Abstract
The purpose of this study was to investigate the relations between regional white matter signal abnormalities (WMSA) and cognitive functioning among individuals being treated for cardiovascular risk factors and/or clinical events. Forty-one participants with cardiovascular disease underwent a comprehensive neuropsychological assessment and MRI. Total WMSAs were quantified using a semi-automated thresholding technique. Unique to this study, total WMSA volume was divided into three separate anatomically related regions: WMSA in the periventricular (PERIWMSA) region, WMSA adjacent to subcortical nuclei (SUBWMSA), and WMSA in the deep white matter (DEEPWMSA). A ratio of these measures to total cerebral brain volume was compared to cognitive measures assessing attention, executive functioning, psychomotor speed, immediate and delayed memory, language, and visuospatial functioning. PERIWMSA, SUBWMSA, and total WMSA were significantly associated with performance on measures of attention/processing speed. No other significant relationships between WMSA and cognition were noted. Secondary analyses suggested that PERIWMSA volume was increased in individuals with clinical evidence of atherosclerosis. These results emphasize the utility of studying the associations between regional WMSA and cognitive/functional performance in patients undergoing cardiovascular treatment.
Keywords: Cardiovascular disease, MRI, Hyperintensities, Cognition
Cardiovascular and cerebrovascular disease have generally been described in the literature as separate diagnostic entities despite the fact that they often share many of the same risk factors, such as hypertension, coronary artery disease, and increased age (Donnan 2003; Smithard 2003; Hypertension 2003). Recently, the literature has demonstrated that patients with cardiovascular disease also experience cognitive decline, suggesting an association between cardiovascular and cerebrovascular disease (Newman and Stygall 1999; Schultz Tremper 2004). For example, recent studies from our laboratory have demonstrated that executive dysfunction is associated with reduced cardiac output among older, non-demented patients with cardiovascular disease suggesting an association between reduced systemic blood flow and negative affects on the brain (Jefferson et al. 2006).
Abnormal findings on magnetic resonance imaging (MRI), especially the presence of white matter signal abnormalities (WMSA), have also been shown to be related to vascular risk factors (for a review see Kuo and Lipsitz 2004). Though the exact pathological etiology of WMSA on MRI is unknown, ischemic and vascular mechanisms have been implicated in both clinical and postmortem studies, especially in the presence of hypertension (Korf et al. 2004; Raz et al. 2003a; Soderlund et al. 2003) and atherosclerosis (Pico et al. 2002; Rosano et al. 2005b). Clinically, WMSA are often associated with increased morbidity (Rosano et al. 2005a), stroke (Wen and Sachdev 2004), and cognitive decline (Mosley et al. 2005). For instance, increased WMSA volume is associated with reduced attention (Paul et al. 2005), slower speed of processing (van den Heuvel et al. 2006), and executive dysfunction (Marshall et al. 2006; Oosterman et al. 2004; Tullberg et al. 2004). Taken together, these data emphasize the utility of studying cardiac risk factors and cognition, particularly among an aging cohort, for which cognitive impairment has emerging relevance (i.e., aging cardiac patients).
Thus, the purpose of this pilot study was to examine the relationship between WMSA and cognitive function in a cohort of older non-demented cardiovascular patients currently undergoing treatment for a variety of cardiovascular risk factors (i.e., hypertension, hypercholesterolemia, diabetes) and/or overt cardiovascular events (i.e., stent placement, myocardial infarction, cardiovascular bypass grafting). We believe this cohort of patients who have multiple risk factors for cognitive decline recruited from cardiovascular clinic cohort without current overt cognitive dysfunction represents an understudied population and as such might provide additional insight into the evolution and progression of WMSA and cognitive decline in older patient populations. We hypothesized that the accumulation of WMSA adjacent to the subcortical nuclei would be associated with cognitive functions mediated by frontal-subcortical circuits, including executive functioning and attention (Gunning-Dixon and Raz 2003; Raz et al. 2003b). We did not expect cognitive domains that are predominantly mediated by cortical gray matter, such as language and immediate and delayed memory, to be associated with WMSA variables. Secondarily, we investigated the relationship between regions of WMSA and cardiovascular severity, as we expected that increasing cardiovascular severity would be related to increased WMSA volume.
