Summary
The gastric pathogen Helicobacter pylori (H. pylori) infects over half the world's population. The lifelong infection induces gastric inflammation but the host fails to generate protective immunity. To study the lack of protective H. pylori immunity, CD4+CD25+ Treg cells were investigated for their ability to down-regulate H. pylori-specific CD4+CD25- cells in a murine model. CD25- lymphocytes from infected mice were hyporesponsive to antigenic stimulation in vitro even in the absence of CD25+ Treg cells unless treated with high dose IL-2. Transfer of CD45RBhi naïve CD25- cells from infected mice into rag1-/- mice challenged with H. pylori resulted in severe gastritis and reduced bacterial loads, whereas transfer of CD45RBlo memory CD25- cells from H. pylori-infected mice resulted in only mild gastritis and persistent infection. CD25- cells stimulated in the absence of CD25+ cells in rag1-/- mice promoted bacterial clearance, but lost this ability when subsequently transferred to wild type mice harboring CD25+ cells. These results demonstrate that CD25+ cells induce anergy in CD25- cells in response to H. pylori infection but are not required to maintain hyporesponsiveness. In addition, CD25+ cells are able to suppress previously activated CD25- cells when responding to H. pylori challenge in vivo.
Keywords: Anergy, Helicobacter pylori, inflammation, regulatory T cells, mouse
Introduction
CD4+CD25+ regulatory T cells (Treg cells) have been identified in the peripheral tissue of mice and humans where they help prevent the development of autoimmunity by down-regulation of self-reactive T cells that escape thymic education [1]. Whereas the level of regulatory CD25hi T cells in human blood ranges from 2 – 4%, a distinct population of regulatory CD25+ cells make up approximately 10% of the mouse peripheral T cell pool [2-4]. Treg cells are further characterized by the expression of the transcription factor Foxp3 and by their anergic response to in vitro stimulation [5, 6]. The suppressive activity of these cells is contact-dependent and requires TCR activation [7]. It has recently been shown that Treg cells are also necessary for the maintenance of tolerance to food antigens and commensal flora of the gut [8, 9] as transfer of CD4+ T cells depleted of Treg cells into immunodeficient mice results in the spontaneous development of colitis and wasting disease [10, 11]. Treg cells were shown to prevent the development of colitis when simultaneously transferred with a CD25+ Treg cell-depleted population [9, 12].
Treg cells have been identified in the host response to specific pathogens [13-16]. Reduced and non-protective immune responses have been described for the parasites Plasmodium yoelii and Leishmania major and the fungus Pneumocystis carinii which contributes to the ability of these microorganisms to establish persistent infection of the host [14-16]. Recently, CD25+ Treg cells have also been identified in the gastric mucosa of Helicobacter pylori (H. pylori)-infected patients [17-19]. H. pylori is a gram-negative bacterium that infects the stomach of more than half the world's population and in many developing nations its prevalence exceeds 80% of the population [20, 21]. Infection persists for the life of the host and is accompanied by active-chronic inflammation and an adaptive immune response that fails to generate protective immunity. Several early studies on H. pylori immunity indicated that infection might induce T cell hyporesponsiveness as both peripheral blood lymphocytes and gastric lymphocytes from H. pylori-positive patients were shown to respond to in vitro stimulation by H. pylori antigen with low cytokine secretion and proliferation relative to H. pylori-negative patients [22, 23]. In mice, the in vitro H. pylori-specific recall response of T cells from experimentally infected animals is significantly weaker than mice immunized with H. pylori antigens [24, 25] and limiting the contribution of CD25+ cells can result in increased gastritis and reduced bacterial loads [26, 27].
In the present study we investigated the extent of the regulatory activity of CD4+CD25+ Treg cells during H. pylori infection in mice. We demonstrate here that the presence of Treg cells at the time of T cell activation in the gastric mucosa results in the generation of a population of CD25- H. pylori-specific anergic T cells. Although several reports have suggested that these H. pylori-specific CD25- T cells become responsive when CD25+ Treg cells are removed [26-28], our in vitro and in vivo data suggest that once stimulated in the presence of Treg cells, this hyporesponsive CD25- population remains anergic even in the absence of CD25+ regulatory T cells. Additionally, we demonstrate that Treg cells are not only capable of inducing H. pylori-specific anergy in naive CD25- cells, but are capable of down-regulating anti-H. pylori immunity in previously activated CD25- cells as well.
Results
Foxp3 expression is increased in the gastric mucosa during H. pylori infection
Prior to investigating the role of CD25+ regulatory T cells during H. pylori infection, we confirmed the recruitment of these cells to the gastric mucosa in our model of H. pylori infection by measuring Foxp3 mRNA levels in gastric biopsies by quantitative PCR. Foxp3 positive Treg cells have been previously reported to reside in the gastric mucosa after H. pylori infection as has been demonstrated in both human and murine tissue [17, 26]. In our model, Foxp3 expression was increased almost three fold compared to naïve control mice four weeks after challenge (data not shown). Although the increase was not statistically significant we observed a comparable increase in two independent experiments.
