Abstract
Objectives
Relaxin induces the matrix metalloproteinase MMP-1 (collagenase-1) in TMJ fibrocartilaginous cells, and this response is potentiated by β-estradiol. We identified the MMP-1 promoter sites and transcription factors that are induced by relaxin with or without β-estradiol in fibrocartilaginous cells.
Material & Methods
Early passage cells were transiently transfected with the pBLCAT2 plasmid containing specific segments of the human MMP-1 promoter regulating the chloramphenicol acyl transferase (CAT) gene and co-transfected with a plasmid containing the β-galactosidase gene. The cells were cultured in serum-free medium alone or medium containing 0.1 ng/ml relaxin, or 20 ng/ml β-estradiol or both hormones, and lysates assayed for CAT and β-galactosidase activity.
Results
Cells transfected with the −1200/−42 or −139/−42 bp MMP-1 promoter-reporter constructs showed 1.5-fold and 3-fold induction of CAT by relaxin in the absence or presence of β-estradiol, respectively. Relaxin failed to induce CAT in the absence of the −137/−69 region of the MMP-1 promoter, which contains the AP-1- and PEA3-binding sites. Using wild type or mutated minimal AP-1 and PEA-3 promoters we found that both these promoter sites are essential for the induction of MMP-1 by relaxin. The mRNAs for transcription factors c-fos and c-jun, which together form the AP-1 heterodimer, and Ets-1 that modulates the PEA-3 site, were upregulated by relaxin or β-estradiol plus relaxin.
Conclusion
These studies show that both the AP-1 and PEA-3 promoter sites are necessary for the induction of MMP-1 by relaxin in fibrocartilaginous cells.
Keywords: AP-1, MMP-1, PEA-3, fibrocartilaginous cells, relaxin
Introduction
Because of the age and gender distribution of temporomandibular joint disorders (TMDs) primarily in women of reproductive age, we have been exploring the role of the female sex hormones estrogen and relaxin in contributing to these diseases. We have found that relaxin induces the tissue degrading matrix metalloproteinases (MMPs) MMP-1 (collagenase-1), -3 (stromelysin-1), -9 (92 kDa gelatinase) and -13 (collagenase-3) in TMJ disc fibrocartilaginous cells from mice and rabbits (1–3). Furthermore, β-estradiol enhances the induction of these proteinases by relaxin. More recently, we have found that TMJ disc cells express both estrogen receptors (ER) -α and -β as well as the relaxin receptors, relaxin family peptide (RXFP) -1 and -2 pointing to a potential cellular activation mechanism by which their ligands, acting via some or all of these receptors, may induce MMPs. While the presence of these receptors on TMJ cells suggests their likely contribution to MMP induction, the signaling mechanisms and basis for the subsequent transcriptional regulation of MMPs by relaxin remains to be characterized.
Previous studies have shed some light on the possible transcriptional regulatory mechanisms of MMP-1 gene expression by hormones and growth factors, suggesting a crucial role of the 5′ flanking sequence containing the TPA and the oncogene responsive unit (TORU) comprised of the activator protein-1 (AP-1) motif [5′-TGAGTCA-3′] and the polyoma enhancer activator protein-3 (PEA-3) motif [5′-GAGGATGT-3′] in the promoter region of MMP-1 gene (4,5). These studies have demonstrated that several stimuli including phorbol esters are capable of modulating MMP-1 gene expression via signaling pathways in which transcriptional factors in the fos and jun families bind to the AP-1 motif while Ets family binds to the PEA-3 motif in the promoter region of MMP-1 thereby inducing the transcription of MMP-1 (5,6). Although both the AP-1 and PEA-3 motifs of the MMP-1 promoter appear to play an important role in the transcriptional regulation of MMP-1, the extent of their involvement varies substantially depending on the cell type and the stimulus used. Thus for example, both AP-1 and PEA-3 motifs are necessary for the induction of MMP-1 promoter activity in fibroblasts or HeLa cells exposed to fibronectin fragments and PMA, respectively (7,8). In contrast, while the AP-1 motif is necessary for mediating the induction of MMP-1 transcriptional activity in TPA-treated fibroblasts and insulin-treated HeLa cells, the PEA-3 motif acts as an enhancer in inducing MMP-1 transcription in these cells (9,10).
Other regulatory mechanisms may also be involved in the transcriptional activity of MMP-1 gene (11–13). Thus, MMP-1 promoter activity is induced and maintained in the absence of a functional AP-1 motif in PMA-treated U937 cells undergoing differentiation from monocytes to macrophages in vitro (6), suggesting that the distal promoter sequence of MMP-1 potentially regulates MMP-1 transcriptional activity in an AP-1 independent manner. Furthermore, the stimulation of MMP-1 promoter activity by v-src in fibroblasts requires PEA-3 and STAT motifs rather than AP-1 motif in MMP-1 promoter (6,14). Together, these findings suggest that the regulatory role of AP-1 and PEA-3 motifs in mediating the transcriptional activity of MMP-1 is likely cell and tissue specific. Moreover, the location of individual AP-1 and PEA-3 motifs and the sequences surrounding them may also contribute to the determination of the function of these elements in regulating the transcriptional activity of the MMP-1 gene.
