Abstract
Objective
To test the hypothesis that the atrophy rate measured from serial magnetic resonance imaging (MRI) studies is associated with time to subsequent clinical conversion to a more impaired state in both cognitively normal elderly subjects and in subjects with amnestic mild cognitive impairment (MCI).
Methods
Ninety one normal elderly and 72 patients with amnestic MCI were identified from the Mayo Alzheimer's Disease Research Center and Alzheimer's Disease Patient Registry who met inclusion criteria. Atrophy rates of four different brain structures - hippocampus, entorhinal cortex, whole brain, and ventricle - were measured from a pair of MRI studies separated by one to two years. The time of the second scan marked the beginning of the clinical observation period.
Results
During follow-up, 13 normal patients converted to MCI or AD while 39 MCI subjects converted to AD. Among those normal at baseline, only larger ventricular annual percent volume change (APC) was associated with a higher risk of conversion (hazard ratio for a 1-SD increase 1.9, p = 0.03). Among MCI subjects both greater ventricular volume APC (hazard ratio for a 1-SD increase 1.7, p<0.001) and greater whole brain APC (hazard ratio for a 1-SD increase 1.4, p = 0.007) increased the risk of conversion to AD. Both ventricular APC (hazard ratio for a 1-SD increase 1.32, p = 0.009) and whole brain APC (hazard ratio for a 1-SD increase1.59, p = 0.001) provided additional predictive information to covariate-adjusted cross-sectional hippocampal volume at baseline about the risk of converting from MCI to AD.
Discussion
Higher whole brain and ventricle atrophy rates 1-2 years prior to baseline are associated with an increased hazard of conversion to a more impaired state. Combining a measure of hippocampal volume at baseline with a measure of either whole brain or ventricle atrophy rates from serial MRI scans provides complimentary predictive information about the hazard of subsequent conversion from MCI to AD. However, overlap among those who did versus those who did not convert indicate that these measures are unlikely to provide absolute prognostic information for individual patients.
Imaging is increasingly recognized as a potentially useful way to measure disease progression as well as disease severity in Alzheimer's disease (AD). Much of the effort in imaging research in AD is centered on validating various imaging measures as biomarkers of disease stage and progression. Acceptance of imaging as a valid biomarker of AD requires convergence of information from multiple types of studies, from multiple research groups, and in a variety of populations. Our approach and that of others has been to use the clinical categorization of subjects cross-sectionally or the clinical course of well-characterized subjects over time in longitudinal studies as the gold standard. Imaging measures are then compared against the clinical gold standard. The types of studies that have been performed in this field can be considered in four general categories. These are cross-sectional case-control comparisons; longitudinal studies in which a single imaging measurement is used to predict subsequent clinical course; and, longitudinal studies in which an imaging study and a clinical assessment are coupled at two or more time points and the rate of change in imaging is correlated with contemporaneous assessments of clinical change. A fourth type of study design is the one employed in this paper and described below.
In the present study, rates of atrophy of four different brain structures were measured from serial magnetic resonance imaging (MRI) studies over a 1-2 year interval. The clinical status of each subject at the time of the second scan was the start point for longitudinal assessment of changes in clinical status. We tested the hypothesis that the atrophy rate measured from serial MRI studies is associated with time to subsequent clinical conversion to a more impaired state among cognitively normal elderly subjects and among subjects with amnestic mild cognitive impairment (MCI). This analysis was performed with four different measures of brain atrophy rate —hippocampus, entorhinal cortex (ERC), whole brain, and ventricle. For each measure, we assessed whether the atrophy rate added significant information about the hazard of conversion beyond information available from a single cross-sectional volumetric measurement.
Methods
Subjects
Ninety one normal elderly and 72 patients with amnestic MCI were identified from the Mayo Alzheimer's Disease Research Center (ADRC), and Alzheimer's Disease Patient Registry (ADPR) who met inclusion criteria (Table 1) (1). This was a study of two parallel cohorts (baseline normal and baseline MCI), and therefore normal and amnestic MCI subjects were not individually matched. All MCI subjects met criteria for amnestic MCI as defined by Petersen et a (2-4). Approximately two-thirds of the subjects in this study were drawn from a community source. MCI subjects were identified as having potential memory impairment during routine general medical visits. This approach results in an older MCI cohort than that employed in many other imaging studies which is to draw on subjects who have been referred (either by a physician or by self) to a memory clinic.
Table 1.