Methods
Clinical sample
Participants consisted of a subset of individuals participating in a prospective study examining the effects of cardiovascular disease on brain functioning in the elderly (55 years and older). Forty-one patients (M=27, F=14) from a larger sample of patients being clinically treated for cardiovascular disease (actively managed or monitored in a large hospital cardiovascular clinic) completed a baseline evaluation including neurocognitive assessment and MRI. Sample demographic data and medical history variables are presented in Table 1. Participants were screened and excluded for (1) neurological disease history (e.g., Alzheimer's disease, stroke, seizure disorder, head injury with loss of consciousness >10 min); (2) major psychiatric illness such as schizophrenia or bipolar disorder; (3) current substance abuse or history of substance abuse treatment; (4) presence of other systemic disease that limited life expectancy to less than 1 year (e.g., terminal cancer); and (5) MRI contraindications (i.e., pacemakers, metal implants, etc.). The association between WMSA and cognition was examined for the entire group of 41 participants.
Table 1.
Sample characteristics
| Total (n=41) | Risk factor only (n=19) | Clinical atherosclerosis + risk factor (n=22) | t value or chi-squared | p value | |
|---|---|---|---|---|---|
| Age (years) | 71.15 (7.43) | 71.47 (7.46) | 70.86 (7.56) | 0.26 | 0.80 |
| Education (years) | 14.03 (2.20) | 13.44 (2.04) | 14.50 (2.26) | −1.54 | 0.13 |
| Sex (% female) | 34% | 58% | 14% | 8.88 | 0.003 |
| Systolic blood pressure (mm/HG) | 130.40 (17.97) | 135.11 (19.29) | 126.87 (16.52) | 1.38 | 0.18 |
| Diastolic blood pressure (mm/HG) | 67.34 (9.24) | 68.43 (9.59) | 66.53 (9.13) | 0.60 | 0.56 |
| HTN | 75.6% | 66.6% | 80.7% | 1.03 | 0.31 |
| Hypercholesterolemia | 43.9% | 46.6% | 42.3% | 0.07 | 0.79 |
| Diabetes | 12.2% | 10.5% | 13.6% | 0.68 | 0.41 |
| Smoking | 31.7% | 26.3% | 36.4% | 0.28 | 0.60 |
| CABG | 36.6% | 0% | 57.7% | 13.65 | <0.001 |
| MI | 46.3% | 0% | 73.1% | 20.43 | <0.001 |
| Stent placement | 12.2% | 0% | 19.2% | 3.28 | 0.07 |
| Angioplasty | 17.1% | 0% | 26.9% | 4.87 | 0.03 |
| MMSE (0–30) | 28.90 (1.16) | 28.74 (1.24) | 29.05 (1.09) | −0.84 | 0.40 |
| Total WMSA ratio | 0.61 (0.99) | 0.31 (0.24) | 0.79 (1.21) | −1.09 | 0.28 |
| DEEPWMSA ratio | 0.33 (0.78) | 0.09 (0.14) | 0.55 (1.03) | −2.37 | 0.03 |
| PERIWMSA ratio | 0.27 (0.25) | 0.18 (0.14) | .36 (.31) | 0.89 | 0.35 |
| SUBWMSA ratio | 0.01 (0.03) | 0.008 (0.02) | 0.01 (0.03) | 0.04 | 0.73 |
For the secondary analysis assessing WMSA and cardiovascular disease severity, participants were dichotomized into two groups (1) participants with cardiovascular risk factors only (hypertension, hypercholesterolemia, diabetes and/or smoking, n=19) and (2) patients with cardiovascular risk factors and clinical evidence of atherosclerosis as defined by a positive clinical history of one or more of the following events; coronary artery bypass grafting (CABG), myocardial infarction (MI), cardiac arrest, angioplasty, and/or stent placement (n=22). Medical history information was based on patient self-report during a structured interview where patients were specifically asked about various cardiovascular risk factors or events. Additionally, where possible we confirmed patient responses through medical chart review of previous diagnostic codes and/or examination of current medicine regimens. See Table 1 for patient demographic and clinical information including incidence of risk factors and cardiovascular events.