Because of the known differences in the MMP-1 promoter elements involved in the transcription of its gene and the lack of information on how relaxin regulates the expression of this gene, here we determined the transcriptional regulatory mechanisms for MMP-1 gene induction by relaxin and the modulation of this response by β-estradiol.
Materials and Methods
Reagents
All animal experiments were conducted with the approval of the Institutional Review Board. Twenty-week-old female New Zealand white rabbits (Oryctolagus cuniculus) were obtained from Nita Bell laboratories (Hayward, California). All reagents were from Sigma Chemical Company (St. Louis, MO) unless otherwise mentioned. Recombinant human relaxin was kindly provided by Connetics Corporation (Palo Alto, CA). All restriction enzymes were purchased from Promega (Madison, WI).
Promoter-Reporter Constructs
Three human MMP-1 promoter constructs, kindly provided by Peter Herrlich and Hans Rahmsdorf (Institution Karlsruhe, Germany), were used in this study. The constructs contain MMP-1 promoter sequences −1200/−42 bp, −139/−42 or −66/−42 bp placed upstream of the minimal tyrosine kinase promoter and the bacterial gene for chloramphenicol acetyl transferase (CAT) in the plasmid pBLCAT2 (15). The plasmids mAPcoltkCAT, mPEA-3col-tkCAT and WTcol-tkCAT were obtained from Dr. Zena Werb (University of California San Francisco, CA, USA) (8). These were previously constructed using synthetic oligonucleotides containing the AP-1 and PEA-3 sites (15). The construct WTcol-tkCAT has a minimal −90/−67 bp segment of the MMP-1 promoter containing the AP-1 and the PEA-3 sites. The constructs mAP-1col-tkCAT contains a mutated AP-1 site with an AT to TG substitution in the minimal −90/−67 bp minimal promoter. mPEA-3col-tkCAT contains a G to A substitution in the PEA-3 site of this minimal promoter. These mutations produce oligonucleotides that do not complex with AP-1 or Ets-1, respectively. A vector with the bacterial lac Z gene that codes for β-galactosidase (β-gal) and is driven by the SV-40 early promoter and enhancer was utilized as a positive control.
Isolation and Culture of Cells
The cells were isolated from rabbit TMJ and cultured as described previously (1,16), and cultured in α-MEM supplemented with 10% fetal calf serum. After 2 weeks, the cells were trypsinized and replated. Cells in passages 3 to 5 plated at a density of 50,000 cells/cm2 were used in subsequent experiments at 70% confluence.
Transfections
Plasmids containing the promoter-reporter constructs were purified by Qiagen Maxi Plasmid purification kit (Qiagen Corporation, Germany). Purified plasmid DNA (2–8 mg) was mixed with 25 ml CaCl2, and incubated with the cells for 4 hr, and washed with phosphate buffered saline (PBS). Cells were maintained in either serum free medium (α-MEM + 0.2% lactalbumin hydrolysate; LAH) (control) or in medium containing β-estradiol (20 ng/ml), or relaxin (0.1 ng/ml) or relaxin plus β-estradiol or 12-O-tetradeconyl-phorbol-13-acetate (TPA; 50 ng/ml) for 12 hr. TPA was used as a positive control in all experiments since its induction of MMP-1 promoter sites has been well characterized (7). For dose response experiments, 0, 0.01, 0.1 and 1 ng/ml of relaxin was utilized on cells transfected with the WTcol-tkCAT construct. Cell culture medium was collected, the cells were washed and incubated with reporter lysis buffer (Promega, Madison, WI) for 15 min and scraped.
β-gal activity was assayed by its hydrolysis of the substrate o-nirophenyl-α-D-galactopyranoside (Promega, Madison, WI) and read at 420 nm (Spectramax M2, Molecular Devices, Sunnyvale, CA) to monitor the efficiency of transfections. CAT activity was determined by anti-CAT-digoxygenin ELISA as per manufacturers instructions (Boehringer Mannheim, Indianapolis, IN).
