Abstract
The adolescent period is characterized by substantial behavioral changes, including increases in novelty-seeking and risk-taking, which may facilitate substance use and experimentation. These behavioral changes co-occur with widespread structural and functional neural developments. Ontogenic changes affecting the neural circuitry subserving inhibitory control and reward-related processes are particularly relevant to adolescent risk-taking behavior. Impairment or immaturity of these processes are shown to contribute to enhanced risk for substance abuse. Additionally, the direct neural action of drugs of abuse in adolescents may have more severe consequences than in adults because of the additional potential effects on development. Functional neuroimaging research is beginning to examine the neural correlates of reward and inhibitory processes in adolescents. However, the study of the consequences of exposure to drugs of abuse on brain function in adolescents is lagging. This review summarizes the functional neuroimaging literature that can inform conceptualizations of risk and consequences of substance use in adolescence.
Introduction
The developmental period of adolescence is distinguished by a transition from the dependent, family-oriented state of childhood, to the independent, peer-oriented state of adulthood. This fundamental shift is accompanied by refinements in cognitive, emotional and social skills that facilitate exploratory, novelty-seeking, and sensation-seeking behaviors (1, 2). While the typical adolescent behavioral profile generally aids in the transition to independence, it comes with a cost often related to excessive risk-taking behavior. Some of these risk-taking behaviors can be seen in the form of substance use, as adolescence is a prime time for experimentation with drugs and alcohol (3). The troubling nature of this experimentation is exemplified by the rising prevalence of alcohol and substance use disorders during adolescence (e.g., 4, 5).
Whereas the notion of enhanced reward-seeking combined with the relatively delayed maturation of cognitive control is a common model for understanding the peak onset of substance abuse in adolescence (Figure 1) (1, 2, 6, 7), the neural mechanisms underlying this hypothesis need to be better delineated. For example, competing hypotheses are proposed regarding the function of the reward system in adolescents. Some hypotheses argue for a hyper-responsive system (e.g., 8, 9), similar to the incentive salience theory (10), and others for a hypo-responsive system (e.g., 2, 11), similar to the reward deficiency theory (12, 13). Analogous questions arise regarding the role of negative reinforcement that is associated with withdrawal symptoms. Whereas negative reinforcement is thought to contribute prominently to the maintenance of addiction (e.g.,14), its importance in adolescent addiction is questioned on the basis of observations that withdrawal symptoms are milder in adolescence than adulthood (e.g., 15). On the other hand, the reduction of withdrawal symptoms in adolescents may prevent the potential brake on drug consumption early in the addiction cycle, leading to larger amounts of drug use, and more severe long-term consequences, including adult addiction.
Figure 1. Schematic representation of the developmental trajectories of reward sensitivity and inhibitory control.
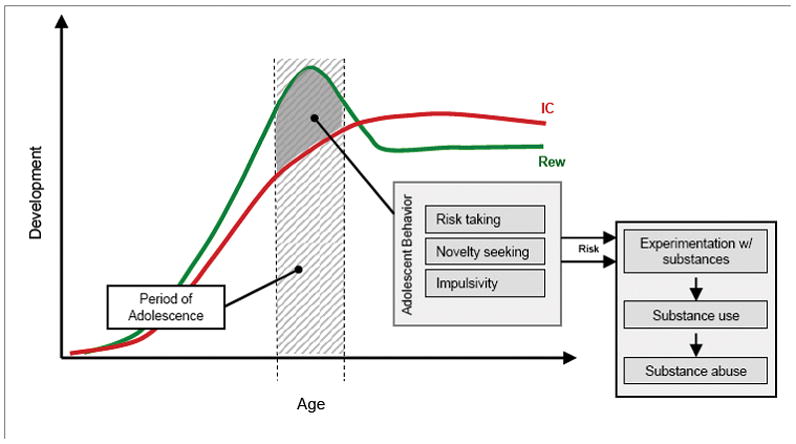
The combination of hypersensitivity to reward (Rew) and still immature inhibitory control (IC) yield a typical adolescent behavioral profile that includes increased risk-taking, novelty-seeking, and impulsivity. This behavioral profile leads to increased risk for adolescents, as these behaviors increase the likelihood that adolescents will experiment with substances. Depending on additional variables (e.g., individual differences in the developmental trajectory; contextual, influences; environmental influences) this experimentation can then lead to regular substance use and abuse. For the sake of this schematic representation, these curves are normalized to a theoretical baseline (y=0).