Neuropsychological assessment
All participants completed a neuropsychological protocol to assess general cognitive status (Mini-mental state examination, MMSE; Folstein et al. 1975) and the following cognitive domains: attention, executive function, motor speed, immediate and delayed memory, language, and visuospatial processing. Individual tests were assigned to each domain using traditional groupings (Lezak 1995) though it should be noted that performance on tests within each domain was reasonably related (average r value within domains >0.40). The attention domain included Trail Making Test Part A (TMA), Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span and Symbol Digit subtests. The executive functioning domain included the Stroop color–word interference trial (Stroop), Trail Making Test Part B (TMB), and Controlled Oral Word Association (FAS). The motor speed domain included the Grooved Pegboard dominant hand (GPB). The memory domain, assessing immediate and delayed memory, included the California Verbal Learning Test (CVLT) and the Rey–Osterrieth Complex Figure Test (ROCF). The language domain included the Boston Naming Test (BNT) and Category Fluency (i.e., animal naming). The visuospatial domain was assessed with the Hooper Visual Organization Test (HVOT), the ROCF copy, and WAIS-III Block Design subtest. Additionally, each of the patients was administered the Beck Depression Inventory to assess current depressive symptoms. For a review of these tests see Lezak (1995). All tests were administered and scored by a trained psychometrician using standard procedures. To facilitate data reduction (given the small sample size), the dependent variable for each individual test measure was converted to a z-score using published age-, sex-, and/or education-matched norms and then an average z-score was calculated for each domain. This was done by summing the z-scores from each individual tests within the domain and dividing the sum by the number of tests in the domain. This average z-score served as the dependent variable for the remaining analyses effectively reducing the number of variables from 14 to six reducing the number of statistical procedures applied.
Brain MRI and WMSA quantification
All participants were imaged using the same Siemens Symphony 1.5 T unit. A standard imaging protocol including axial T1-, T2-, and FLAIR weighted (FOV=24.7; Matrix=192X256; TR/TE= 6,000/105; 5-mm thick slices/2 mm gap) images were obtained for each participant. The FLAIR images were used to quantify WMSA utilizing semi-automated threshold methods. For each participant, WMSA were quantified separately for three neuroanatomical regions: (1) WMSA adjacent to the boundary of the lateral ventricles (PERIWMSA), (2) WMSA adjacent to subcortical gray matter nuclei [i.e., basal ganglia and thalamus (SUBWMSA)], and (3) WMSA observed outside of the preceding defined boundaries (i.e., primarily white matter of the corona radiata, DEEPWMSA). The raw FLAIR imaging data was imported into the commercially available Mayo Clinic software program Analyze 5.0® (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN, USA) where the skull was manually stripped leaving only the brain parenchyma. Second, using the threshold tool, we manually isolated WMSA from surrounding parenchyma for each imaging slice by adjusting the range of histogram values to include pixel intensity values consistent with signal abnormalities. Each slice was visually reviewed by trained raters and edited as needed. Additionally, using the neuroanatomical landmarks described above, WMSA's were separated and labeled according to the three regions (see Fig. 1). The total number of pixels representing WMSA from each neuroanatomical region was summed across the slices separately to calculate the WMSA volume. We used two raters, who achieved a high intra- and inter-rater reliability (>0.90 and >0.85 respectively) regardless of region examined.
Fig. 1.
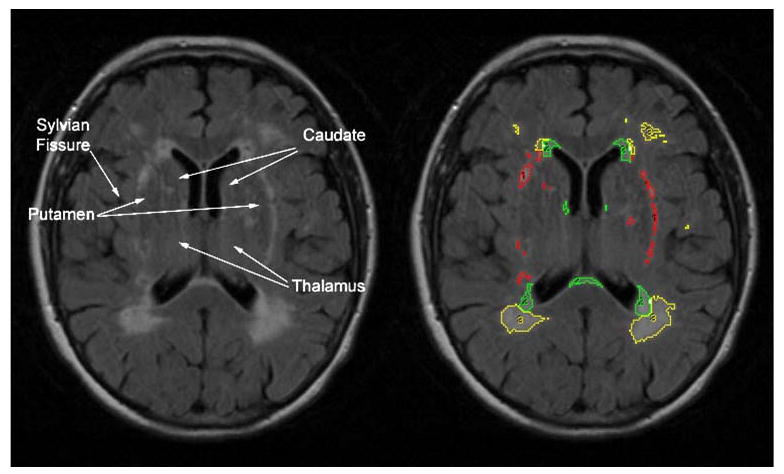
Illustration of MRI method. PERIWMSA were defined as WMSA immediately adjacent to the lateral ventricles within 4 mm. SUBWMSA were defined by the following neuroanatomical boundaries: The superior boundary was defined as the axial slice where the lateral ventricles and the body of the caudate were first visualized; the inferior boundary was defined as the slice where the thalamus and third ventricle were no longer visible; the lateral boundary included the Sylvian fissure and/or the insular cortex; the anterior boundary was the most anterior tip of the lateral ventricles; and the posterior boundary was the most posterior tip of the trigome of the lateral ventricles. DEEPWMSA were defined as those remaining WMSA not included in the aforementioned boundaries containing PERIWMSA or SUBWMSA and were primarily relegated to the corona radiata. Intra- and inter-rater reliability for volume calculation was consistently greater than 0.85 for all WMSA areas. Yellow (3)=DEEPWMSA, green (2)= PERIWMSA, and red (1)=SUBWMSA
Total brain volume (TBV) for each participant was calculated similarly using threshold histogram values that were consistent with brain parenchyma. TBV served as a correction factor for each of the three WMSA values providing a ratio of WMSA to TBV. To ease the interpretation, we calculated the percentage of WMSA in the brain by multiplying each ratio by 100 and these percentages were the primary imaging variables examined [formula = (WMSA pixel total/TBV pixel total) × 100). Applying TBV as a correction factor to the WMSA provides a ratio or percentage (as calculated) of WMSA load relative to the total amount of brain tissue for each participant and reflects the degree of total brain volume impacted by WMSA.