Preparation of 32P-labeled cDNA probe
The 2.2–2.3 kbp c-fos cDNA was released from the plasmid pSP64 (Promega, Madison, WI) by restriction digestion with EcoRI. The c-jun cDNA was obtained from plasmid T7c-jun by restriction digestion with EcoRI/HindIII to release a 2.3 kbp piece. PEA-3 cDNA was prepared by releasing 1.6 kbp EcoRI fragment from the plasmid pGEX-PEA-3 (American Type Culture Collection, Manassus, VA). The cDNAs were purified on a 1% low melting agarose, and 10 ng each of cDNA was labeled with 32P-dCTP using Rediprime DNA labeling kit (Amersham Life Sciences, Arlington Heights, IL). The mixture was incubated for 10 min and the reaction stopped by adding EDTA. The probes were purified using Chroma Spin columns (Clontech, Palo Alto, CA).
RNA Extraction and Northern Analysis
Total RNA was extracted from disc fibrocartilaginous cells after 30 min in control or hormone-containing media as described previously (1), and the yield and purity of RNA determined by UV spectroscopy. The RNA (15 ng) was loaded and electrophoresed on a 1 % denaturing agarose-formaldehyde gel and transferred to a nylon membrane. The membrane was hybridized with the 32P-labeled cDNA probes for c-fos, c-jun, Ets-1 and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) and washed. The signal was detected by autoradiography and quantitated by video densitometry.
Substrate Zymography
Collagenase in cell-conditioned medium was monitored by gelatin substrate zymograms as described previously (1). In brief, the medium standardized by total protein was mixed with 4X sample buffer and electrophoresed on a 10% SDS-polyacrylamide gel containing 2 mg/ml gelatin. After washing the gel with 2.5% Triton X-100, the gels were incubated for 20 hours at 37°C. The gels were stained with Coomassie blue, and destained to visualize proteolytic activity.
Statistical Analysis
Each experiment was performed in quadruplicate. The mean (± SE) fold induction of CAT standardized to β̃-gal for each of the treatments relative to the control baseline was analyzed with an ANOVA and intergroup differences determined by Scheffe’s multiple comparison test with the level of significance set at p < 0.05.
Results
Relaxin induces c-fos, c-jun and Ets-1 mRNAs
We initially studied the modulation of the mRNAs for the transcription factors, Fos and Jun, which form the AP-1 heterodimer, and that for Ets-1. Relaxin and β-estradiol plus relaxin caused a 1.7- and 1.8-fold increase in c-fos levels over controls, respectively (Fig. 1A and B). c-jun mRNA was increased 2-fold over control levels by relaxin both in the presence and absence of β-estradiol (Fig. 1A and C). β-estradiol alone also caused an increase in both c-fos and c-jun mRNAs, although the magnitude of this increase was somewhat less than that with relaxin. Ets-1 mRNA was increased slightly by β-estradiol or relaxin alone, while it showed a 1.8-fold induction with relaxin and β-estradiol combined (Fig. 1A and D). As expected, TPA produced substantial increases in the levels of mRNAs for all three-transcription factors.
Figure 1.
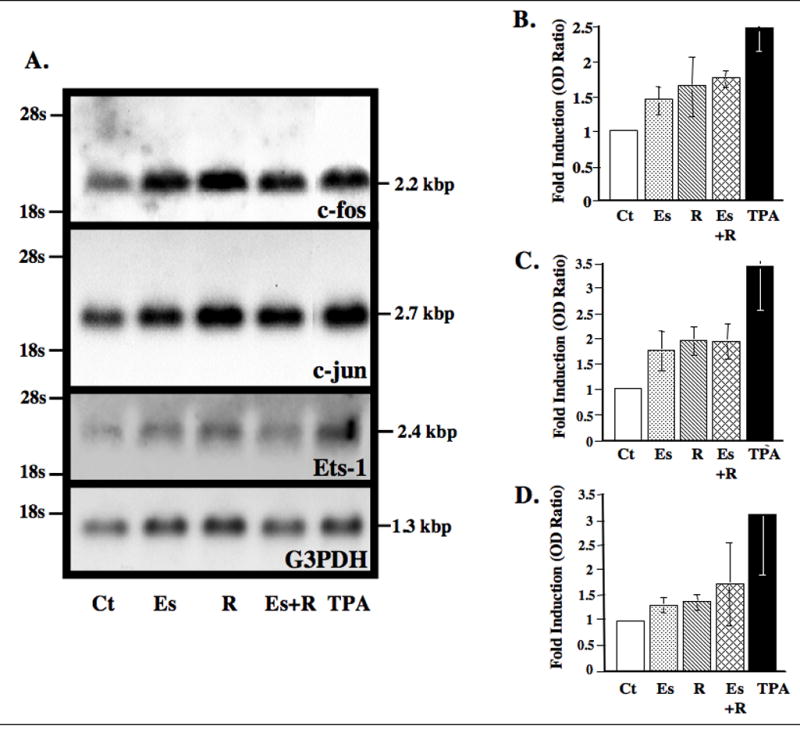
Relaxin with or without β-estradiol upregulates c-fos, c-jun and Ets-1 mRNAs in fibrocartilaginous cells. (A) RNA extracted from cells exposed to control medium (Ct) or medium with β-estradiol (Es, 20 ng/ml) and/or relaxin (R, 0.1 ng/ml) or TPA (50 ng/ml) was subjected to Northern blot analysis for c-jun, c-fos, Ets-1 or G3PDH. Histograms of the mean (±SE) fold-induction of c-fos (B), c-jun (C) and Ets-1 (D) determined by video densitometry and standardized to G3PDH by the various treatments relative to control baseline levels were plotted from four experiments.