Hyporesponsive CD25- cells are activated by high dose IL-2 and antigen
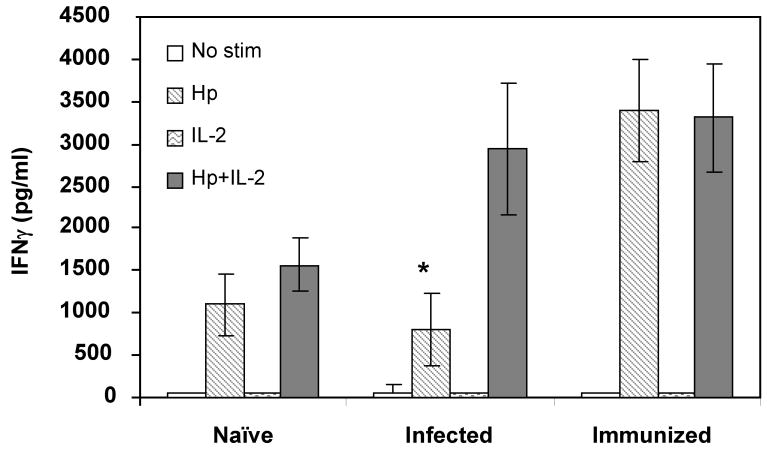
We have previously demonstrated H. pylori-specific anergy in bulk spleen cells from H. pylori-infected mice that could be induced to produce IFNγ in the presence of H. pylori and high dose IL-2 (1000 U/ml) [24]. To control for the possibility that other cell types in the spleen cell population might contribute to the responsiveness of the T cells, purified CD4+ cells were cultured in the presence or absence of high dose IL-2. Figure 1 shows that the CD4+ cells from infected mice displayed activity comparable to naïve mice when stimulated by antigen alone. This activity was significantly less than observed for antigen-stimulated CD4+ cells from immune mice (P < 0.001). The addition of IL-2 resulted in IFNγ production by cells from infected mice comparable to the response of the cells from immune mice.
Figure 1. H. pylori-specific hyporesponsiveness of CD4+ cells from infected mice is reversed in the presence of high dose IL-2.
CD4+ cells were isolated from the spleens of naïve, infected, or immunized mice and stimulated in vitro for 24 hours with 10 μg/ml H. pylori lysate (Hp), 1000 U/ml rhIL-2, or both. T cell activation was determined by production of IFNγ as quantified by ELISA. * P < 0.01 compared to immunized mice similarly treated. Each value shown is the mean of each group ± the standard deviation.
We then assessed the role of CD25+ cells in preventing the T cells from responding to antigen exposure in vitro. Spleen cell populations from infected mice in which the CD25+ cells had been depleted by either complement-mediated killing or by fluorescence activated cell sorting remained anergic when stimulated with H. pylori lysate antigen. However, activity could be recovered only when cells were simultaneously exposed to antigen and high dose IL-2 (data not shown).
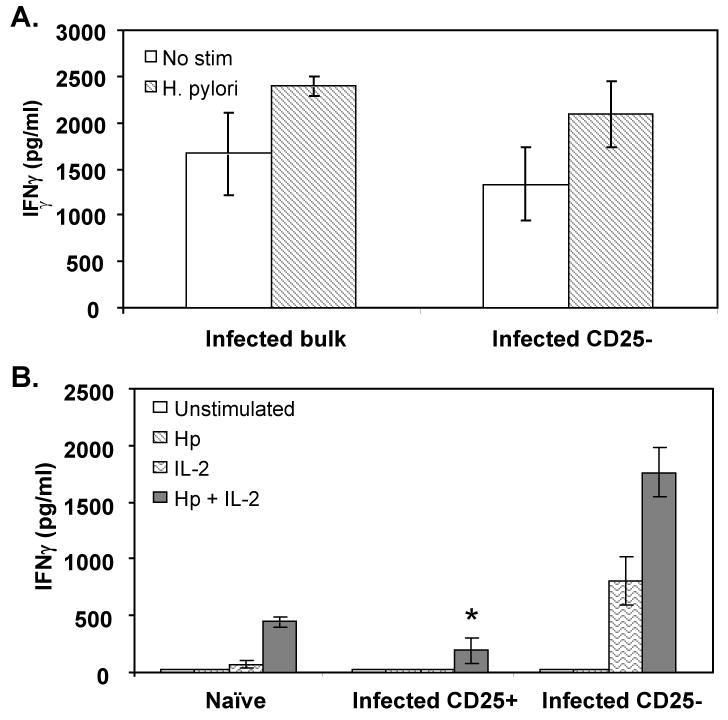
The cellular response of the infected mice was further analyzed by specifically measuring the activity of the CD4+ cells in the presence or absence of CD25+ cells. Initially, CD25+ cells were depleted from bulk spleen cells by complement-mediated killing and the CD25- cells were stimulated in vitro with H. pylori lysate antigen. Removal of the CD25+ cells did not result in the ability of the CD25- cells to respond to antigen (Figure 2A). Flow cytometric assessment of cell populations depleted of CD25+ cells by complement-mediated lysis showed that less than one percent of the cells continued to stain positively for CD25 compared to approximately 11% for untreated cells (data not shown). We then purified CD4+ cells from spleens by positive selection and then further fractionated the cells into CD25+ and CD25- populations by affinity column isolation. The direct stimulation of CD4+ cells from naïve mice with H. pylori antigen generated low levels of activity (Figure 1). Similar observations have been made by others [29]. Therefore we stimulated the fractionated cells with antigen-pulsed macrophages in which soluble antigen was removed by washing prior to co-culture with the fractionated spleen cells. CD4+CD25- cells failed to respond to H. pylori antigen but were induced to produce significantly greater quantities of IFNγ when co-stimulated with high dose IL-2 (P = 0.008) (Figure 2B).
Figure 2. H. pylori-specific CD25- T cells remain hyporesponsive in the absence of CD25+ cells but are activated by the addition of exogenous high dose IL-2.
(A) Splenocytes isolated from H. pylori-infected mice were depleted of CD25+ cells by complement-mediated killing and stimulated with 10 μg/ml H. pylori lysate antigen. (B) CD4+ cells from infected mice fractionated into CD25- or CD25+ T cell populations by flow cytometry were stimulated with antigen pulsed macrophages in the presence or absence of high dose (1000U/ml) rhIL-2. IFNγ was measured as a marker of T cell activation. CD4+ cells from naïve mice were unfractionated. *P < 0.01 compared to CD25- cells stimulated in a comparable manner. Each value shown is the mean of each group ± the standard deviation.
