Jochum C, Beste M, Stone D, Graves SS, Storb R. Development and in vitro characterization of canine CD40-Ig. Vet Immunol Immunopathol. 2008;123:260–265. doi: 10.1016/j.vetimm.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steen H, Mann M. The ABC's (and XYZ's) of peptide sequencing (Review) Nat Rev Mol Cell Biol. 2004;5:699–711. doi: 10.1038/nrm1468. [DOI] [PubMed] [Google Scholar]
- 14.Georges GE, Lesnikova M, Storb R, Yunusov M, Little MT, Nash RA. Minor histocompatibility antigen-specific cytotoxic T lymphocytes generated with dendritic cells from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9:234–242. doi: 10.1053/bbmt.2003.50023. [DOI] [PubMed] [Google Scholar]
- 15.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- 16.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 18.Jochum C, Beste M, Zellmer E, Graves SS, Storb R. CD154 blockade and donor-specific transfusions in DLA-identical marrow transplantation in dogs conditioned with 1-Gy total body irradiation. Biol Blood Marrow Transplant. 2007;13:164–171. doi: 10.1016/j.bbmt.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temple L, Kawabata TT, Munson AE, White KL., Jr Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundamental & Applied Toxicology. 1993;21:412–419. doi: 10.1006/faat.1993.1116. [DOI] [PubMed] [Google Scholar]
- 20.Sorror ML, Leisenring W, Mielcarek M, et al. Intensified postgrafting immunosuppression failed to assure long-term engraftment of dog leukocyte antigen-identical canine marrow grafts after 1 gray total body irradiation. Transplantation. 2008;85:1023–1029. doi: 10.1097/TP.0b013e318169be24. [DOI] [PubMed] [Google Scholar]
- 21.Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 22.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 23.Wallace PM, Rodgers JN, Leytze GM, Johnson JS, Linsley PS. Induction and reversal of long-lived specific unresponsiveness to a T-dependent antigen following CTLA4Ig treatment. J Immunol. 1995;154:5885–5895. [PubMed] [Google Scholar]
- 24.Tan P, Anasetti C, Hansen JA, et al. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB-1. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 26.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–4810. [PubMed] [Google Scholar]
- 27.Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]