Regulation of the human MMP-1 gene promoter by relaxin requires the −139/−67 bp region
Cells transfected with the plasmid pBLCAT2 containing the −1200/−42 bp segment of the collagenase promoter showed statistically significant 1.7-fold and 2.8-fold induction of CAT by relaxin alone or by β-estradiol plus relaxin, respectively (Fig. 2B). In cells transfected with the −139/−42 construct, there was a statistically significant 1.6-fold induction of CAT expression by relaxin alone and a 3.6-fold induction by β-estradiol plus relaxin. TPA produced substantial induction, while β-estradiol alone failed to induce the CAT gene linked to the −1200/−42 bp and −139/−42 bp promoter regions. CAT expression was not induced by any of the hormone treatments or by TPA in cells transfected with constructs containing only −66/−42 bp region, suggesting that the deleted −139 to −67 bp promoter region contains sites responsive to relaxin. Findings showing the induction of 53/58 kDa gelatinolytic activity (collagenase) by relaxin in the absence or presence of β-estradiol and by TPA, but not by β-estradiol alone were consistent with the CAT promoter data and also shows that this MMP is indeed modulated by these treatments (Fig. 2C).
Figure 2.
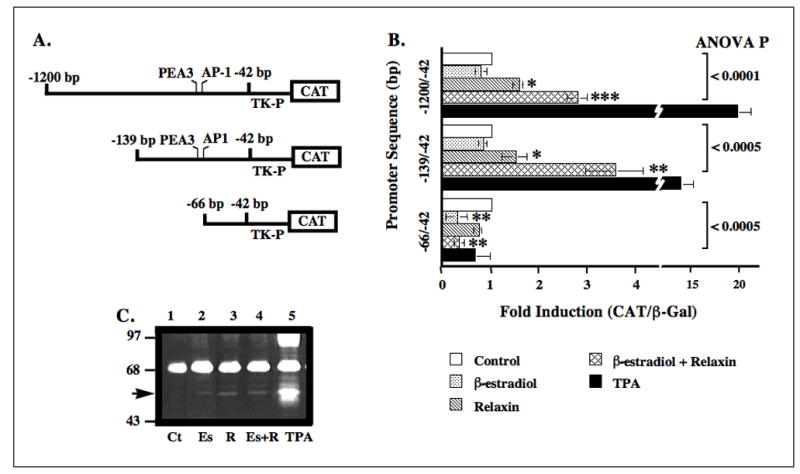
Regulation of human MMP-1 gene promoter by relaxin without or with β-estradiol requires the −139/−67 bp motif. Cell lysates from cells transiently transfected with the −1200/−42 or −139/−42 or −66/−42 bp human MMP-1 promoter-CAT constructs (A) and exposed to control medium (Ct) or medium containing β-estradiol (Es) and/or relaxin (R) or TPA were assayed for β-gal and CAT. (B) The histogram for mean (±SE) fold-induction of CAT/β-gal for each treatment relative to controls was plotted for four experiments. (C) Gelatin-substrate zymography on cell-conditioned medium was used to visualize the changes in levels of endogenously expressed presumptive MMP-1 (arrow). (*p < 0.05, **p < 0.005, ***p < 0.0005).
MMP-1 induction by relaxin requires both the AP-1 and PEA-3 promoter sites
Since the −139/−43 bp region of the MMP-1 promoter contains the AP-1 and PEA-3 motifs, and because our findings demonstrated that relaxin induces the transcription factors for these sites, we studied the precise contribution of these two sites in the regulation of this gene by relaxin by utilizing the mutated forms of these cis-acting elements within the context of a minimal −90/−67 bp promoter (Fig. 3A). Cells transfected with the wild type construct containing the minimal MMP-1 AP-1 and PEA-3 promoter sites responded to relaxin in the absence or presence of β-estradiol with a 1.7-fold and a 2.6-fold increase in CAT expression, respectively, over control levels (Fig. 3B). There was no induction of the wild type promoter in response to β-estradiol alone. In disc cells transfected with the construct mAP-1col-tkCAT or mPEA-3col-tkCAT, there was no modulation of CAT activity in response to any of the hormone treatments or TPA.