Data analyses
Descriptive statistics were calculated for all three MRI regions of interest and cognitive indices. Because the three regional WMSA ratio data were positively skewed, the data were log-transformed and inspected for possible outliers. Visual and quantitative inspection of the transformed data indicated more normally distributed data with no significant outliers. A zero-order correlation matrix was generated between the total WMSA, the three regional WMSA variables, and the six cognitive domain scores to assess the association between the log-transformed WMSA measures and neuropsychological domain performances.
A secondary analysis, using four independent samples t tests, assessed between-group differences (risk factors only vs. clinically apparent atherosclerosis) for each of the three regional WMSA and the total WMSA percentages. Finally, independent samples t tests were used to assess between-group differences for neuropsychological domains. A priori significance was set at alpha=0.05 for all analyses.
Results
Descriptive statistics for WMSA and cognition
Demographic and clinical characteristics are presented in Table 1 for the entire sample as well as the two subgroups utilized in the secondary analyses (risk factors only vs. clinically apparent atherosclerosis). Between-group comparisons revealed no significant differences for age (t=0.26, p= 0.80), education (t=−1.54, p=0.13), MMSE performance (t=−0.84, p=.40), current mean systolic blood pressure (t= 1.38, p=0.18) or diastolic blood pressure (t=0.60, p=0.56). There was a between group difference for gender ratios with the risk factor only group having nearly three times as many females as the clinically evident atherosclerosis group (χ2=8.88, p=0.003). No between-group differences were noted for medical history variables such as hypertension (χ2=0.08, p=0.78), hypercholesterolemia (χ2=1.94, p= 0.16), tobacco use (χ2=0.28, p=0.60), diabetes (χ2=0.68, p=0.41) or depression (χ2=0.04, p=0.85).
Regional WMSA ratios and neuropsychological functioning
Descriptive statistics for the three regional and total WMSA is presented in Table 1. On average, DEEPWMSA appeared to occupy more volume of the brain than PERIWMSA and SUBWMSA though this measure also had the most variability. More participants had measurable PERIWMSA (40/41) than DEEPWMSA (34/41) and SUBWMSA (13/41).
Table 2 contains a correlation matrix for the regional WMSA values and neuropsychological domain scores. DEEPWMSA, PERIWMSA and SUBWMSA were all inversely associated with the attention domain (r=−0.30, p<0.05, r=−0.43, p<0.01; r=−0.48, p<0.03, respectively). SUBWMSA was also inversely associated with the executive function domain (r=−0.36, p=0.05) and PERIWMSA was inversely associated with the motor domain (r=−0.28, p<0.05). Though not significant, medium effect sizes were noted between SUBWMSA and the visuospatial and immediate memory domains (r=−0.32 and 0.30 respectively).
Table 2.
Correlation matrix between WMSA regions and neuropsychological domains
| Total WMSA | DEEPWMSA | PERIWMSA | SUBWMSA | |
|---|---|---|---|---|
| Attention/information processing domain | −0.58** | −0.45* | −0.65** | −0.47** |
| Executive domain | −0.39* | −0.30 | −0.37* | −0.35* |
| Language domain | 0.14 | 0.27 | 0.007 | −0.10 |
| Visuospatial domain | −0.21 | −0.22 | −0.24 | −0.34 |
| Motor domain | −0.04 | 0.13 | −0.24 | −0.07 |
| Immediate memory domain | 0.19 | 0.32 | −0.11 | 0.26 |
| Delayed memory domain | 0.01 | 0.07 | −0.15 | −0.06 |
p<0.05
p<0.01
Cardiovascular disease severity, WMSA, and cognition
Between-group comparisons revealed a significant difference between the clinically apparent atherosclerosis and risk factor only groups for PERIWMSA (t=−2.37, p<0.03). Only the language and immediate memory domains were significantly different between the two groups (t=−2.50, p<0.02; t=−2.440, p=0.02 respectively) with the clinically apparent atherosclerosis group performing worse than the risk factors only group. However, performance for the language and immediate memory domains remained within the average range (z score=−1 to 1) of function for each group compared to normative samples (z=0.61 and −0.10; z=0.66 and −0.18 respectively). No other significant differences between the groups were noted for the remainder of the cognitive domains.
