Summary
Tapasin is a key molecule in the major histocompatibility complex (MHC) class I peptide-loading complex, interacting with several other proteins in the complex. An amino acid substitution at a free cysteine position in tapasin has been shown to disrupt the covalent association of tapasin with ERp57. In this study, we mutated the free cysteine in mouse tapasin, and analyzed the effects on the cell surface expression of the mouse MHC class I molecules Kd and Kb. The C95S substitution in mouse tapasin increased the proportion of open forms relative to folded forms for both types of MHC class I molecules at the cell surface. Furthermore, the C95S substitution resulted in increased association of folded Kd with tapasin. Overall, our studies with these mouse MHC class I allotypes have revealed that the free cysteine 95 in mouse tapasin influences stable expression at the plasma membrane for both MHC class I allotypes, and have shown that tapasin's interaction with folded Kd is elevated by the C95S substitution in tapasin.
Tapasin is one of a group of proteins referred to jointly as the peptide-loading complex, which is required for the normal assembly of MHC class I heavy chains with antigenic peptides in the endoplasmic reticulum (Pamer & Cresswell, 1998; Farmery et al., 2000; Harris et al., 2001a). Tapasin has been proven to be important to MHC class I assembly by the deletion of the tapasin gene in mice and examination of the resultant phenotype. Mice with a tapasin gene knockout have a reduced number of MHC class I molecules at the plasma membrane and the MHC molecules reaching the surface are unstable, which results in poor T cell responses (Grandea et al., 2000; Garbi et al., 2000).
Thus it is known that tapasin is essential to MHC class I assembly, but the means by which tapasin assists the peptide loading of MHC class I heavy chains are not fully comprehended. Structurally, tapasin is a type I transmembrane protein, and the C-terminus binds to TAP (Li et al., 1997; Ortmann et al., 1997; Lehner et al., 1998; Li et al. 1999; Grandea et al. 1998; Deverson et al., 2001; Tan et al., 2002; Petersen et al., 2005). Tapasin acts as a physical link between TAP and the MHC class I heavy chain (Sadasivan et al., 1996). At position 95 in tapasin there is a conserved free cysteine that is not required for an internal disulfide bond (Li et al., 1997; Li et al., 1999; Deverson et al., 2001; Dick et al., 2002). In addition to interacting with TAP and the MHC class I heavy chain, human tapasin has been shown to associate with another protein, ERp57, in the peptide-loading complex, forming a disulfide bond that includes tapasin's cysteine at position C95 (Dick et al., 2002; Peaper et al., 2005; Garbi et al., 2007).
The principal questions that we sought to address in this study were whether mouse tapasin C95 influences the proportion of open (peptide-free), compared to folded, mouse MHC class I molecules, and whether the impact of this tapasin cysteine varies among mouse MHC class I molecules. We found that both Kd and Kb exhibited a higher ratio of open/folded cell surface forms after assembly in cells expressing mouse tapasin C95S. Furthermore, more mouse tapasin C95S than wild type tapasin remained associated with folded Kd molecules. Overall, these studies suggest that mouse MHC class I allotypes are dependent on the presence of the mouse tapasin cysteine at position 95 for normal, stable cell surface expression.