Figure 3.
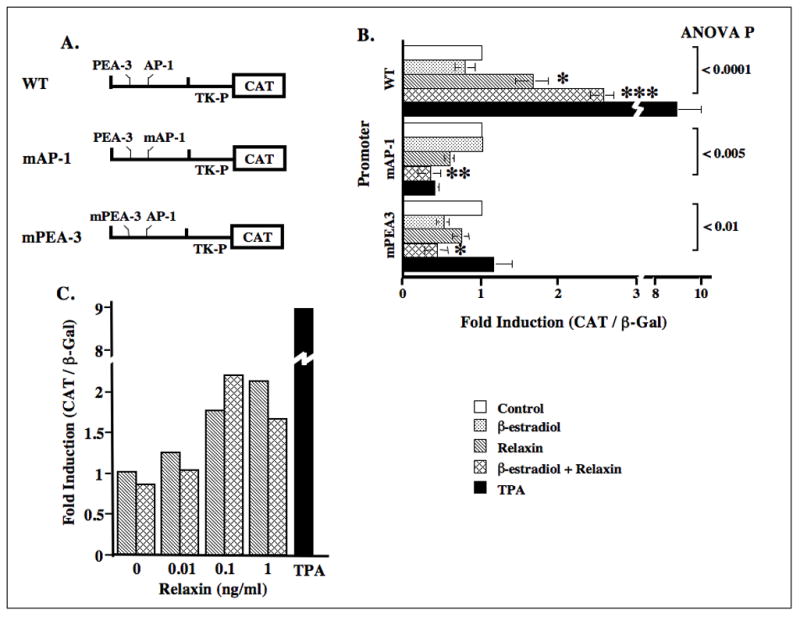
Regulation of the human MMP-1 gene promoter by relaxin without or with β-estradiol requires both the AP-1 and PEA-3 motifs. Cell lysates from cells transiently transfected with the wild type minimal −90/−67 bp segment of the MMP-1 promoter containing the AP-1 and the PEA-3 sites (WTcol-tkCAT, WT) or this promoter with a mutated AP-1 site (mAP-1col-tkCAT, mAP-1) or mutated PEA-3 sites (mPEA-3col-tkCAT, mPEA-3) (A) and cotransfected with pSV-β-gal vector were assayed as described in figure 2. (B) Histogram for mean (± SE) fold induction of CAT/β-gal for each of the treatments relative to controls was plotted for four experiments. (C) Relaxin dose-response experiments were performed by transfecting disc fibrocartilaginous cells with the WTcol-tkCAT promoter, exposing them to increasing concentrations of relaxin with or without β-estradiol and assaying as described above. (*p < 0.05, **p < 0.005, ***p < 0.0005).
Relaxin’s regulation of the minimal wild type MMP-1 promoter by relaxin is dose dependent
The specificity of the responsiveness of the minimal WTcol-tkCAT construct to relaxin was demonstrated by the dose-dependent induction of CAT activity by relaxin and β-estradiol plus relaxin (Fig. 3C). Consistent with our previous findings on the dose dependent induction of collagenase by relaxin or β-estradiol plus relaxin (1), the peak induction of CAT occurred at 1 ng/ml of relaxin in the absence of β-estradiol and 0.1 ng/ml relaxin when β-estradiol was present in the medium.
Discussion
In this study we examined the transcriptional regulatory mechanisms of MMP-1 gene induction by relaxin, a member of the insulin superfamily, in the absence or presence of β-estradiol in disc fibrocartilaginous cells. Our data demonstrated that relaxin’s induction of the transcriptional activity of −1200/−43 bp or −139/−43 bp CAT-linked human MMP-1 promoters is preceded by the upregulation of c-fos, c-jun and Ets-1 genes. Additionally, the presence of β-estradiol increased the sensitivity of the fibrocartilaginous cells to relaxin such that the induction of these transcription factors and promoter constructs by relaxin was higher in the presence than in the absence of β-estradiol. Using wild type minimal AP-1 and PEA-3 promoter or this promoter with mutated AP-1 or mutated PEA-3 motifs, we found that both AP-1 and PEA-3 regions of the MMP-1 promoter are required and essential for full activation of MMP-1 transcriptional activity by relaxin. The response of MMP-1 to relaxin in fibrocartilaginous cells transfected with the wild type promoter construct was dose-dependent and potentiated by β-estradiol. β-estradiol alone failed to induce CAT expression in any of the promoter-reporter constructs. These findings are consistent with our previous observations on the modulation of MMP-1 expression by relaxin in unprimed and β-estradiol-primed fibrocartilaginous cells (1).