Discussion
Many studies have examined regional WMSA; however, to our knowledge, this study is the first to examine the relationship between WMSA adjacent to the subcortical nuclei and cognitive function. Findings from this study demonstrated regional associations between total WMSA, PERIWMSA, SUBWMSA, DEEPWMSA, and attention/processing speed performance among older patients with cardiovascular disease. Our findings are consistent with the current literature documenting an association between WMSA in and around the lateral ventricles with attention/information processing speed among other patient populations. However, this study extends previous findings by demonstrating a similar relationship between WMSA in and around subcortical nuclei and measures of attention/information processing speed as well as unique association between SUBWMSA and measures of executive function. This association likely results from disruption of both long projective and associative pathways that are predominately found in the internal, external, and extreme capsules.
It is also noteworthy that SUBWMSA occupied relatively less brain volume than PERIWMSA while producing nearly equivalent associations. This finding may indicate the strategic nature of lesions in these areas whereby large numbers of projective fibers from various areas of the brain are impacted by much smaller lesions. This association also emphasizes the clinical importance of such lesions and the need to understand what medical/pathological factors might contribute to the evolution and progression of WMSA in subcortical areas. This finding is consistent with functional neuroanatomy as these cognitive domains depend on the effective integration of subcortical nuclei, especially the caudate and putamen, with frontal areas of the brain.
Secondary analyses examining the impact of vascular disease severity on WMSA location suggest significant differences between patients who have clinically apparent atherosclerotic disease and those with risk factors only for measure of PERIWMSA. There was also a trend toward significant increase in DEEPWMSA for patients with clinically apparent atherosclerosis. This finding is consistent with the literature as WMSA are more prevalent within populations with know vascular risk factors. What is disappointing is the lack of significance with regards to SUBWMSA between the two groups. This could be related to the small number of participants in the sample with measurable WMSA in these areas of the brain or the heterogeneity of the sample. However, this preliminary finding may suggest differential progression of WMSA that could be examined more thoroughly in longitudinal population based studies, as studies that include a large number of participants might demonstrate some clinical difference between patients with and without SUBWMSA.
Examination of the cognitive domains between the groups also revealed significant differences for the language and immediate memory domains. However, as noted, the average performance for these two domains remained well within the average range of function compared to normative data for each test. Though performance across the domains was generally worse in the clinically evident atherosclerosis group, these findings are difficult to interpret given the fact that performance still remains within the average range of function regardless of domain examined.
Though there are several limitations to this study, these results do suggest several possible directions that future research could take to examine the clinical and functional utility of similar WMSA distributions. Studies investigating group difference between patients with preclinical disease such as hypertension alone versus overt cardiovascular disease or patients with myocardial infarction versus the possible confounding effects of cardiopulmonary bypass may yield additional insights into regional brain change/functional relationships. The findings from this study also demonstrate a need for additional research using longitudinal methods. These types of studies are likely to yield promising insights into the evolution and progression of cerebrovascular injury associated with cardiovascular risk factors. Additional lines of research investigating WMSA location using vascular maps or atlases may also prove to be useful as there may be unique effects of cardiovascular disease on different regions due to vascular architecture, alterations in perfusion, or a combination of the two. Also, more sensitive neuroimaging measures of the integrity of white matter pathways, such as diffusion tensor imaging, may allow researchers and clinicians to develop early imaging markers that would allow for better prediction of cognitive outcome as well as provide potential neuroanatomical targets for medical treatments of cardiovascular disease.
Acknowledgments
This manuscript was supported in part by the following grant: K23-MH073416 (D.F.T.), F32-AG022773 (A.L.J.), K23-AG030962 (A.L.J.), K23-MH065857 (R.P.H.), F32-HL74568 (J.G.), F32-AG024708 (A.M.B.), R01AG017975 (R.A.C.), and P30-AG013846 (D.F.T. and A.L.J.).