For these studies, we utilized a mouse fibroblast cell line (MF) generated from tapasin−/− mice (Grandea et al., 2000) that were made by Drs. A. Grandea and L. Van Kaer and colleagues (Vanderbilt University, Nashville, TN). A tapasin-positive control cell line was also made using a mouse wild type tapasin cDNA (Li et al., 1999), a kind gift from Dr. P. Wang (Barts and London School of Medicine). The tapasin cDNA was cloned into the pMIN vector, packaged using 293E cells, and transduced into mouse tapasin MFs. MF cell lines were created expressing no tapasin, wild type tapasin, or tapasin C95S, along with epitope-tagged Kd or Kb in the pLXSN retroviral vector (Clontech, Mountain View, CA, USA). The Kd and Kb had an epitope tag for the 64-3-7 antibody (Ab), so that open, peptide-free Kd and Kb could be recognized by 64-3-7 in flow cytometry, and so that Kd and Kb could be recognized by 64-3-7 on Western blots. This epitope tag has been shown not to affect peptide binding and trafficking of MHC class I molecules (Yu et al., 1999; Myers et al., 2000; Harris et al., 2001b; Lybarger et al., 2001). Mouse tapasin C95S was made by site-directed mutagenesis using the QuikChange kit (Stratagene, La Jolla, CA, USA) with the wild type mouse tapasin cDNA (Li et al., 1999) as a template. All cells were maintained at 37°C in 5% CO2 in DMEM containing 10% fetal bovine serum, 4 mM HEPES, 2 mM L-glutamine, 1X sodium pyruvate, 1X non-essential amino acids, penicillin (100 U/ml), streptomycin (100 μg/ml), 3 × 10−6 vol/vol β-mercaptoethanol, and 400 μg/ml G418. The media reagents were purchased from Invitrogen with the exception of the fetal bovine serum, which was obtained from Atlanta Biologicals (Lawrenceville, GA, USA). Prior to some flow cytometry procedures, cells were cultured in medium containing Nutridoma-SP serum substitute (Roche Applied Science, Indianapolis, IN) instead of fetal bovine serum and containing 300 rather than 400 μg/ml G418. For brefeldin A assays, the brefeldin A was added to the medium at a concentration of 2 μg/ml for varied time periods before the cells were harvested for flow cytometry.
The 64-3-7 monoclonal Ab binds to the α1 domain of open, peptide-free Ld (Smith et al., 1993), and, as mentioned above, the 64-3-7 mAb can also detect open forms of MHC class I molecules such as Kd and Kb into which the 64-3-7 epitope has been incorporated by site-directed mutagenesis (Yu et al., 1999; Myers et al., 2000; Harris et al., 2001b; Lybarger et al., 2001). The 34-1-2 Ab binds to folded Kd on the α1 domain (Ozato et al., 1983). Additional information supporting 34-1-2 Ab recognition of the peptide-binding region is that weak cross-reactive binding of 34-1-2 to Ld is strongly increased by Ld association with human β2m or particular peptide ligands, or by mutation of Ld at positions in the peptide-binding region (Nieto et al., 1989; Solheim et al., 1995). The B8-24-3 Ab recognizes folded Kb molecules (American Type Culture Collection). A hamster anti-mouse tapasin mAb (provided by Dr. T. Hansen) was used to probe Western blots (Harris et al., 2001a). Immunoprecipitations, Western blotting, and flow cytometry were performed as previously described (Turnquist et al., 2001; Solheim et al., 1995).