The insulin superfamily, including insulin, insulin-like growth factors, and relaxin, is a family of small, structurally related peptides that act on a variety of mammalian cells in endocrine, paracrine and autocrine manner to regulate cell proliferation, apoptosis, transformation and differentiation (17–20). Relaxin has also been shown to play an essential role in extracellular matrix turnover in the uterus, pubic symphysis, and fibrocartilage of synovial joints by modulating the expression of MMPs (1,3,21). Our data provides the first evidence that the transcriptional regulation of MMP-1 by relaxin requires both the AP-1 and PEA-3 binding motifs. Our finding that the AP-1 motif is required and essential for the induction of MMP-1 by relaxin is consistent with previous studies showing that the transcriptional regulation of MMP-1 by insulin and phorbol esters in HeLa cells requires the AP-1 site (9). However, in contrast to our findings that the mutation of the Ets-1 transcriptional factor-binding PEA-3 motif results in a complete abrogation of MMP-1 transcription by relaxin in fibrocartilaginous disc cells, the mutation of this motif failed to completely neutralize the stimulatory effect of insulin on MMP-1 expression in HeLa cells. Recent studies also indicate that while the transcriptional regulation of MMP-1 by insulin is largely mediated through an AP-1 motif, multiple MMP-1 promoter elements are required for the full stimulatory effect of insulin on MMP-1 expression (22). The variability in MMP-1 transcriptional regulatory mechanisms between insulin and relaxin may result for several reasons, such as differences in cell types, stimulating agent, downstream signaling cascade, promoter context, AP-1 flanking sequences, and accessory elements that modulate the signaling via these two motifs (22).
The AP-1 motif, to which the members of Fos and Jun transcription factor families bind, and PEA-3 motif, to which the Ets transcription factor family bind, are typically present in the promoters of inducible MMPs and TIMP genes, and are essential elements for their transcriptional activation. Individual AP-1 and PEA-3 elements often play distinct roles in basal and induced transcription, and this may be a function of specific transcription factors that bind to these motifs. The MMP-1 gene contains adjacent AP-1 and PEA-3 motifs that are proximal to the transcriptional start codon. These motifs are required for activation of the promoter by transforming oncogenes and tumor promoting phorbol esters (4,6,14). In the present study, we found that the induction of MMP-1 transcriptional activity was preceded by the upregulation of the genes for the transcriptional factors, c-fos, c-jun and Ets-1 in fibrocartilaginous disc cells stimulated by relaxin or relaxin plus β-estradiol. These results indicate that these transcription factors likely act as mediators that converge the variety of stimulatory signals to the DNA binding motifs AP-1 and PEA-3 in the MMP-1 promoter, and cause the subsequent increase in MMP-1 transcriptional activity. This concept is confirmed by previous studies in which the AP-1 motif in the MMP-1 promoter has been shown to play a pivotal role in the convergence of different signaling pathways to the AP-1 motif, and subsequent induction of MMP-1 transcription. For example, insulin stimulation activates the protein kinases JNK-1 and JNK-2 in rat fibroblasts, which is followed by phosphorylation and activation of c- Jun (23,24). In contrast, in murine kidney mesangial-derived cells the mitogen-activated protein (MAP) kinase pathway is required for insulin signaling through the AP-1 motif (25). It is plausible that relaxin stimulation results in similar convergence of as yet undetermined signaling pathways that activate transcription factors such as c-fos, c-jun and Ets-1, eventually leading to the binding of these transcription factors to their respective motifs in the MMP-1 promoter to initiate the transcriptional activity of this gene. Recent studies point to several possible signaling pathways involved in the regulation of various genes by relaxin (26–29). The contribution of these signaling pathways to relaxin’s modulation of AP-1 and PEA-3 promoter sites and induction of MMP-1 remain to be determined.
Our findings also show that exposure of the disc fibrocartilaginous cells to β-estradiol potentiates relaxin’s transcriptional activation of MMP-1 and slightly increases the gene expression of c-fos and c-jun transcription factors. The mechanisms by which estrogen enhances relaxin’s transcription of MMP-1 are not currently known. However, the current understanding of estrogen signaling may provide some insights into its modulation of the cell’s responses to relaxin. Estrogen signaling is known to occur through at least two distinct ligand-dependent pathways, namely (1) ligand-activated estrogen receptor (ER) binding specifically to DNA at estrogen-responsive elements (EREs) through ER’s DNA binding domain that brings coactivators and corepressors to the transcription site, and (2) transcription factor cross talk involving the interaction of liganded ER with transcription factors such as AP-1, nuclear factor-κB or SP-1 that then modulates the activities of these transcription factors by stabilizing their DNA binding and/or recruiting coactivators to the complex (30,31). With regard to the latter mechanism, it is plausible that β-estradiol priming could lead to an indirect recruitment of liganded ERs to AP-1-responsive DNA elements via heterodimers of fos and jun that subsequently stimulate MMP-1 transcription. Also, EREs have been shown to be present in the promoter of the receptor for insulin-like growth factor (32), a family of hormones to which relaxin belongs. If EREs are similarly found to be present in the promoter of relaxin receptors, it may indicate a basis for estrogen’s modulation of MMP-1 induction by relaxin. These and other potential mechanisms for estrogen’s modulation of cellular responses to relaxin need further exploration.