Neurobiological models of addiction provide useful frameworks for studying adolescent substance use. A number of these models have been proposed, each emphasizing distinct aspects of addiction (e.g., role of hedonic experience, aversive responses, or repetitive behavior), and each originating from unique perspectives (e.g., behavioral phenotype, neurocognitive formulation, neural systems model, molecular machinery, genetics). Review of these models goes well beyond the scope of the current work. However, brief references may be made wherever relevant.
Here, we adopt a cognitive neuroscience framework, and focus on three cognitive processes that contribute to addiction. Alterations in two of these processes, reward and inhibitory control, are believed to be mainly causal. Alteration in the third process, working memory, is believed to be secondary to drug exposure. Additionally, inhibitory control processes may also be directly affected by substances of abuse. Of note, the majority of developmental functional neuroimaging work concerned with substance use to date, focuses on alcohol use. For this reason, work on alcohol and alcohol abuse will dominate this review.
Developmental vulnerability to substance abuse: Reward-related processes and inhibitory control
Reward-related processes
General description
Reward-related processes are involved in the response to positive salient outcomes and the motivation to achieve these outcomes. These processes appear to develop in a curvilinear manner, which is characterized by a hypersensitivity to rewards peaking during adolescence (for overview, 16). This peak facilitates reward- and sensation-seeking behavior (1, 17), which, in turn, confers vulnerability for substance experimentation, use, and dependence.
A large body of neuroimaging work has examined the neural substrates of reward processing and addiction (e.g.,18–20). Surprisingly however, very little of this research has focused on reward pathways or the development of addiction in adolescence. The neural pathway supporting reward processing is perhaps one of the best delineated neural networks outside of those underlying basic sensory processing. This system comprises primarily subcortical structures and dopaminergic projections to medial and orbital regions of the frontal cortex (21–23). More specifically, the receipt of rewarding outcomes commonly involves the ventral striatum (including nucleus accumbens; NAcc) (24–27). The medial prefrontal cortex (mPFC) appears to track rewarding monetary outcomes (28), and also responds more strongly to positive rather than negative monetary reward (28). The orbitofrontal cortex (OFC) is involved in the representation of stimulus-reward value (25, 29, 30), and contributes to updating reward values as they change with time (31, 32). Additionally, although most known for its essential function in response to threat, the amygdala is also implicated in reward-related processing (33).
Adolescent reward sensitivity
The neurodevelopmental studies of reward processing conducted with typically developing youths generally support enhanced sensitivity to reward during adolescence. For example, during monetary-based decision making, healthy adolescents have shown ventral striatal activation in response to monetary gains (34). This finding matches that reported for healthy adults performing the same task (24). These response patterns suggest that adolescents and adults recruit similar reward-related circuitry.. However, the extent of activation in these regions differs between age groups. Specifically, ventral striatal response to monetary gains during a decision-making task was stronger in adolescents compared to adults (26). A similar NAcc hyper-response to a monetary reward was also reported when adolescents were compared to both young children and adults during a delayed response task (35). However, some discrepancies exist, with at least one developmental neuroimaging study failing to detect stiatal hyper-response in adolescents during the receipt of a reward (36). In fact, during the anticipation of a potential reward in this study, striatal response was greater in adults than in adolescents (36). The lack of an increased striatal response by adolescents in this specific study has led to the hypothesis of a hypo-functional reward system in adolescence that requires extra stimulation to be maintained at a homeostatic level. An important difference between the studies reporting striatal hyper-response and those reporting striatal hypo-response in adolescents concerns the specific reward-related process under scrutiny. Striatal hyper-response occurred during reward receipt, whereas striatal hypo-response occurred during reward anticipation.