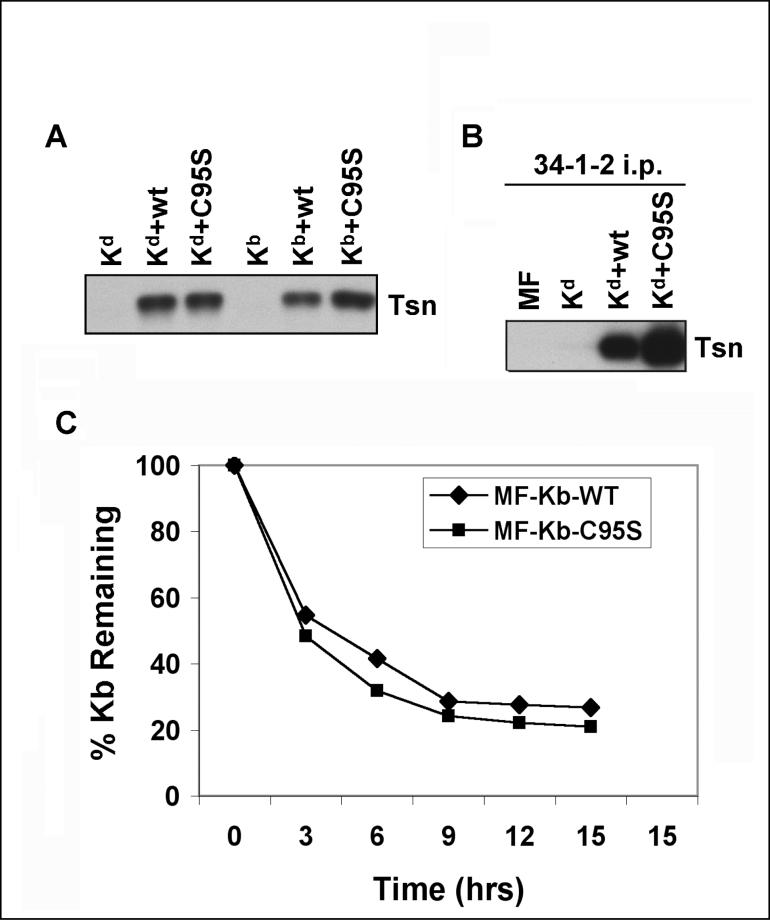
To compare the phenotypes of intracellular Kd and Kb assembled in the presence of tapasin C95S, we utilized the cell lines described above which expressed Kd or Kb together with wild type tapasin or tapasin C95S. The tapasin expression of these transfectants was assessed by Western blotting and the expression of wild type and mutant tapasin C95S was confirmed to be similar, as shown in Figure 1A. We have previously noted that some folded Kd molecules remain associated with wild type tapasin (Simone et al., submitted), and Li et al. (1999) reported that anti-tapasin Ab co-precipitated TAP and peptide-occupied MHC class I from mouse RMA cells. In this study, we found that immunoprecipitation of folded Kd molecules results in co-immunoprecipitation of even more mouse tapasin C95S than wild type tapasin molecules (Figure 1B). (Note that these studies were performed with iodoacetamide, rather than with N-ethylmaleimide or S-methyl methanethiosulfonate; therefore, all the tapasin molecules associated with folded Kd are non-covalently bound and there are no higher molecular weight disulfide-bonded complexes that include tapasin.) The C95S mutation in tapasin may result in slower assembly of Kd molecules, and thereby lead to the accumulation of more folded intermediates that maintain interaction with the peptide-loading complex. However, an alternative theory could be that the C95S mutation causes the Kd molecules to associate more tightly and/or for a longer time with tapasin.
Figure 1.
(A) Wild type and mutant tapasin expression levels were matched among paired transfectants expressing each type of MHC class I heavy chain (Kd or Kb). Aliquots of whole cell lysates were electrophoresed on a 10% acrylamide Tris-glycine gel. The proteins were subsequently transferred from the gel to a blot and probed with a mAb specific for mouse tapasin (Tsn). (B) More folded Kd was associated with tapasin C95S than with wild type tapasin. Immunoprecipitations were performed with monoclonal Ab 34-1-2 on lysates of the indicated cell types. The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed with anti-tapasin Ab (indicated as Tsn). (C) Cell surface Kb molecules on cells expressing mouse tapasin C95S had a slightly faster turnover rate, relative to cell surface Kb molecules on cells expressing wild type tapasin. MF cells that expressed mouse wild type tapasin or tapasin C95S were cultured in medium containing 2 μg/ml brefeldin A for 0, 3, 6, 9, 12, or 15 hours. Following incubation in the brefeldin A-containing medium, the cells were stained with anti-Kb mAb B8-24-3 and phycoerythrin-labeled secondary antibody, and analyzed on a FACS Calibur.