Patient characteristics
| Characteristic | Normal | MCI |
|---|---|---|
| No. of patients | 91 | 72 |
| No. of females (%) | 55 (60) | 31 (43) |
| Mean (SD) age at second scan, years | 81.9 (7.5) | 80.0 (7.7) |
| Mean (SD) education, years of schooling at baseline | 13.5 (3.0) | 13.7 (3.6) |
| Median (IQR) MMSE score at time of second scan | 29 (28, 30) | 26 (25, 28) |
| Mean (SD) time between scans, years | 1.4 (0.3) | 1.3 (0.4) |
| No. of observed conversions* | 13 | 39 |
| Median (95% CI) time to conversion, years | ---† | 3.2 (2.1, 3.7) |
| Median (range) length of conversion-free follow-up‡, years | 3.4 (0.9, 10.9) | 1.9 (0.9, 9.0) |
, This quantity cannot be estimated because the survivorship curve did not drop below 0.5 due to the small number of events.
, Follow-up time defined as time from second scan to conversion, censoring, or last follow-up among those who remained stable.
Cognitively normal and amnestic MCI subjects (2-4) were selected who had undergone serial MRI studies at a 1-2 year interval and subsequently had additional approximately annual clinical follow-up. We used the time of scan 2 as the baseline point when assessing the time to clinical conversion. Subjects had to be clinically unchanged between the two MRI scans used to calculate atrophy rate prior to baseline. That is, cognitively normal subjects must have remained within the normal category and MCIs must have remained within the MCI category between the two MRI studies. Subjects were excluded from the study if they had secondary clinical diagnoses that could potentially interfere with the volumetric measurement process or that introduced ambiguity about the patient's clinical progression. For example, subjects with renal failure or severe congestive heart failure were excluded, as were subjects who exhibited symptoms of dementias other than AD.
MRI Methods
T1-weighted 3D volumetric SPGR sequence scans were utilized for all measurements. An identical scan acquisition protocol was used for all scans. Different scanners were used, but all were GE Signa 1.5 with body resonance module gradient sets and transit-receive single channel head coils. And, all scanners undergo a standardized quality control calibration procedure every morning which monitors geometric fidelity over a 200 mm volume along all 3 cardinal axes, signal to noise ratio, and transmit gain. Four atrophy rate measurements were performed in all subjects: hippocampus, ERC, whole brain, and ventricle (5). Image processing steps were performed by a research associate who was blinded to all clinical information. Rates of hippocampal and ERC atrophy between scan 1 and scan 2 were measured by first spatially registering scan 2 to scan 1. The temporal lobes for both scans were then sub-volumed, and all clinical and chronologic ordering information was removed from the image files prior to manual tracing. Hippocampus and ERC volume were measured by manually tracing their anatomic boundaries for each image slice (six image slices for ERC) sequentially from posterior to anterior (6). When tracing, scan 1 and 2 were viewed side by side. Spatially registering and simultaneously viewing each scan pair allowed the research associate to make identical boundary decisions when tracing serial scans. This reduces operator dependent variability in subjective decisions about hippocampal and ERC boundaries. Right and left hippocampal and ERC values were summed. Whole brain and ventricular atrophy rates were measured with the boundary shift integral (BSI) technique (7-9). Following spatial and intensity normalization of scan 2 to scan 1, intensity differences between the two scans at the brain-CSF boundary are used to compute change in volume. The whole brain atrophy rate reflects shrinkage of the brain on scan 2 relative to scan 1 - from out to in at the cortical surface and from in to out at the ventricular surface. The ventricular atrophy rate was derived by creating a binary mask for each subject that selectively extracted ventricular change. The binary mask was an approximate area overlaying the ventricles within which the BSI was measured. Change in volume between the two serial MRI scans was expressed as annualized percent change (APC) using the formula
Statistical Methods
Univariate Association Between Conversion Risk and Atrophy Rate
We used time-to-event methods to evaluate whether APC in the volume measure of interest was associated with time to clinical conversion. Censoring was done at last follow-up or upon evidence of a diagnosis that could complicate the accurate determination of whether the patient converted clinically. For example, patients who were amnestic MCI at baseline and subsequently experienced a hemispheric infarction were censored at the time of infarction. Hazard ratios, 95% confidence intervals and one-sided Wald tests from a Cox proportional hazards model were used to evaluate whether APC was associated with time to clinical conversion. One-sided tests were used because we hypothesized a priori that higher rates of brain atrophy would only increase risk of conversion. Tests for non-proportional hazards were conducted for each model and were non-significant in each case. Hazard ratios for a 1-SD decrease in APC were used to quantify the magnitude of the effect. (In the case of ventricle, hazard ratios were based on a 1-SD increase.) Due to the limited number of conversion events among normals and because it was found to be unnecessary among MCIs, we did not adjust the models for demographic covariates. Although we analyzed APC as a continuous variable, in order to graphically illustrate a result of the proportional hazards models we divided the MCI cohort into tertiles based on the ventricular APC. We present separate Kaplan-Meier curves for the three (artificial) MCI groups. The choice of tertiles as opposed to two or four groups was arbitrary – but it did mirror the approach used in an earlier publication on hippocampal volume as a predictor of conversion (16).