Age-related differences of neural activation during the receipt of a reward are also seen in regions of the prefrontal cortex (PFC). Specifically, adolescents, relative to adults, showed a weaker dorsolateral PFC (DLPFC) response (26), and a more diffuse pattern of activation in the orbital frontal cortex when receiving a monetary reward (35). Similarly, when adolescents made risky decisions that could lead to a large reward, they showed lower activation of the ventrolateral PFC compared to adults (37). This pattern of reduced PFC activation for adolescents relative to adults suggests poorer top-down cognitive control when adolescents are receiving a reward and showing highly appetitive behavior.
Collectively, these findings can be conceptualized as representing the protracted development of frontally regulated cognitive control, resulting in poor top-down regulatory/inhibitory control over a hyper-responsive reward system. This developmental neural response pattern likely underlies adolescent impulsivity, risk-taking, and sensation-seeking behavior, coalescing into heightened propensity for substance use.
Inhibitory control
General description
Inhibitory control processes are involved in the ability to suppress behavior that is prepotent, over-learned, or irrelevant (38–40). These processes show a gradual linear developmental trajectory that begins in childhood and continues throughout adolescence (41). The protracted development of inhibitory control processes likely hinders the ability of adolescents to control reward-related processes, resulting in increased sensation-seeking and increased risk for experimentation with drugs and alcohol.
Experimental tasks probing these processes include the antisaccade eye movement, go/no-go, and stop signal tasks. Functional neuroimaging studies conducted with healthy adults implicate prefrontal cortical regions, particularly the inferior prefrontal cortex (39, 42), in the regulation of inhibitory processes (43). These findings are especially relevant here, considering the steep trajectory of PFC development occurring in adolescence (44, 45), which is paralleled by substantial performance improvement on inhibitory control tasks (44).
Typical development of inhibitory control
A handful of functional neuroimaging studies have explored the normal development of inhibitory control. In studies that employ paradigms requiring a manual response, such as the go/no-go or the stop signal tasks, adolescents show inferior frontal cortex (IFC) activation during inhibition, suggesting similar patterns of regional activation as those reported in adults (46, 47). During late childhood and adolescence, age increases are positively correlated with IFC response during inhibition (48–50). Likewise, better inhibitory performance by children and adolescents is associated with a greater response in IFC (46). Taken together, these findings suggest that, while adolescents engage similar neural circuitry as adults during inhibitory control, recruitment of this circuitry evolves throughout late childhood and adolescence.
Although IFC changes across adolescence are relatively well documented, studies diverge in the direction of these changes relative to adults. Experiments using go/no-go tasks report greater IFC response in adults than in children and adolescents (48–50). These findings are interpreted as a functional “underdevelopment” or immaturity in adolescents, indicating that the adolescent IFC is unable to be fully mobilized. Other studies report greater IFC response in children and adolescents than in adults (47, 51). These findings are interpreted as a “less efficient” inhibitory system in adolescents, indicating that the adolescent IFC must “work harder” compared to adults.
The discrepancy in neural activation findings may also reflect differences between adults and adolescents on task performance. In general, performance is worse for adolescents than adults. Thus, age-related differences in neural responses may be an epiphenomenon of task difficulty or of performance strategy, rather than a direct reflection of neural maturation. Parametric task designs can help in dissociating the effects of task difficulty from those of age effects. However, a number of methodological issues accompany parametric approaches, the substantial increase in task duration being one of the most preemptive (52). Alternatively, adjusting task difficulty to minimize individual differences can also be used. This strategy has been employed during reward processing tasks in which cue duration was adjusted individually to yield an average of 60% correct responses for all participants (52–54).
Inhibitory control and adolescents at-risk for substance abuse
In contrast to the dearth of reward-related neuroimaging studies with adolescents at risk, studies of inhibitory control have been conducted with adolescents who were at higher risk by virtue of positive familial history for addiction. These studies have yielded a number of behavioral and functional neuroimaging findings.