To assess the effect of mouse tapasin C95S on the stability of cell surface MHC class I molecules, we analyzed the cell surface expression of folded Kb molecules by flow cytometry after the arrival of additional Kb molecules had been blocked by brefeldin A. Brefeldin A has been previously shown to prevent protein transport from the Golgi to the plasma membrane (Yewdell and Bennink, 1989). After culture in brefeldin A, the cells exhibited a rate of surface Kb turnover that was about 10% more rapid if they expressed mouse tapasin C95S rather than wild type tapasin (Figure 1C). In previous experiments, we have monitored the rate of loss of folded Kd from the cell surface after brefeldin A treatment, and found it to be about 20% more rapid for cells expressing mouse tapasin C95S instead of wild type mouse tapasin (Simone et al., submitted).
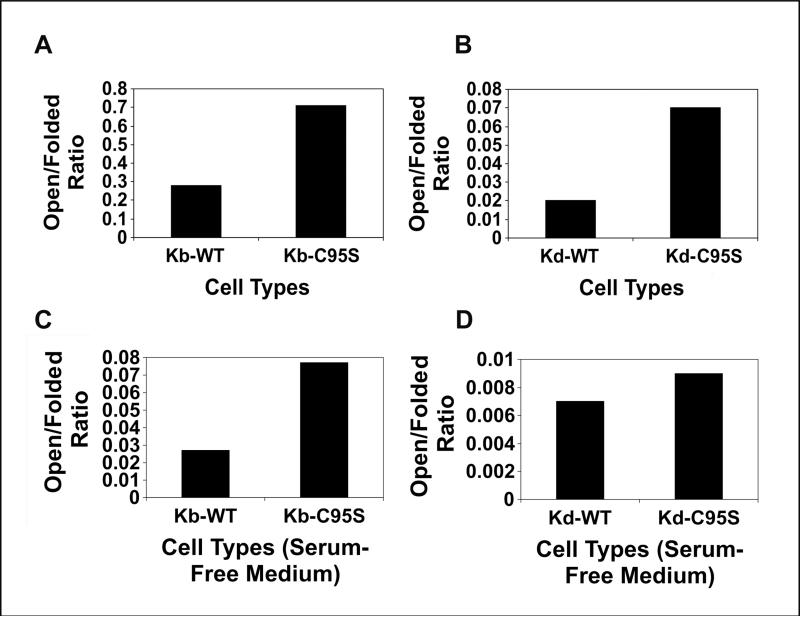
We also compared the ratios of open to folded Kb and Kd molecules on the cells expressing mouse wild type tapasin versus C95S tapasin, both after culture in regular medium and after culture in serum-free medium (Figure 2). (Because the Kd and Kb heavy chains expressed in our cell lines included an epitope tag for the 64-3-7 monoclonal Ab, we were able to monitor the surface expression of the open forms of these mouse MHC class I heavy chains with the 64-3-7 Ab.) In complete medium containing fetal bovine serum, the expression of the mouse tapasin C95S mutation resulted in an increased proportion of open forms at the cell surface for both Kb and Kd (Figure 2A,B). The difference between cells expressing tapasin C95S and wild type tapasin was slightly greater for Kd than for Kb, consistent with our results with the brefeldin A assay (Figure 2A,B, Figure 1C and Simone et al., submitted). In cells cultured in medium containing a serum substitute instead of fetal bovine serum, the mouse tapasin C95S mutation also increased the ratio of open to folded Kb and Kd molecules (Figure 2C,D), but the difference was substantially narrowed for Kd relative to the result obtained in serum-containing medium (compare Figure 2D to 2B). Evidently the availability of exogenous β2m and/or other factors in bovine serum have a greater effect on the stable cell surface expression of Kd than Kb.
Figure 2.
Flow cytometric analysis was performed with the 64-3-7 mAb (for open forms) and either mAb B8-24-3 (for Kb folded forms) or mAb 34-1-2 (for Kd folded forms) and a phycoerythrin-conjugated secondary Ab on MF cells expressing wild type tapasin or tapasin C95S. Samples were assayed on a FACS Calibur flow cytometer (BD Biosciences). The ratios of the mean fluorescence intensity values for Kb and Kd were calculated and are shown on the graphs. Background staining with secondary Ab only was <10 channels for all the cell types included in the analysis. The results shown in A and B were obtained with cells cultured in medium containing fetal bovine serum, and the results shown in C and D were obtained with cells cultured in medium containing a serum substitute.