CD4+CD25- cells from infected mice promote gastritis and reduce bacterial load
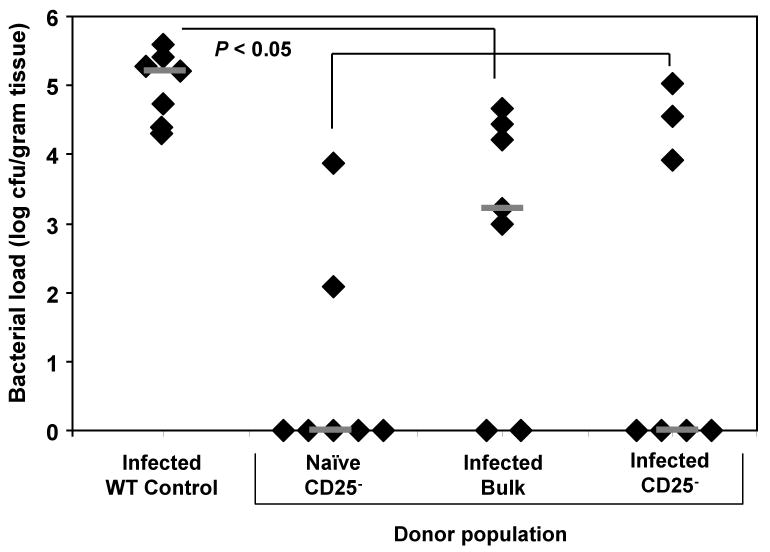
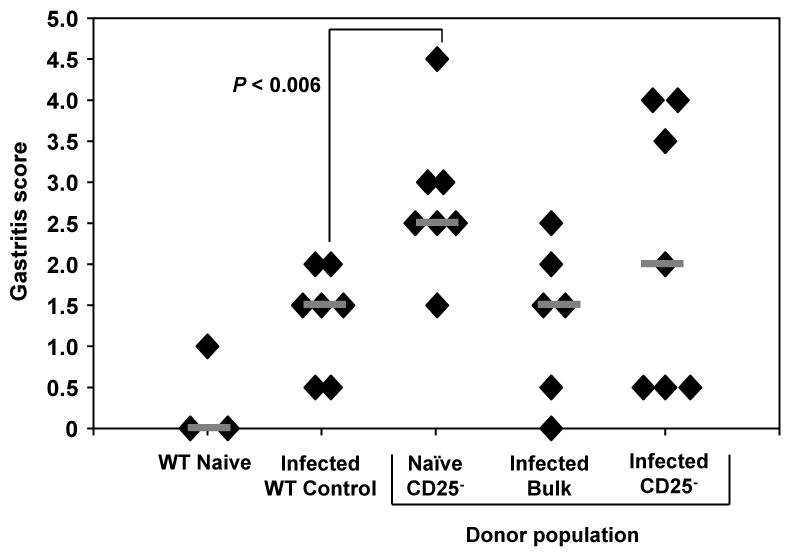
Since CD25- T cells from H. pylori-infected mice were hyporesponsive in vitro, we transferred these cells into immunodeficient rag1-/- mice prior to challenge with live H. pylori to measure their responsiveness in vivo in the absence of regulatory T cells. Since mice deficient in the rag1 enzyme lack mature T cells and B cells, the CD25- cells from the donor mice would be stimulated in the absence of any recipient mouse lymphocytes. We observed significant reductions in bacterial load in rag1-/- mice reconstituted with CD25- cells from naïve donors (Figure 3). These mice had the lowest average number of bacteria and displayed the most severe inflammation, significantly greater than experimentally infected WT control mice (Figure 4, P < 0.006). Transfer of bulk CD4+ cells from H. pylori-infected mice into rag1-/- recipients resulted in a significant decrease in bacterial load compared to infected WT control mice (Figure 3, P < 0.05) and mild gastric inflammation (Figure 4). Contrary to our expectations, we also observed decreased bacterial load in rag1-/- mice receiving CD4+CD25- cells from H. pylori-infected mice that was statistically equivalent to groups transferred with CD4+CD25- cells from naïve donors. Histologic examination of the gastric mucosa demonstrated that rag1-/- mice reconstituted with CD4+CD25- cells from H. pylori-infected mice responded with mild inflammation (Figure 4).
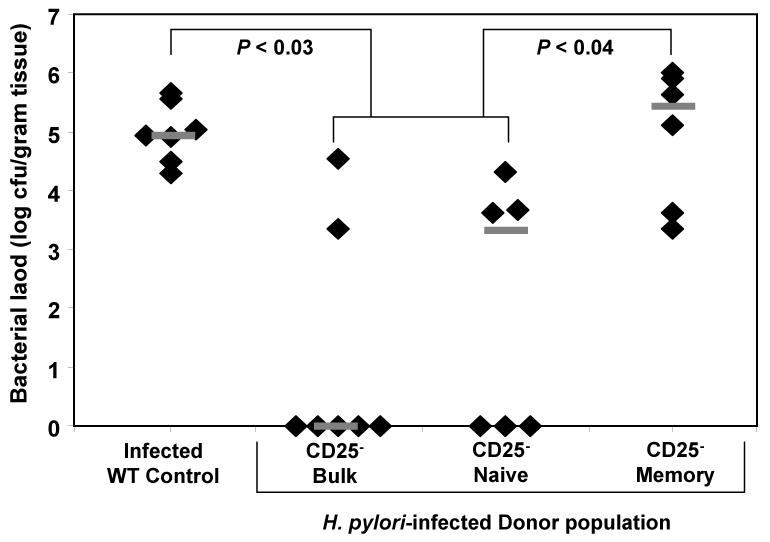
Figure 3. Adoptive transfer of bulk CD4+ or CD4+CD25- cells from H. pylori-infected donors results in decreased bacterial colonization.
Bulk CD4+ or purified CD4+CD25- spenocytes isolated from naïve or H. pylori-infected mice were adoptively transferred into immunodeficient recipient mice on day zero. All mice were then challenged with live H. pylori on days one and two. Mice were sacrificed on day 28 and the level of bacterial colonization was compared to infected WT control mice. Grey bars represent the median for each group. N = seven mice per group. * P < 0.05 for all three transfer groups compared to infected WT control.