Behavioral studies reveal that adolescents, who are at higher than normal risk for developing a substance use disorder, show even greater inhibitory deficits than non-risk adolescents. Adolescent offspring of alcoholic parents show poorer inhibitory control than normal-risk adolescents (55). Additionally, poor behavioral inhibitory control in children and adolescents is associated with an increased risk for substance use. For example, in a large sample of adolescents from families with high substance abuse profiles, poor response inhibition on a Stop task was associated with high levels of both alcohol and drug use by these youths (56). Electrophysiological measures that are linked to poor inhibitory control are also associated with, and predict substance use in the adolescent children of alcoholic parents (57, 58). These findings suggest that, while developmental deficits of inhibitory control in non-risk adolescents may contribute to a high likelihood of experimenting with substances, an even greater deficit in high-risk adolescents could facilitate the progression to disordered use.
Functional neuroimaging studies with at-risk youths are consistent with neuropsychological findings. At-risk adolescents from families with a history of alcohol use disorder (AUD) tend to show weaker IFC response relative to normal-risk adolescents during inhibition on a go/no-go task (59). These group differences are seen despite a report of no differences in task performance. The absence of performance differences in this particular study could be a reflection of the small sample size (n< 15/group). This limitation is a common concern in functional neuroimaging studies, which usually involve samples of n<15/group compared to neuropsychological experiments of n>20/group samples. The absence of group differences on behavioral measures usually raises the question of how relevant the task is to the diathesis under investigation. Here, the link between inhibitory control and risk for substance abuse is fortunately well established (60, 61). Therefore, the absence of performance differences may indeed reflect the relatively low statistical power in neuroimaing studies to detect behavioral group differences, rather than the lack of relevance. Alternatively, the at-risk adolescents may be using an alternative strategy to maintain their performance at a normal level, despite impaired IFC function.
Taken as a whole, reward-related and inhibitory control findings paint a picture of adolescent risk for substance use that is established by a developmental discordance between these two sets of processes (see Figure 1). Specifically, reward-related processes appear to be hyperactive in adolescence, which may facilitate adolescent experimentation with substances (see table 1). Conversely, inhibitory control processes appear to be functionally underdeveloped with limited regulatory control over reward-related processes. This developmental divergence appears to peak in adolescence, and thus be a significant contributor to risk for substance use.
Table 1.
Overview of neural response patterns during normal adolescent development
| Response in Typical Development | ||
|---|---|---|
| IFC | Striatum | |
| Adolescents 30–32, 50, 60–61 | ⬇ | ⬆ |
| At-risk Adolescents 39 | ⬇⬇ | ? |
⬇ = less neural response; ⬆ = greater neural response;? = no known results
Overview of neural response patterns during normal adolescent development. Relative to adults, typical adolescents show less in inferior frontal cortex (IFC) response during inhibitory control tasks, but a greater striatal response during reward processing. Relative to typical adolescents, adolescents at-risk for substance abuse show less response in the IFC during inhibitory control.
Consequences of drug action on brain function
In addition to the physiological increase of vulnerability for substance use in adolescence, the direct neural effects of substances on development may potentiate risk for dependence and worsen the consequences of exposure by disrupting typical neurodevelopment (62). This possibility is supported by studies that suggest the adolescent brain is more sensitive than the adult brain to the effects of substances. Translational studies in rodents find that adolescents present unique responses to alcohol and other common substances of abuse. For example, adolescent animals appear to be less sensitive to the negative effects of alcohol, such as sedation and motor-impairment (63). This lack of sensitivity can facilitate substance use by removing a natural signal to stop or limit consumption (64). In a similar vein, alcohol seems to ease social interaction more potently in adolescents than in adults, thus yielding stronger incentives for consumption (65). These apparent incentives for the adolescent to continue consuming substances (alcohol) becomes even more troubling as there is evidence to suggest neuroplastic/neural reorganization and cognitive deficits with adolescent exposure to substances such as alcohol and nicotine (66–69). Additional evidence suggests that the neurotoxic effects of alcohol are worse in adolescence than in adults. This is particularly true of the hippocampus, which shows alcohol-induced damage of NMDA receptor systems with deleterious consequences on learning and working memory in adolescents (67, 70).