Previously, effects of mutations at this position had been studied in the context of human tapasin (Dick et al., 2002; Howarth et al., 2004; Turnquist et al., 2004; Stepensky et al., 2007; Kienast et al., 2007; Peaper & Cresswell, 2008). In our study, we have found that the mouse MHC class I allotypes Kd and Kb are also affected by the mutation of the cysteine at position 95 of mouse tapasin. Our demonstration that mouse tapasin C95S expression leads to an increase in the ratio of open to folded mouse MHC class I molecules at the cell surface is consistent with the finding that human tapasin C95A expression resulted in an increased turnover rate and decreased thermostability for folded forms of B44 (Dick et al., 2002).
The cell surface ratio of open/folded Kb and Kd at the cell surface was altered by the presence of tapasin C95S. Our results suggest that, relative to Kb, Kd may have somewhat more dependency on wild type tapasin structure and function, which could relate to the structure of Kd itself and/or to the pool of Kd peptide ligands available in the endoplasmic reticulum. The peptide-binding groove of Kd is unusual in that it possesses five deep pockets, and the anchor residue for Kd peptide ligands is a large amino acid (tyrosine) at the P2 position (43-47: Maryanski et al., 1991; Romero et al., 1991; Quesnel et al., 1995; Mitaksov & Fremont, 2006; Suri et al., 2006); these features presumably allow very tight binding of peptide ligands to folded Kd.
Our findings suggest that mutation of mouse tapasin C95 affects the association of the MHC class I molecule with tapasin and the ultimate stability of the assembled mouse MHC class I molecules. The expression of tapasin C95S, in comparison with wild type tapasin, caused an increase in the open/folded ratio for the cell surface forms of both Kd and Kb (Figure 2). A high level of tapasin is associated with folded Kd, as we have previously observed (Simone et al., submitted and Figure 1B), and this association was further increased by the C95S mutation (Figure 1B). Human tapasin C95 interacts with ERp57 via a disulfide bond (Dick et al., 2002; Peaper et al., 2005; Garbi et al., 2007), and additional studies in our laboratory are specifically addressing the effect of the C95 position in mouse tapasin on tapasin's biochemical interactions with ERp57 and the relationship of those biochemical interactions to the effects we have noted in this report on mouse MHC class I molecule surface stability. Overall, our findings show that extension of tapasin analysis to additional species and additional types of MHC class I molecules can provide broader understanding of the complexity and variability in tapasin's ability to regulate antigen presentation by MHC class I molecules.
Acknowledgments
We thank Dr. Ping Wang for the gift of the mouse tapasin cDNA, and Drs. T. Hansen, A. Grandea, and L. Van Kaer for gifts of cell lines. We also gratefully acknowledge the assistance of the University of Nebraska Medical Center Cell Analysis Facility, Monoclonal Antibody Facility, and DNA Sequencing Core Facility. This work was supported by NIH/NIGMS R01 Grant GM57428 (to J.C.S.), by an NIH/NCI Training Grant T32 CA009476 Fellowship (to L.C.S.), and UNMC Graduate Studies Fellowships (to L.C.S. and A.T.). Core facilities at the University of Nebraska Medical Center receive support from the NIH/NCI Cancer Center Support Grant P30CA036727 (to the Eppley Cancer Center) and the Nebraska Research Initiative. The UNMC DNA Sequencing Core Facility also receives partial support from the NIH/NCRR INBRE Program P20 RR016469 Grant.