Figure 4. Adoptive transfer of bulk CD4+ or CD4+CD25- cells from H. pylori-infected donors results in mild gastric inflammation.
Bulk CD4+ or purified CD4+CD25- splenocytes isolated from naïve or H. pylori-infected mice were adoptively transferred into immunodeficient recipient mice on day zero. All mice were then challenged with live H. pylori on days one and two. Mice were sacrificed on day 28 and the level of gastric inflammation was compared to infected WT control mice. N = seven mice per group.
Memory CD25- cells promote mild gastritis and persistent H. pylori colonization
Our data demonstrate that adoptive transfer of purified CD25- cells from H. pylori-infected donors into immunodeficient mice promotes inflammation that is hostile to H. pylori upon challenge (Figures 3 and 4). This is in contrast to the hyporesponsiveness we observed in vitro. We next determined whether transfer of bulk CD25- cells from infected mice, as performed above, might include a memory and naïve population of CD25- cells capable of generating distinct responses. The CD25- population from H. pylori-infected mice was fractionated into CD45RBhi (naïve) and CD45RBlo (memory) populations and then separately transferred into rag1-/- recipients followed by challenge with H. pylori. As demonstrated in Figure 5, H. pylori challenge of rag1-/- mice reconstituted with either bulk CD25- cells or purified CD4+CD25-CD45RBhi naïve cells from H. pylori-infected mice resulted in significantly decreased levels of bacterial colonization relative to infected WT controls (P < 0.05). In contrast, challenge of rag1-/- mice reconstituted with purified CD4+CD25-CD45RBlo memory cells from H. pylori-infected mice failed to reduce bacterial colonization and had bacterial loads that were significantly greater than from transfer of unfractionated CD25- cells or purified CD4+CD25-CD45RBhi naïve cells from infected mice (P < 0.05). The increased levels of bacteria in these mice were comparable to H. pylori-infected WT control mice. These data demonstrate that after in vivo challenge with H. pylori, the H. pylori-specific memory CD25- cells remain unresponsive despite the absence of CD25+ regulatory T cells resulting in persistent colonization.
Figure 5. Memory CD4+CD25- cells from H. pylori-infected mice remain hyporesponsive after in vivo challenge.
Bulk CD25- cells, CD25- CD45RBhi (naïve) cells or CD25- CD45RBlo (memory) cells were prepared from the spleens of H. pylori infected mice and transferred into immunodeficient recipient mice on day zero. Mice were challenged with live H. pylori on days one and two and sacrificed 28 days post-challenge. Grey bars represent the median for each group. N = six mice per group.
Histologic examination of the gastric mucosa in these mice revealed an inverse relationship between the bacterial load and the degree of gastritis (Figure 6). Whereas protected rag1-/- mice that had been reconstituted with CD25- bulk cells or CD25- naïve cells from infected donor mice displayed severe gastritis compared to infected WT control mice (P < 0.003), mice reconstituted with H. pylori-specific CD25- memory cells had a heavy bacterial load and only minimal inflammation. The gastric inflammation was equivalent to infected WT controls and statistically reduced relative to mice reconstituted with bulk or naïve CD25- cells (P < 0.003) These results are consistent with previous reports suggesting that increased gastritis correlates with decreased bacterial colonization [24, 30, 31]. These data suggest that after anergy is induced in H. pylori-specific CD25- cells, the CD25+ regulatory T cells are no longer necessary to maintain persistent infection.
Figure 6. Memory CD4+CD25- cells induce mild gastritis in response to H. pylori infection.
Bulk CD25- cells, CD25- CD45RBhi (naïve) cells or CD25- CD45RBlo (memory) cells were prepared from the spleens of H. pylori-infected mice and transferred into immunodeficient recipient mice on day zero. Mice were challenged with live H. pylori on days one and two and sacrificed 28 days post-challenge. Gastric sections were removed and the degree of inflammation based on amount and degree of cellular infiltrate and changes in tissue architecture was measured. Transfer of splenocytes from infected WT mice served as a control. N = six-seven mice per group.
Treg cells suppress previously activated H. pylori-specific CD25- cells in vivo
The data presented above indicate the presence of Treg cells during activation of H. pylori-specific CD25- cells is sufficient to induce a permanent state of anergy in some H. pylori-specific cells. Because H. pylori-infected mice can be protected by therapeutic immunization [32, 33] we next tested whether previously-activated, CD25- T cells are capable of promoting inflammation that reduces the bacterial load when introduced to wild type mice either before or after H. pylori infection, a system in which the recipient mice have a full complement of CD25+ T cells. First, rag1-/- mice reconstituted with CD25- cells from naïve wild type mice were challenged with H. pylori. Challenge of these mice resulted in a population of CD25- T cells associated with a significant reduction in bacterial load relative to our infected WT control group (Figure 7, P < 0.05). These CD25- cells were then isolated from the spleens of the immunodeficient mice and transferred again into either naïve WT mice or H. pylori-infected WT recipients that contain a normal repertoire of CD25+ regulatory T cells. The naïve recipients were challenged one week after transfer. Mice were harvested at four weeks post-transfer and gastric sections were removed to determine the level of bacterial colonization and gastric inflammation as described in Methods.
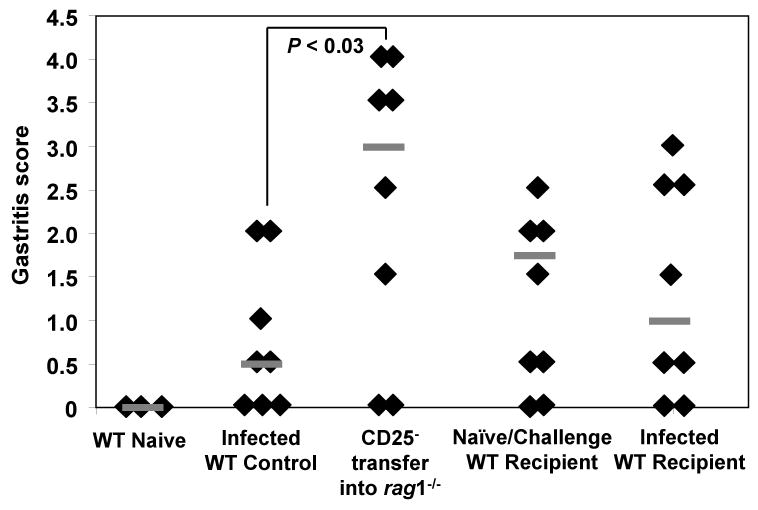
Figure 7. Previously-activated CD25- cells are not capable of reducing bacterial load in WT recipients.