Building on the findings from studies conducted primarily with animal models, the neuroimaging studies with adolescent substance users tend to focus on working memory and inhibitory control processes. In general, these neuroimaging studies report functional differences between adolescent substance users and non-users, supporting the idea of functional disruptions that result from substance use by adolescent substance users. However, four caveats complicate this line of research. The first two limitations concern the need to account for: 1) the disease stage (i.e., experimentation vs. abuse vs. dependence); and 2) the state within the addictive cycle (i.e., craving vs. current use vs. withdrawal vs. abstinence). Systematic studies in adolescents that examine specific cognitive processes across these stages and states have not been conducted, and findings that concern differences between theses stages remain patchy. A third caveat involves the distinct neural effects of different drugs of abuse. Here again, systematic studies conducted with adolescents that compare classes of addictive substances (e.g., opiates, stimulants, nicotine) have not been conducted. A fourth caveat concerns the issue of demonstrating abnormalities present in adolescent substance users are secondary to the direct neural action of the substances, rather than preceding chronic drug exposure. In other words, it is difficult to separate neural function related to risk factors (such as those discussed above) from those related to direct drug action. The best strategy to address this issue is to conduct longitudinal studies that follow high and low risk adolescents across multiple time points in their progression towards substance use or non-use.
Substance-related effects on working memory
Working memory refers to the short-term retention of information that no longer exists in the external environment, and the use of this information to aid in goal-directed behavior (71). The prefrontal cortex is involved in working memory processes, with dorsolateral regions being particularly implicated (71). Although deficits in working memory do not seem to confer risk for substance abuse, they do appear to result from chronic exposure to drugs. Indeed, impaired working memory is observed in adult substance abusers, suggesting a direct action of drugs on memory systems. Such cognitive impairment could worsen the progression of substance abuse, in addition to increasing the burden of addiction.
Neurofunctional differences between substance-using adolescents and non-using adolescents are apparent in spatial working memory tasks (see table 2). For example, adolescents who are current cannabis users show a greater DLPFC and lower IFC activation during performance of spatial working memory tasks compared to non-using adolescents (72). This suggests a greater working memory load or decreased efficiency of working memory processes for adolescent cannabis users during these tasks. In contrast, cannabis-using adolescents who have been abstinent for one month show a decreased DLPFC response during spatial working memory performance relative to non-using adolescents (73). Together, these finding suggest that cannabis has a direct action on adolescent neural function. Additionally, this effect appears to be differentially modulated by the presence or absence of the drug. However, alternative interpretations, such as pre-existing deficits or withdrawal effects, cannot be ruled out (73).
Table 2.
Overview of neural response patterns in adolescent substance users
| Response in Substance Users | |||||
|---|---|---|---|---|---|
| IFC | DLPFC | Parietal | |||
| Working memory tasks | Cannabis users 71 | ⬇ | ⬆ | – | |
| Cannabis users 72 (abstinent) | – | ⬇ | – | ||
| Alcohol users 71, 73 | – | – | ⬆ | ||
| Alcohol users 74 (long term use) | – | – | ⬇ | ||
| Alcohol users vs. Comorbid alcohol/cannabis users 72 | ⬆ | – | – | ||
| IFC | DLPFC | ||||
| Inhibitory control tasks | Cannabis users 79, 80 | –* | ⬆ | ||
| IFC | OFC | Limbic | ACC | ||
| Substance signaling stimulus tasks | Alcohol users 85–86 | ⬇ | ⬆ | ⬆ | ⬆ |
⬇ = less neural response; ⬆ = greater neural response; – = no neural response difference
Overview of neural response patterns in adolescent substance users. Unless otherwise noted, all groups represent the comparison between adolescent substance users and non-user adolescents. The IFC is typically implicated in inhibitory control processes, DLPFC in working memory function, and parietal cortex in attention. The OFC, as well as limbic system structures, are involved in reward processes, and the ACC in both emotion and cognitive (e.g., conflict monitoring) processes.
Though no inhibition related differences is reported, cannabis users showed a greater IFC response during the no-inhibition trials in this study. This pattern may represent a hyperactive basal tone in the IFC of cannabis users.