References
- Deverson EV, Powis SJ, Morrice NA, Herberg JA, Trowsdale J, Butcher GW. Rat tapasin: cDNA cloning and identification as a component of the class I MHC assembly complex. Genes and Immunity 2001. 2001;2:48. doi: 10.1038/sj.gene.6363727. [DOI] [PubMed] [Google Scholar]
- Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Farmery MR, Allen S, Allen AJ, Bulleid NJ. The role of ERp57 in disulfide bond formation during the assembly of major histocompatibility complex class I in a synchronized semipermeabilized cell translation system. Journal of Biological Chemistry. 2000;275:14933. doi: 10.1074/jbc.275.20.14933. [DOI] [PubMed] [Google Scholar]
- Garbi N, Tan P, Diehl AD, Chambers BJ, Ljunggren H-G, Momburg F, Hämmerling GJ. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nature Immunology. 2000;3:234. doi: 10.1038/79775. [DOI] [PubMed] [Google Scholar]
- Garbi N, Hammerling G, Tanaka S. Interaction of ERp57 and tapasin in the generation of MHC class I-peptide complexes. Current Opinions in Immunology. 2007;19:1. doi: 10.1016/j.coi.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Grandea AG, III, Comber PG, Wenderfer SE, Schoenhals G, Fruh K, J. J. Monaco JJ, Spies T. Sequence, linkage to H2-K, and function of mouse tapasin in MHC class I assembly. Immunogenetics. 1998;48:260. doi: 10.1007/s002510050430. [DOI] [PubMed] [Google Scholar]
- Grandea AG, III, Golovina TN, Hamilton SE, Sriram V, Spies T, Brutkiewicz RR, Harty JT, Eisenlohr LC, Van Kaer L. Impaired assembly yet normal trafficking of MHC class I molecules in tapasin mutant mice. Immunity. 2000;13:213. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- Harris MR, Lybarger L, Yu YYL, Myers NB, Hansen TH. Association of ERp57 with mouse MHC class I molecules is tapasin dependent and mimics that of calreticulin and not calnexin. Journal of Immunology. 2001a;166:6686. doi: 10.4049/jimmunol.166.11.6686. [DOI] [PubMed] [Google Scholar]
- Harris MR, Lybarger L, Myers NB, Hilbert C, Solheim JC, Hansen TH, Yu YY. Interactions of HLA-B27 with the peptide loading complex as revealed by heavy chain mutations. International Immunology. 2001b;13:1275. doi: 10.1093/intimm/13.10.1275. [DOI] [PubMed] [Google Scholar]
- Howarth M, Williams A, Tolstrup AB, Elliott T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11737. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast A, Preuss M, Winkler M, Dick TP. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nature Immunology. 2007;8:864. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- Li S, Sjögren H-O, Hellman U, Pettersson RF, Wang P. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8708. doi: 10.1073/pnas.94.16.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Paulsson KM, Sjogren H-O, Wang P. Peptide-bound major histocompatibility complex class I molecules associate with tapasin before dissociation from transporter associated with antigen processing. Journal of Biological Chemistry. 1999;274:8649. doi: 10.1074/jbc.274.13.8649. [DOI] [PubMed] [Google Scholar]
- Lybarger L, Yu YYL, Chun T, Wang C-R, Grandea AG, III, Van Kaer L, Hansen TH. Tapasin enhances peptide-induced expression of H2-M3 molecules, but is not required for the retention of open conformers. Journal of Immunology. 2001;167:2097. doi: 10.4049/jimmunol.167.4.2097. [DOI] [PubMed] [Google Scholar]
- Maryanski JL, Romero P, Van Pel A, Boon T, Salemme FR, Cerottini JC, Corradin G. The identification of tyrosine as a common key residue in unrelated H-2Kd restricted antigenic peptides. International Immunology. 1991;3:1035. doi: 10.1093/intimm/3.10.1035. [DOI] [PubMed] [Google Scholar]
- Mitaksov V, Fremont DH. Structural definition of the H-2Kd peptide-binding motif. Journal of Biological Chemistry. 2006;281:10618. doi: 10.1074/jbc.M510511200. [DOI] [PubMed] [Google Scholar]