CD25- splenocytes isolated from naïve WT donor mice were transferred into rag1-/-recipient mice on day zero followed by challenge with live H. pylori on days one and two. Bulk splenocytes isolated from these recipient mice were isolated on day 29 and transferred into either naïve or H. pylori-infected WT mice. Naïve mice were challenged with live H. pylori on days 35 and 36. Mice were sacrificed on day 64 and the level of bacterial colonization was compared to infected WT control mice. Grey bars represent the median for each group. N = five-seven mice per group.
Transfer of the CD25- cells into naïve wild type mice that were challenged after adoptive transfer resulted in high levels of bacterial colonization, statistically greater than transfer into rag1-/- mice (P < 0.05) and equivalent to that of infected control mice (Figure 7). Similar results were observed when these CD25- cells were transferred into previously-infected wild type mice. Bacterial colonization levels remained high, not significantly distinct from infected WT controls. These data suggest that in addition to naïve CD25- cells, previously-activated CD25- cells succumb to the regulatory control of CD25+ T cells in vivo.
Gastric sections were isolated from these mice to assess the level of inflammation. As shown in Figure 8, infected WT control mice developed mild gastritis as we have observed previously. The control group of immunodeficient mice that had received CD25- cells associated with reduced bacterial loads resulted in significantly increased levels of inflammation relative to infected controls (P < 0.03). Transfer of these CD25- cells into either infected wild type or naïve wild type mice followed by challenge resulted in statistically equivalent levels of gastritis (1.4 ± 1.0, 1.3 ± 1.2 respectively). Further, both groups of wild type recipients of the CD25- cells displayed mild gastritis statistically similar to infected controls. These data are in accordance with other models of H. pylori immunity in which high gastritis correlates with decreased colonization [24, 30, 31].
Figure 8. Transfer of previously-activated CD25- cells into WT recipients results in mild gastric inflammation.
CD25- splenocytes isolated from naïve WT donor mice were transferred into rag1-/- recipients on day zero followed by challenge with live H. pylori on days one and two. Bulk splenocytes isolated from these recipients were isolated on day 29 and transferred into either naïve or H. pylori-infected WT mice. Naïve mice were challenged with live H. pylori on days 35 and 36. Mice were sacrificed on day 64 and gastric inflammation was measured as described in Methods and compared to infected WT control mice. N = eight mice per group.
Discussion
This study was designed to investigate the extent of CD25+ T cell regulatory activity during H. pylori infection. T cells from H. pylori-infected mice remained hyporesponsive to H. pylori antigens in vitro even in the absence of CD25+ Treg cells. The ability of high dose IL-2 together with H. pylori antigen to activate these CD25- T cells is indicative of anergy. We confirmed these observations in vivo as transfer of H. pylori-specific CD25- memory cells fractionated from the T cell population of infected mice failed to promote gastritis or reduce the bacterial load when transferred into rag1-/- mice that were subsequently challenged with H. pylori. Therefore, using both in vitro and in vivo techniques, we demonstrate here that CD25+ regulatory T cells induce a CD25- cell hyporesponse that helps give rise to the mild inflammation and persistent colonization observed during chronic H. pylori infection in mice.
Several reports have documented the involvement of CD4+CD25+ regulatory T cells in the host immune response of human subjects to H. pylori [17, 26-28, 34, 35]. Foxp3 expression, a surrogate marker for CD25+ Treg cells, has been documented in the gastric mucosa of infected humans [17, 26] and the number of CD25+ Treg cells is elevated in children compared to adults which is consistent with reduced levels of gastritis in the pediatric population [35].
In vivo studies by Raghavan et al. in the mouse model have shown that transfer of lymph node CD25- cells into immunodeficient nu/nu mouse recipients followed by H. pylori challenge results in severe gastritis and significantly decreased bacterial colonization relative to control nu/nu mice reconstituted with unfractionated lymph node cells [27]. More recently, Rad et al. depleted the CD25+ cell population in vivo using CD25-specific antibodies to achieve significantly increased gastritis in H. pylori infected mice and a reduction in bacterial load [26]. The results obtained by these two laboratories are similar to other models of pathogenic microbial infections in which CD25- cells activated in the absence of regulatory T cells mount a protective immune response [14, 15]. These studies, and others by Eaton et al. discussed below are significant in that they provide evidence that Treg cells are part of the natural host response to H. pylori infection and that they contribute to the persistence of H. pylori at the gastric mucosa. However, while these studies have investigated the potential of CD25- T cells to respond to H. pylori infection in the absence of Treg cells, they have not addressed the potential activity of CD25- T cells activated in the presence of Treg cells.
As discussed above, Raghavan et al. transferred CD25- cells from naïve mice into immunodeficient recipients prior to challenge [27]. Rad et al. employed a different strategy in that CD25+ cells were depleted in vivo by application of CD25-specific antibodies [26]. This depletion however was accomplished before infection was established and therefore the H. pylori-specific CD25- cells were activated in the absence of Treg cells [26]. Eaton et al. have developed a useful model for studying host immunity against H. pylori infection using adoptive transfer of wild type T cells into SCID mouse recipients that subsequently get challenged with H. pylori [36-38]. These mice develop severe gastritis and over time significantly reduce and in some cases eliminate the H. pylori load from the gastric mucosa [37]. When fractionated populations were transferred into SCID mice, naïve T cells (CD45RBhigh) were capable of promoting severe gastritis that depleted the Helicobacter population while memory T cells (CD45RBlow) did not [36]. Similar to the study by Raghavan et al., the donor cells used by Eaton et al. were obtained from naïve mice. The present report is distinct from these prior studies in that the donor populations employed to investigate the potential activity of CD25- T cells were obtained from H. pylori-infected mice and therefore the H. pylori-specific memory cells were originally activated in the presence of Treg cells.