IFC: Inferior Frontal Cortex; DLPFC: Dorsal Lateral Prefrontal Cortex; OFC: Orbital Frontal Cortex; ACC: Anterior Cingulate Cortex
The neural effects of different classes of addictive substances, or of their combination, are beginning to be explored on working memory in adolescent substance users. Results of this work suggest these effects may be substance specific. For example, differences in brain activation pattern during working memory performance are reported between current alcohol-only-using adolescents and adolescents who are current users of both alcohol and cannabis (72). Working memory activates IFC and bilateral temporal cortex more strongly in adolescents using only alcohol than in adolescents using both alcohol and cannabis. As previously mentioned, IFC function is implicated in inhibitory control. Greater IFC activity in alcohol-only-using adolescents suggests a larger cognitive load is placed on inhibitory control processes for these adolescents during working memory performance. Alcohol-only-using adolescents also show a larger response in parietal regions during comparison to non-using adolescents (72, 74). The parietal cortex is implicated in spatial attention, and greater parietal activity suggests a higher load may be placed on spatial attention processes for alcohol-using adolescents when performing a (spatial) working memory task. Alternatively, the weaker activation in dual-drug users may reflect the inability of these adolescents to recruit these regions for working memory. Such differences in neural activation may translate as subtle cognitive impairments that hinder academic achievement in these adolescents. Another interpretation concerns different levels of motivation to perform the task, with these differences being reflected as decreased effort and reduced engagement of structures supporting task performance.
As this work illustrates, existing studies in adolescents raise many questions that warrant not only replications, but the development of a systematic body of research. Compared to the relative wealth of functional neuroimaging studies that examine working memory in addicted adults, the current dearth of work in adolescents is remarkable.
Substance-related effects on inhibitory control
In addition to the deficits observed during typical adolescent development and in adolescents who are at high risk for a substance use disorder, perturbations of inhibitory control are also reported for adolescents that have a history of substance use (75) (see table 2). Disinhibition or the inability to control inappropriate behavioral responses is frequently described in individuals with history of substance use (76, 77). Deficits in inhibitory processes are evidenced both in response to traditional tasks of inhibition, such as the Stroop task or the go-nogo task, but also with tasks probing responses to substance-related cues.
With respect to traditional tasks of inhibition, neuroimaging studies in both adults and adolescents suggest impaired processing of inhibitory control. Adults with current heavy cannabis use show more diffuse DLPFC, increased mid-cingulate, and decreased anterior cingulate activation relative to non-using adults during response inhibition in a Stroop task(78). Although group differences also emerged between users and non-users in adolescents, findings differ from those reported in adults. The distinct findings in adults and adolescents cannot be easily interpreted because of task differences, other individual differences such as co-morbidity, and nature of substance use. Adolescent substance users are reported to present greater superior frontal gyrus (Brodman areas 10 and 11) and bilateral parahippocampal responses to a Stroop task relative to non-using adolescents (79). However, the substance-using adolescents in this work also had a comorbid diagnosis of conduct disorder, and the contribution of conduct disorder to the group differences can not be ruled out. Such comorbidity is common among substance-using adolescents and presents a challenge for the study of adolescent substance use.
Additional evidence of deficits in inhibitory control is found in adolescent cannabis users. After 1-month abstinence, adolescent cannabis users have shown greater recruitment of frontal regions relative to non-using adolescents during go/no-go task performance (80). This suggests that the abnormal recruitment of frontal regions by substance-using adolescents persists beyond the period of use, and may represent alterations of development in these regions. One possible interpretation relies on compensatory mechanisms. Greater frontal activation for adolescent substance users may reflect compensatory processes aimed at maintaining behavioral performance at a level similar to that of non-using adolescents (80). However, these findings may also represent a greater load placed on working-memory processes for the substance-using adolescents. Given the involvement of DLPFC in working memory (81), and reports of greater working memory-related DLPFC recruitment by cannabis-using adolescents relative to non-using adolescents (82), it is conceivable that the greater frontal response by substance-using adolescents reflects deficient memory-related processes rather than deficient inhibitory control.