Contributor Information
David F. Tate, Department of Radiology, Center for Neurological Imaging, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Neurology, Alzheimer's Disease Center, Boston University School of Medicine, Boston, MA, USA.
Angela L. Jefferson, Department of Neurology, Alzheimer's Disease Center, Boston University School of Medicine, Boston, MA, USA
Adam M. Brickman, Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Department of Neurology, College of Physicians and Surgeons, Columbia University, New York, NY, USA
Karin F. Hoth, Department of Medicine, Division of Psychosocial Medicine, National Jewish Health, Denver, CO, USA Department of Psychiatry, University of Colorado Denver, Denver, CO, USA.
John Gunstad, Department of Psychology, Kent State University, Kent, OH, USA.
Kathryn Bramley, Department of Radiology, Center for Neurological Imaging, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Robert H. Paul, Department of Psychology, Behavioral Neuroscience, University of Missouri-St. Louis, St. Louis, MO, USA
Athena Poppas, Section of Cardiology, Rhode Island Hospital, Warren Alpert School of Medicine at Brown University, Providence, RI, USA.
Ronald A. Cohen, Department of Psychiatry and Human Behavior, Butler Hospital, Warren Alpert School of Medicine at Brown University, Providence, RI, USA
References
- Donnan G. Stroke: prediction, prevention, and outcome. The Lancet Neurology. 2004;3(1):9. doi: 10.1016/S1474-4422(03)00609-4. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/S0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hypertension WE. Nursing Standard. 2003;18(13):45–53. doi: 10.7748/ns2003.12.18.13.45.c3517. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of Aging. 2006;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44(1):29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz L. Cerebral white matter changes and geriatric syndromes: is there a link. Journal of Gerontology. 2004;59(8):818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. 3rd. New York: Oxford University Press; 1995. [Google Scholar]
- Marshall GA, Hendrickson R, Kaufer DI, Ivanco LS, Bohnen NI. Cognitive correlates of brain MRI subcortical signal hyperintensities in non-demented elderly. International Journal of Geriatric Psychiatry. 2006;21(1):32–35. doi: 10.1002/gps.1419. [DOI] [PubMed] [Google Scholar]
- Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64(12):2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- Newman S, Stygall J. Changes in cognition following cardiac surgery. Heart (British Cardiac Society) 1999;82(5):541–542. doi: 10.1136/hrt.82.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman JM, Sergeant JA, Weinstein HC, Scherder EJ. Timed executive functions and white matter in aging with and without cardiovascular risk factors. Reviews in the Neurosciences. 2004;15(6):439–462. doi: 10.1515/revneuro.2004.15.6.439. [DOI] [PubMed] [Google Scholar]
- Paul RH, Gunstad J, Poppas A, et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovascular Diseases (Basel, Switzerland) 2005;20(2):129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico F, Dufouil C, Levy C, et al. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the EVA-MRI cohort. Cerebrovascular Diseases (Basel, Switzerland) 2002;14(2):109–115. doi: 10.1159/000064741. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue K, Acker J. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003a;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003b;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. Journal of the American Geriatrics Society. 2005a;53(4):649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Naydeck B, Kuller LH, Longstreth WT, Jr, Newman AB. Coronary artery calcium: associations with brain magnetic resonance imaging abnormalities and cognitive status. Journal of the American Geriatrics Society. 2005b;53(4):609–615. doi: 10.1111/j.1532-5415.2005.53208.x. [DOI] [PubMed] [Google Scholar]
- Schultz Tremper R. Cognitive deficits following cardiac surgery: a brief review of the literature. Dimensions of Critical Care Nursing. 2004;23(2):93–95. doi: 10.1097/00003465-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Smithard D. Management of stroke: acute, rehabilitation and long-term care. Hospital Medicine (London, England) 2003;64(11):666–672. doi: 10.12968/hosp.2003.64.11.2349. [DOI] [PubMed] [Google Scholar]
- Soderlund H, Nyberg L, Adolfsson R, Nilsson LG, Launer LJ. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex. 2003;39(45):1093–1105. doi: 10.1016/S0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(2):149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Sachdev PS. Extent and distribution of white matter hyperintensities in stroke patients: the Sydney Stroke Study. Stroke. 2004;35(12):2813–2819. doi: 10.1161/01.STR.0000147034.25760.3d. [DOI] [PubMed] [Google Scholar]