In vitro analysis of CD25+ T cell regulatory activity often is performed by depleting CD25+ T cells from the lymphocyte population to assess the ability of the CD25- cell to respond to antigen in their absence [7]. Evidence that the CD25+ T cells are required for ongoing suppression is obtained when the CD25- T cells proliferate or produce cytokine in recall assays. We recently demonstrated that H. pylori-specific CD25- cells from infected mice remain hyporesponsive even in the absence of CD25+ T cells, an observation consistent with the presence of anergic cells [24]. We relied upon the adoptive transfer model in immunodeficient mice developed by Eaton et al to test these observations in vivo [36-38]. Initially, adoptive transfer of CD25- cells from H. pylori-infected wild type donor mice resulted in significant gastritis and a reduction in bacterial load. This observation was contrary to expectations given the anergic response of these cells noted in vitro. Our subsequent transfer in which CD25- memory cells were compared to the CD25- naïve cells from H. pylori infected mice demonstrated that the naïve CD25- cells were associated with bacterial clearance whereas memory CD25- cells from the same donor mice remained hyporesponsive upon infection. Although Eaton et al have described the role of CD45RBhigh cells in promoting inflammation capable of killing H. pylori [36], this is the first study to demonstrate that the H. pylori-specific CD25- cells induced during infection of wild type mice are in fact hyporesponsive and do not require ongoing suppression to remain down-regulated.
The present study also demonstrates that CD25- T cells activated in the absence of Treg cells and which reduce the bacterial load in the SCID adoptive transfer model become ineffective when transferred into wild type mice. Transfer into wild type recipients reintroduces dominant regulatory T cells into the response and results in mild inflammation and persistent infection. Using this population of CD25- cells, we were able to determine that Treg cells are not only capable of influencing naïve CD25- cells, but are also capable of rendering previously-activated CD25- cells ineffective. These findings are in accordance with previous studies investigating the extent of CD25+ regulatory control in a murine model of colitis [39]. Transfer of CD4+CD25+ regulatory T cells four weeks after established CD4+CD45RBhi-induced colitis resulted in resolution of disease as early as two weeks after transfer of regulatory T cells. Similarly, we demonstrate here that CD25+ Treg cells maintain the ability to influence previously-activated responsive CD25- cells during H. pylori infection.
The ability of resident Treg cells to suppress previously activated donor CD25- T cells is in contrast with studies demonstrating that animals harboring an existing Helicobacter infection can be protected by therapeutic immunization [32, 33, 40]. Therapeutic immunization studies indicate that activation of T cells under certain circumstances results in a population of T cells that is not influenced by the Treg cells that are part of the host response to infection. Therefore either the nature or frequency of proinflammatory T cells produced here in the absence of Tregs must be different than the T cell response induced by immunization. Further analysis will be required to characterize these two types of responses.
Several recent reports have begun to elucidate the development of the host T cell response to H. pylori infection. This response consists, in part, of immunoregulatory CD25+ Treg cells that actively suppress other H. pylori-specific cells from promoting heightened inflammation [26, 27, 41]. In the mouse model, we have demonstrated that the presence of CTLA-4 on Tregs is necessary to suppress H. pylori associated protective gastritis [24]. The presentation of antigen by the gastric epithelium and the involvement of the co-receptor B7H1 may also be promoting the induction of suppressive CD4+CD25+ Treg cells that have been demonstrated in vitro to decrease the proliferative activity of activated T cells [42]. Mechanistically, it appears that IL-10 production by Treg cells may play a role in the suppressive activity of these cells [36, 43, 44] although IL-10 deficient Treg cells were also capable of significantly reducing the proinflammatory effects of H. pylori-specific CD25- cells in an adoptive transfer model [41].
CD25+ regulatory T cells are typically associated with active suppression. The present study however demonstrates an alternate mechanism of immune down-regulation by this cell type. The observation that H. pylori-specific CD25- T cells remain unresponsive even in the absence of CD25+ T cells both in vitro and in vivo provides compelling evidence for the induction of anergy. These observations should enhance our understanding of H. pylori immunopathogenesis, and may be relevant to our understanding of the immunopathogenesis of other microbial infections and the maintenance of immunologic homeostasis in the gastrointestinal tract.
Materials and Methods
Reagents
Complete cell media consisted of RPMI 1640 supplemented with 10% fetal bovine serum (Gibco Life Sciences, Carlsbad, CA). MACS CD4+ cell purification reagents and columns were purchased from Miltenyi Biotech (Auburn, CA) and used according to manufacturer's instructions. Low-tox M rabbit complement and Lymphocyte-M were purchased from Cedarlane Laboratories (Hornby, Ontario). Anti-CD28 antibody and IFNγ ELISA reagents were purchased from ebioscience (San Diego, Ca) and IL-2 ELISA reagents purchased from R & D (Minneapolis, MN) were used according to manufacturer's instructions. Anti-CD25, anti-CD45RB, and PE-conjugated anti-CD25 and anti-FCγRIII antibodies were purchased from BD Biosciences (Franklin Lakes, NJ). H. pylori lysates were prepared by probe sonication of H. pylori suspensions in PBS. Sonicate was sterile filtered using 0.2 μm acrodisc filters (Pall Corporation, Ann Arbor, Mi). The Foxp3 assay on demand (Mm00475156_m1) was purchased from ABI (Foster City, Ca)
Mice
Six to ten week old C57BL/6 female mice and lymphocyte-deficient rag1-/- male mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed under specific pathogen free conditions in microisolator units. All studies involving the use of mice were reviewed and approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Bacteria
H. pylori Sydney Strain 1 (SS1) [45] was grown on Columbia agar (Difco, Detroit, MI) supplemented with horse blood and antibiotics at 37°C for 96 h under microaerobic conditions (5% O2, 10% CO2). For inoculation of mice, bacteria were transferred to 10 ml Brucella broth (Difco Laboratories, Detroit) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and amphotericin B (2.5 μg/ml). Liquid cultures were established in T25 flasks and maintained at 37°C with 5% CO2.