With respect to tasks probing responses to substance-related cues, findings suggest the presence of neural dysfunction underlying a difficulty in substance abusers for shifting attention away from substance-related cues (83). Such difficulty would be consistent with the recruitment of inhibitory control processes when substance-users encounter and attempt to resist environmental cues that are associated with their substance use. In addition, substance-using adults exhibit enhanced arousal with the presentation of substance-related cues, consistent with stronger salience of these cues. In line with the proposal of deficient inhibitory control and increased reward-processing in addiction, substance-using adults show hyperactivation of frontal, limbic (i.e., amygdala), and anterior cingulate regions to substance-associated cues (84, 85). Similar responses to substance-related cues are seen in substance-using adolescents. Relative to non-using adolescents, substance-using adolescents show increased responses in frontal (including orbitofrontal cortex), limbic (i.e., amygdala), and anterior cingulate regions when viewing substance-related words and images (86, 87). In keeping with the notion of compromised inhibitory control function, substance-using adolescents also show deficient recruitment of the IFC during presentation of substance-related cues (87). The coupling of greater reward system response and deficient IFC response by substance using adolescents suggests an inability to inhibit appetitive responses to substance-related cues.
Conclusion
Perhaps the most striking fact that emerges from this review is the paucity of neuroimaging work conducted with adolescent substance use problems. At present, the strongest findings originate from studies of risk factors, likely because these studies are generally simpler to conduct since they do not have to control for the type of substances used, or the addictive stage (e.g., abstinence, withdrawal). These studies point to a greater than normal discrepancy in the developmental trajectories of reward and inhibitory control processes for adolescents at-risk of developing a substance use disorder. This excessive discrepancy consists of an exaggerated hyper-responsive reward system, and an abnormally protracted development of inhibitory processes. Only few studies directly compare adolescents with adults, even though such works can be critical to understand the impact of drugs on brain development. Alternatively, longitudinal studies can provide robust data, and a number of developmental laboratories are now well-positioned to conduct this type of work.
In the context of such a wide open area of research, the use of neurobiological models to guide research programs can be invaluable. A number of neurobiological models of reward systems/addiction have been proposed for the mature brain (88–93). These models fall into several categories and are built around a number of themes: neurotransmitter function (e.g., 93); molecular alterations (e.g., 94); neurocognitive conceptualizations such as conditioning (88); homeostatic/allostatic equilibrium (e.g.,90); cognitive impulsivity (e.g., 89, 95); compulsion/drive (e.g., 93); or incentive salience theory (e.g., 92). However, only a few models have been formulated from a developmental perspective. These are based on ontogenic changes in brain function during adolescence and are derived primarily from findings of animal studies (8, 64, 96, 97) and functional neuroimaging work (9, 98, 99). The major challenge for the future of adolescent substance use research is to generate models that best integrate current research findings and, in turn, to design research programs that systematically test these models.
Acknowledgments
This work was funded by the Intramural Research Program at the National Institute of Mental Heath
References
- 1.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 2.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 3.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. The Monitoring the Future national results on adolescent drug use: Overview of key findings, 2005. National Institute on Drug Abuse; Bethesda, MD: 2006. [Google Scholar]
- 4.Chen Z, Knutson E, Kurosky A, Albrecht T. Degradation of p21cip1 in cells productively infected with human cytomegalovirus. J Virol. 2001;75(8):3613–25. doi: 10.1128/JVI.75.8.3613-3625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohde P, Lewinsohn PM, Kahler CW, Seeley JR, Brown RA. Natural course of alcohol use disorders from adolescence to young adulthood. J Am Acad Child Adolesc Psychiatry. 2001;40(1):83–90. doi: 10.1097/00004583-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12:339–337. [Google Scholar]
- 7.Steinberg L. A Neurobehavioral Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 11.Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27(18):4839–49. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5(3):121–41. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Gardner EL. The neurobiology and genetics of addiction: Implications of the reward deficiency syndrome for therapeutic strategies in chemical dependency. In: Elster J, editor. Addiction: Entries and Exits. Russell SageFoundation; New York: 1999. pp. 57–119. [Google Scholar]
- 14.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 15.O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186(4):612–9. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 16.Ernst M, Spear LP. Development of reward systems in adolescence. In: de Haan M, Gunnar MR, editors. Handbook of Developmental Social Neuroscience. Guilford Press; In Press. [Google Scholar]
- 17.Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–8. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 18.Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008;95(1–2):115–28. doi: 10.1016/j.drugalcdep.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: A review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008 doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28(18):4583–91. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 22.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 23.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 24.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 25.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–7. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27(18):4826–31. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 29.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 30.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 31.O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–21. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 33.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 34.May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55(4):359–66. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13(3):214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 39.Curtis CE, D’Esposito M. The inhibition of unwanted actions. In: Bargh J, Gollwitzer P, Moresella E, editors. The Psychology of Action. Vol. 2 Guildford Press; New York: 2008. [Google Scholar]