Interestingly, our data also showed that β-estradiol alone produces some degree of induction of c-jun and c-fos gene expression but at the concentration used, failed to induce transcriptional activity of MMP-1. This might be because the small increases in mRNA of the c-jun and c-fos genes is not sufficient to result in a significant increase of transcription factor protein level that is essential for the transactivation of MMP-1. On the other hand, the antagonistic interaction between ER-α and ER-β on AP-1 mediated transactivation of MMP-1 could also be a key element that determines the final effect of β-estradiol treatment on the MMP responses of fibrocartilaginous disc cells (33).
Acknowledgments
This study was supported by NIH R29 DE11993 and RO1 DE018455. We wish to thank Dr. Peter Herrlich and Dr. Hans Rahmsdorf of Institution Karlsruhe, Germany and Dr. Zena Werb of UCSF for providing the MMP-1 promoter-reporter constructs, and Connetics Corporation for providing the recombinant human relaxin.
References
- 1.Kapila S, Xie Y. Targeted induction of collagenase and stromelysin by relaxin in unprimed and beta-estradiol-primed diarthrodial joint fibrocartilaginous cells but not in synoviocytes. Lab Invest. 1998;78:925–38. [PubMed] [Google Scholar]
- 2.Kapila S, Wang W, Uston K. MMP induction by relaxin causes cartilage matrix degradation in target synovial joints: Receptor profiles correlate with matrix turnover. Annals NYAS. 2008 doi: 10.1111/j.1749-6632.2009.03830.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi T, Duong TT, Hashem G, Shiga M, Zhang Q, Kapila S. Relaxin’s induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res Ther. 2005;7:R1–11. doi: 10.1186/ar1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tower GB, Coon CI, Brinckerhoff CE. The 2G single nucleotide polymorphism (SNP) in the MMP-1 promoter contributes to high levels of MMP-1 transcription in MCF-7/ADR breast cancer cells. Breast Cancer Res Treat. 2003;82:75–82. doi: 10.1023/B:BREA.0000003948.14026.7c. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti MP, White LA, Schroen DJ, Benbow U, Brinckerhoff CE. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): Mechanisms that control enzyme activity, transcription and mRNA stability. Crit Rev Eukaryot Gene Expr. 1996;6:391–411. doi: 10.1615/critreveukargeneexpr.v6.i4.40. [DOI] [PubMed] [Google Scholar]
- 6.Doyle GA, Pierce RA, Parks WC. Transcriptional induction of collagenase-1 in differentiated monocyte-like (U-937) cells is regulated by AP-1 and an upstream C/EBP-beta site. J Bio Chem. 1997;272:11840–11849. doi: 10.1074/jbc.272.18.11840. [DOI] [PubMed] [Google Scholar]
- 7.Auble DT, Brinckerhoff CE. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. 1991;30:4629–4635. doi: 10.1021/bi00232a039. [DOI] [PubMed] [Google Scholar]
- 8.Tremble P, Damsky CH, Werb Z. Components of the nuclear signaling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol. 1995;129:1707–1720. doi: 10.1083/jcb.129.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman SC, Ayala JE, Streeper RS, Culbert AA, Eaton EM, Svitek CA, et al. Multiple promoter elements are required for the stimulatory effect of insulin on human collagenase-1 gene transcription. Selective effects on activator protein-1 expression may explain the quantitative difference in insulin and phorbol ester action. J Biol Chem. 1999;274:18625–18634. doi: 10.1074/jbc.274.26.18625. [DOI] [PubMed] [Google Scholar]
- 10.Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, et al. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunology. 2003;170:838–845. doi: 10.4049/jimmunol.170.2.838. [DOI] [PubMed] [Google Scholar]
- 12.Reunanen N, Westermarck J, Hakkinen L, Holmstrom TH, Elo I, Eriksson JE, et al. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J Biol Chem. 1998;273:5137–5145. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- 13.Uria JA, Jimenez MG, Balbin M, Freije JM, Lopez-Otin C. Differential effects of transforming growth factor-beta on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J Biol Chem. 1998;273:9769–9777. doi: 10.1074/jbc.273.16.9769. [DOI] [PubMed] [Google Scholar]
- 14.Vincenti MP, Schroen DJ, Coon CI, Brinckerhoff CE. V-src activation of the collagenase-1 promoter through PEA3 and STAT: requirement of extracellular signal-regulated kinases and inhibition by retinoic acid receptors. Molecular Carcinogenesis. 1998;21:194–204. [PubMed] [Google Scholar]