Infection/Immunization
Infections were performed with H. pylori SS1 by gavage using flexible tubing on the end of an 18 G needle. Each mouse received 500 μl of an actively growing bacterial culture of at least 0.3 OD450nm on two consecutive days. Immunization was performed by intranasal administration of 100 μg H. pylori lysate plus 5 μg cholera toxin adjuvant in 20μl PBS on day 0, 7, 14, and 28 as previously described [25].
Complement-mediated lysis of CD25+ cells
Single cell suspensions were prepared from spleens and red blood cells were removed by lysis in a hypotonic solution. Cells were resuspended in 2% FBS/PBS and incubated for 30 minutes at 4°C with anti-mouse CD25+ antibody at 0.65 μg per 1 × 107 cells. Cells were then washed and resuspended in PBS with Low-tox M rabbit complement (20:1 v/v) and incubated at 37°C for 45 minutes. Viable lymphocytes were concentrated using a Lymphocyte-M gradient followed by several washes in PBS. Deletion of CD25+ cells was confirmed by staining with PE-conjugated anti-CD25 antibody and assessment by flow cytometry.
Foxp3 Quantitative PCR
The ABI Foxp3 assay was set up in accordance with the manufacturer's instructions and run against all the samples in the study using GAPDH as an endogenous control on a 384-well plate. RNA was accurately quantified using a nanodrop-1000 spectrophotometer (Nanodrop Industries). Archive cDNA was made for all samples by means of an RT reaction using ABI high-Capacity cDNA archive kit and using similar amounts of total RNA as starting material in a 100ul reaction in an ABI 9700 PCR unit. Results were generated using ABI SDS software and are presented as relative fold changes versus a designated calibrator sample. Results include 95% confidence limits.
Isolation of CD25+ and CD25- cell populations by flow cytometry
CD4+ splenocytes were positively selected according to manufacturer's instructions using the MACS CD4+ cell purification reagents and medi-MACS columns on a magnetic support. Purified CD4+ cells were incubated with FCγRIII for 15 minutes followed by incubation with PE-conjugated anti-CD25 antibody. Sorting of CD25+ and CD25- cells was performed using a BD FACSAria (Franklin Lakes, NJ) at the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University.
In vitro recall assay
Bulk spleen cells or CD25- spleen cells from immunized or infected mice were prepared as described above and plated in 96 well plates in 200 μl complete media and stimulated with 10 μg/ml H. pylori lysate. Supernatants were removed at 36 hours to determine the amount of IFNγ secretion. Designated groups were also treated with high dose (1000U/ml) IL-2 for one hour prior to stimulation with antigen. Similar assays were also performed with CD4+ spleen cells prepared by positive selection using the MACS CD4+ cell purification reagents and columns purchased from Miltenyi Biotech (Auburn, CA), and in some cases with CD4+ cells further fractionated into CD25+ and CD25- population by affinity isolation using a CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotech). Stimulation of CD4+ cells fractionated by CD25 status was accomplished with bone marrow derived macrophages from C57BL/6 mice. Briefly, bone marrow cells isolated from hind leg femurs and tibias were grown in DMEM supplemented with 10% FBS and supplemented with 20% GM-CSF-conditioned media from Ladmac cell culture. Each well was seeded with 1 × 104 macrophages and pulsed with 10 μg/ml H. pylori lysate antigen for six hours. The macrophages were washed three times and then the fractionated cells were added to the wells for stimulation as described above.
Evaluation of inflammation and CFU determination
For all adoptive transfer studies, recipient mice were sacrificed 28 days post-infection by CO2 asphyxiation and gastric biopsies from the greater curvature of the stomach was collected to assess degree of gastritis and bacterial load. To determine gastric inflammation, a biopsy strip was fixed in 10% buffered formalin. H&E staining was performed at the Willard Alan Bernaum Cystic Fibrosis Research Center core facility at the CWRU School of Medicine (Cleveland, OH). As previously described [46, 47] the area of the tissue section displaying the most severe inflammation was evaluated blindly and assigned a global score from 0 – 5 based upon the following parameters: 0, no significant lesions; 1, mild infiltrate of inflammatory cells, typically along the base of the glands; 2, larger focus of inflammation extending between glands and/or in submucosa; 3, patch(es) of inflammation extending between glands toward the lumen and in the underlying submucosa. Moderate mucous cell metaplasia and mild to moderate epithelial hyperplasia may be present. 4, intense transmucosal inflammatory infiltrate extending across the field, distorting glandular architecture, marked epithelial hyperplasia and extensive mucous cell metaplasia often present; 5, extensive mucosal and submucosal inflammation with disruption of glandular architecture and ulceration. To determine bacterial colonization, a biopsy strip was placed in a pre-weighed tube of 200 μl Columbia broth, the wet weight was determined and the tissue was homogenized using a disposable pellet pestle (Kontas Glass Company, Vineland, NJ). Serial dilutions were prepared and 10 μl aliquots were plated for growth as described above. Bacterial load was determined as CFU/gram of stomach tissue.
Adoptive transfer studies
Transfer of CD25- cells to rag1-/- mice (Figures 3 and 4)
Bulk CD4+ or CD4+CD25- cells were resuspended in PBS and 2 × 106 cells were injected i.p. into each rag1-/- mouse on Day 0. Mice were challenged with H. pylori SS1 on days 1 and 2.