- 40.Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126(2):220–46. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- 41.Casey BJ, Getz S, Galvan A. Developmental Review. The adolescent brain. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 44.Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–57. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Booth JR, Burman DD, Meyer JR, Trommer BL, Davenport ND, Parrish TB, Gitelman DR, Mesulam MM. Brain-behavior correlation in children depends on the neurocognitive network. Hum Brain Mapp. 2004;23(2):99–108. doi: 10.1002/hbm.20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16(2):449–53. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- 48.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 49.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28(11):1163–77. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1231–8. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–51. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 52.Levita L, Jones M, Casey BJ. BOLD fMRI: An update with emphasis on pediatric application. In: Rumsey J, Ernst M, editors. Neuroimaging in Developmental Clinical Neuroscience. Cambridge University Press; Cambridge: In Press. [Google Scholar]
- 53.Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Habeych ME, Folan MM, Luna B, Tarter RE. Impaired oculomotor response inhibition in children of alcoholics: The role of attention deficit hyperactivity disorder. Drug Alcohol Depend. 2006;82(1):11–7. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468–75. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 57.Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adulthood. Biol Psychiatry. 2005;57(1):76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 58.Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625–34. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–4. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- 60.Clark DB, Cornelius JR, Kirisci L, Tarter RE. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 2005;77(1):13–21. doi: 10.1016/j.drugalcdep.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160(6):1078–85. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 62.Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–89. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- 64.Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–6. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- 65.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 66.Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann N Y Acad Sci. 2004;1021:234–44. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- 67.Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24(11):1712–23. [PubMed] [Google Scholar]
- 68.DeBry SC, Tiffany ST. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res. 2008;10(1):11–25. doi: 10.1080/14622200701767811. [DOI] [PubMed] [Google Scholar]
- 69.Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29(2):207–20. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- 70.White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–20. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- 71.D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):761–72. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79(2):201–10. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28(10):1577–86. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- 75.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 76.Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, Chorlian DB, Stimus AT, Begleiter H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31(1):156–65. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 77.Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12(3):405–15. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- 78.Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23(1):107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Banich MT, Crowley TJ, Thompson LL, Jacobson BL, Liu X, Raymond KM, Claus ED. Brain activation during the Stroop task in adolescents with severe substance and conduct problems: A pilot study. Drug Alcohol Depend. 2007;90(2–3):175–82. doi: 10.1016/j.drugalcdep.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194(2):173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zald DH. Orbital versus dorsolateral prefrontal cortex: anatomical insights into content versus process differentiation models of the prefrontal cortex. Ann N Y Acad Sci. 2007;1121:395–406. doi: 10.1196/annals.1401.012. [DOI] [PubMed] [Google Scholar]
- 82.Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176(3–4):239–47. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- 83.Stormark KM, Field NP, Hugdahl K, Horowitz M. Selective processing of visual alcohol cues in abstinent alcoholics: an approach-avoidance conflict? Addict Behav. 1997;22(4):509–19. doi: 10.1016/s0306-4603(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 84.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 85.Hommer DW. Functional imaging of craving. Alcohol Res Health. 1999;23(3):187–96. [PMC free article] [PubMed] [Google Scholar]
- 86.Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29(1):33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60(7):727–35. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- 88.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 89.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146(4):373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 90.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 91.Verdejo-Garcia A, Perez-Garcia M, Bechara A. Emotion, decision-making and substance dependence: a somatic-marker model of addiction. Curr Neuropharmacol. 2006;4(1):17–31. doi: 10.2174/157015906775203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78(3):610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 94.Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 95.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 96.Anderson KG, Schweinsburg A, Paulus MP, Brown SA, Tapert S. Examining personality and alcohol expectancies using functional magnetic resonance imaging (fMRI) with adolescents. J Stud Alcohol. 2005;66(3):323–31. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 98.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]