- 15.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf HJ, Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapila S, Lee C, Richards DW. Characterization and identification of proteinases and proteinase inhibitors synthesized by temporomandibular joint disc cells. J Dent Res. 1995;74:1328–1336. doi: 10.1177/00220345950740061301. [DOI] [PubMed] [Google Scholar]
- 17.Grimberg A, Cohen P. Role of Insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–98. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallberg LM, Ikeno Y, Englander E, George H. Effects of aging and caloric restriction on IGF-I, IGF-I receptor, IGFBP-3 and IGFBP-4 gene expression in the rat stomach and colon. Regul Pept. 2000;89:37–44. doi: 10.1016/s0167-0115(00)00095-1. [DOI] [PubMed] [Google Scholar]
- 19.Leng SL, Leeding KS, Whitehead RH, Bach LA. Insulin-like growth factor (IGF)-binding protein-6 inhibits IGF-II-induced but not basal proliferation and adhesion of LIM 1215 colon cancer cells. Mol Cell Endocrinol. 2001;174:121–127. doi: 10.1016/s0303-7207(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhou R, Diehl D, Hoeflich A, Lahm H, Wolf E. IGF-binding protein-4: biochemical characteristics and functional consequences. J Endocrinol. 2003;178:177–193. doi: 10.1677/joe.0.1780177. [DOI] [PubMed] [Google Scholar]
- 21.Samuel CS, Butkus A, Coghlan JP, Bateman JF. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137:3884–3890. doi: 10.1210/endo.137.9.8756561. [DOI] [PubMed] [Google Scholar]
- 22.Ayala JE, Streeper RS, Svitek CA, Goldman JK, Oeser JK, O’Brien RM. Accessory elements, flanking DNA sequence, and promoter context play key roles in determining the efficacy of insulin and phorbol ester signaling through the malic enzyme and collagenase-1 AP-1 motifs. J Bio Chem. 2002;277:27935–27944. doi: 10.1074/jbc.M203682200. [DOI] [PubMed] [Google Scholar]
- 23.Bennett BL, Satoh Y, Lewis AJ. JNK: a new therapeutic target for diabetes. Current Opinion in Pharmacology. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA. Insulin-regulated gene expression. Biochemical Society Transactions. 2001;29:552–558. doi: 10.1042/bst0290552. [DOI] [PubMed] [Google Scholar]
- 25.Ayala JE, Boustead JN, Chapman SC, Svitek CA, Oeser JK, Robey RB, et al. Insulin-mediated activation of activator protein-1 through the mitogen-activated protein kinase pathway stimulates collagenase-1 gene transcription in the MES 13 mesangial cell line. J Molecular Endocrinology. 2004;33:263–280. doi: 10.1677/jme.0.0330263. [DOI] [PubMed] [Google Scholar]
- 26.Anand-Ivell R, Heng K, Bartsch O, Ivell R. Relaxin signalling in THP-1 cells uses a novel phosphotyrosine-dependent pathway. Mol Cell Endocrinol. 2007;272:1–13. doi: 10.1016/j.mce.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Halls ML, Bathgate RA, Summers RJ. Comparison of signaling pathways activated by the relaxin family peptide receptors, RXFP1 and RXFP2, using reporter genes. J Pharmacol Exp Ther. 2007;320:281–290. doi: 10.1124/jpet.106.113225. [DOI] [PubMed] [Google Scholar]
- 28.Ho TY, Yan W, Bagnell CA. Relaxin-induced matrix metalloproteinase-9 expression is associated with activation of the NF-kappaB pathway in human THP-1 cells. J Leukoc Biol. 2007;81:1303–1310. doi: 10.1189/jlb.0906556. [DOI] [PubMed] [Google Scholar]
- 29.Ivell R, Heng K, Anand-Ivell R. Diverse signalling mechanisms used by relaxin in natural cells and tissues: the evolution of a “neohormone”. Adv Exp Med Biol. 2007;612:626. doi: 10.1007/978-0-387-74672-2_3. [DOI] [PubMed] [Google Scholar]
- 30.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 31.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κB and C/EBPβ. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Arencibia M, Davila N, Camion J, Carranza MC, Calle C. Identification of two functional estrogen response elements complexed with AP-1-like sites in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2005;94:1–14. doi: 10.1016/j.jsbmb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama S, Fujimoto N, Asano K, Ito A. Suppression by estrogen receptor beta of AP-1 mediated transactivation through estrogen receptor alpha. J Steroid Biochem Mol Biol. 2001;78:177–184. doi: 10.1016/s0960-0760(01)00083-8. [DOI] [PubMed] [Google Scholar]