- Myers NB, Harris MR, Connolly JM, Lybarger L, Yu YY, Hansen TH. Kb, Kd, and Ld molecules share common tapasin dependencies as determined using a novel epitope tag. Journal of Immunology. 2000;165:5656. doi: 10.4049/jimmunol.165.10.5656. [DOI] [PubMed] [Google Scholar]
- Nieto MC, Song ES, McKinney D, McMillan M, Goodenow RS. The association of H-2Ld with human β-2 microglobulin induces localized conformational changes in the α-1 and -2 superdomain. Immunogenetics. 1989;30:361. doi: 10.1007/BF02425276. [DOI] [PubMed] [Google Scholar]
- Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- Ozato K, Evans GA, Shykind B, Margulies D, Seidman JG. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:2040. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annual Review of Immunology. 1998;16:323. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimmer within the MHC class I peptide-loading complex. EMBO Journal. 2005;24:3613. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaper DR, Cresswell P. The redox activity of ERp57 is not essential for its functions in MHC class I peptide loading. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10477. doi: 10.1073/pnas.0805044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JL, Hickman-Miller HD, McIlhaney MM, Vargas SE, Purcell AW, Hildebrand WH, Solheim JC. A charged amino acid residue in the transmembrane/cytoplasmic region of tapasin influences MHC class I assembly and maturation. Journal of Immunology. 2005;174:962. doi: 10.4049/jimmunol.174.2.962. [DOI] [PubMed] [Google Scholar]
- Quesnel A, Casrouge A, Kourilsky P, Abastado JP, Trudelle Y. Use of synthetic peptide libraries for the H-2Kd binding motif identification. Peptide Research. 1995;8:44. [PubMed] [Google Scholar]
- Romero P, Corradin G, Luescher IF, Maryanski JL. H-2Kd-restricted antigenic peptides share a simple binding motif. Journal of Experimental Medicine. 1991;174:603. doi: 10.1084/jem.174.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- Smith JD, Myers NB, Gorka J, Hansen TH. Model for the in vivo assembly of nascent Ld class I molecules and for the expression of unfolded Ld molecules at the cell surface. Journal of Experimental Medicine. 1993;178:2035. doi: 10.1084/jem.178.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim JC, Carreno BM, Myers NB, Lee DR, Hansen TH. Peptide-induced rescue of serologic epitopes on class I MHC molecules. Journal of Immunology. 1995;154:1188. [PubMed] [Google Scholar]
- Stepensky D, Bangia N, Cresswell P. Aggregate formation by ERp57-deficient MHC class I peptide-loading complexes. Traffic. 2007;8:1530. doi: 10.1111/j.1600-0854.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Suri A, Walters JJ, Levisetti MG, Gross ML, Unanue ER. Identification of naturally processed peptides bound to the class I MHC molecule H-2Kd of normal and TAP-deficient cells. European Journal of Immunology. 2006;36:544. doi: 10.1002/eji.200526235. [DOI] [PubMed] [Google Scholar]
- Tan P, Kropshofer H, Mandelboim O, Bulbuc N, Hämmerling GJ, Momburg F. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. Journal of Immunology. 2002;168:1950. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Solheim JC. Analysis of MHC class I interactions with endoplasmic reticulum proteins. Methods in Molecular Biology. 2001;156:165. doi: 10.1385/1-59259-062-4:165. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Petersen JL, Vargas SE, McIlhaney MM, Bedows E, Mayer WE, Grandea AG, III, Van Kaer L, Solheim JC. The immunoglobulin-like domain of tapasin influences intermolecular interactions. Journal of Immunology. 2004;172:2976. doi: 10.4049/jimmunol.172.5.2976. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989;244:1072. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- Yu YYL, Myers NB, Hilbert CH, Harris MR, Balendiran GK, Hansen TH. Definition and transfer of a serological epitope specific for peptide-empty forms of MHC class I. International Immunology. 1999;11:1897. doi: 10.1093/intimm/11.12.1897. [DOI] [PubMed] [Google Scholar]