Transfer of naïve or memory CD25- cells into rag1-/- mice (Figures 5 and 6)
CD4+CD25- bulk, CD4+CD25-CD45RBhi (brightest 20%) or CD4+CD25-CD45RBlo (dullest 10%) cells were resuspended in PBS and 1.5 × 105 cells were injected i.p. into each rag1-/- mouse on Day 0. On Days 1 and 2, mice were infected with H. pylori SS1.
Transfer of CD25- cells from reconstituted rag1-/- mice into wild type mice (Figures 7 and 8)
On Day 0, 4.5 × 106 CD25- spleen cells were injected i.p. into rag1-/- recipient mice. Mice were challenged with H. pylori SS1 on Days 1 and 2. On Day 29, mice were sacrificed and splenocytes were removed and prepared for a second round of adoptive transfer by i.p. injection of 1 × 107 bulk splenocytes (16% CD4+ measured by flow staining) into naïve or infected wild type mice. Naïve recipients were then challenged on Days 35 and 36.
Statistics
Differences between experimental groups in each experiment was evaluated by Student's T test. Differences were considered statistically significant if P values were less than 0.05.
Acknowledgments
This research was supported by NIH grants AI055710 (T.G.B.) and DK046461 (S.J.C.) and by the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland grant #P30 CA43703.
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 2.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 7.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 8.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 10.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–656. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 12.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol. 2002;169:3232–3241. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 14.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 15.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Lundgren A, Stromberg E, Sjoling A, Lindholm C, Enarsson K, Edebo A, Johnsson E, Suri-Payer E, Larsson P, Rudin A, Svennerholm AM, Lundin BS. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifarth C, Funk A, Reich K, Dahne I, Classen M, Deusch K. Selective increase of CD4+ and CD25+ T cells but not of gamma delta T cells in H. pylori associated gastritis. Adv Exp Med Biol. 1995;371B:931–934. [PubMed] [Google Scholar]
- 19.Stromberg E, Lundgren A, Edebo A, Lundin S, Svennerholm AM, Lindholm C. Increased frequency of activated T-cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol Med Microbiol. 2003;36:159–168. doi: 10.1016/S0928-8244(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 20.Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994;35:742–745. doi: 10.1136/gut.35.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 22.Fan XJ, Chua A, Shahi CN, McDevitt J, Keeling PW, Kelleher D. Gastric T lymphocyte responses to Helicobacter pylori in patients with H. pylori colonisation. Gut. 1994;35:1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karttunen R. Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses of subjects with and without antibodies to H. pylori. Clin Exp Immunol. 1991;83:396–400. doi: 10.1111/j.1365-2249.1991.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson K, Czinn S, Redline R, Blanchard T. Induction of CTLA-4 mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. Journal of Immunology. 2006;176:5306–5313. doi: 10.4049/jimmunol.176.9.5306. [DOI] [PubMed] [Google Scholar]
- 25.Rahn W, Redline RW, Blanchard TG. Molecular analysis of Helicobacter pylori-associated gastric inflammation in naive versus previously immunized mice. Vaccine. 2004;23:807–818. doi: 10.1016/j.vaccine.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, Reindl W, Dossumbekova A, Friedrich M, Saur D, Wagner H, Schmid RM, Prinz C. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Raghavan S, Fredriksson M, Svennerholm AM, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin Exp Immunol. 2003;132:393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–1762. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malfitano AM, Cahill R, Mitchell P, Frankel G, Dougan G, Bifulco M, Lombardi G, Lechler RI, Bamford KB. Helicobacter pylori has stimulatory effects on naive T cells. Helicobacter. 2006;11:21–30. doi: 10.1111/j.0083-8703.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 30.Ermak TH, Giannasca PJ, Nichols R, Myers GA, Nedrud J, Weltzin R, Lee CK, Kleanthous H, Monath TP. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garhart CA, Redline RW, Nedrud JG, Czinn SJ. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect Immun. 2002;70:3529–3538. doi: 10.1128/IAI.70.7.3529-3538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuenca R, Blanchard TG, Czinn SJ, Nedrud JG, Monath TP, Lee CK, Redline RW. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1996;110:1770–1775. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 33.Doidge C, Crust I, Lee A, Buck F, Hazell S, Manne U. Therapeutic immunisation against Helicobacter infection. Lancet. 1994;343:914–915. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 34.Raghavan S, Suri-Payer E, Holmgren J. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scand J Immunol. 2004;60:82–88. doi: 10.1111/j.0300-9475.2004.01447.x. [DOI] [PubMed] [Google Scholar]
- 35.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, Schmitz JM, Lorenz RG, Novak L, Smythies LE, Smith PD. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–7461. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 37.Eaton KA, Mefford ME. Cure of Helicobacter pylori infection and resolution of gastritis by adoptive transfer of splenocytes in mice. Infect Immun. 2001;69:1025–1031. doi: 10.1128/IAI.69.2.1025-1031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eaton KA, Ringler SR, Danon SJ. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 40.Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthesy-Theulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath TP, Blum AL. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 41.Lee CW, Rao VP, Rogers AB, Ge Z, Erdman SE, Whary MT, Fox JG. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2-/- mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–2707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE. B7-H1 Expression on gastric epithelial cells after Helicobacter pylori exposure promotes the development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun. 2007 doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol. 2003;170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto Y, Blanchard TG, Drakes ML, Basu M, Redline RW, Levine AD, Czinn SJ. Eradication of Helicobacter pylori and resolution of gastritis in the gastric mucosa of IL-10-deficient mice. Helicobacter. 2005;10:407–415. doi: 10.1111/j.1523-5378.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 46.Blanchard TG, Czinn SJ, Redline RW, Sigmund N, Harriman G, Nedrud JG. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 47.Gottwein JM, Blanchard TG, Targoni OS, Eisenberg JC, Zagorski BM, Redline RW, Nedrud JG, Tary-Lehmann M, Lehmann PV, Czinn SJ. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J Infect Dis. 2001;184:308–314. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]