Adjustment of Cross-Sectional Measures in Mci
A secondary question of interest was whether APCs provide additional predictive information beyond that of a single cross-sectional volumetric measurement. Although APCs automatically control for variability in baseline volume due to age, total intracranial volume (TIV) and sex, cross-sectional volumes do not. Thus, we adjusted cross-sectional volumes at scan 2 among MCI patients by first fitting a least squares regression model among normals who had not converted during follow-up. Volume at scan 1 was the dependent variable and age, TIV, and sex were the independent variables. Our method was to fit a full model with these three covariates and to remove terms one at a time that did not approach significance based on a Wald test using p<0.20 for inclusion in the model. The relatively high p-value threshold was used because of the a priori knowledge that these variables were important factors in individual brain volume variability. After main effect terms were excluded from the model as necessary, higher order terms and interactions were tested and included in the model using a more strict criteria (p<0.05).
Once the regression equation describing the relationship between volume and the covariates among normals was estimated, we used it to obtain a predicted cross-sectional volume for each MCI patient based on his or her covariates. An adjusted cross-sectional volume was defined as the difference between the observed volume at scan 2 and the predicted volume. This adjusted volume can be thought of as measuring the MCI patient's deviation in volume from that expected under normal aging, taking into account age, TIV, and sex as necessary. We have used a related methodology to obtain W scores in the past (10). For clarity, in this report we refer to these covariate-adjusted measures of volume at scan 2 as cross-sectional measures, which should be distinguished from the rate of volume change (APC) calculated between scan 1 and scan 2. Due to the limited number of conversion events among the normal cohort, cross-sectional volumes were analyzed only in MCI subjects. For each of the four brain structures under consideration, we assessed whether cross-sectional volume was associated with time to conversion from MCI to AD using hazard ratios, 95% CIs, and one-sided Wald tests from a Cox model.
Bivariate Models of Conversion Risk in Mci
As noted above, a secondary question of interest was whether APCs provide additional predictive information about the hazard of conversion beyond that of a single cross-sectional volumetric measurement. We fit four bivariate Cox models consisting of cross-sectional volume at scan 2 and APC between scan 1 and scan 2; one each for hippocampus, ERC, whole brain, and ventricle. As will be seen, baseline adjusted hippocampal volume and APC of both ventricle and whole brain proved to be highly significant predictors of conversion in MCI. Therefore as a final step we fit bivariate Cox models with cross-sectional hippocampal volume plus ventricular APC, and with cross-sectional hippocampal volume plus whole brain.
Results
The mean interval between the two MRI exams used to calculate rates was 1.4 years (range, 0.9 to 2.0 years) in elderly normal subjects and 1.3 years (range, 0.7 to 2.6 years) in MCI subjects (Table 1). As would be expected, rates of atrophy for the MCI cohort were greater that those of the normal elderly cohort for all four MRI measures (Table 2). During follow-up, 39 MCI subjects converted to AD while 13 normal subjects converted (11 to MCI and two directly to AD) (Table 1). We treated all 13 normal converters as a single group. The two normal-to-AD converters were not notably different from the 11 normal-to-MCI converters in age, or baseline MMSE score. We assume that everyone who progresses from normal to AD passes through an MCI phase. The fact that two of the 13 normal-converter subjects did not have a scheduled clinical assessment during the time they were in the MCI phase is most likely a chance occurrence due to the timing of the clinical assessments with respect to the clinical course in that particular person. Among the normal cohort only larger ventricle volume APC was associated with a higher hazard of conversion (HR (CI) of 1.88 (1.0, 3.6), p = 0.026) (Table 3).
Table 2.
Annual percent change (APC) by group at baseline
| Normal | MCI | |||
|---|---|---|---|---|
| Mean (SD) | Min., Max. | Mean (SD) | Min., Max. | |
| Hippocampus | -1.7 (1.4) | -4.8, 1.9 | -3.3 (2.7) | -11.7, 0.0 |
| ERC | -5.0 (3.6) | -13.7, 3.6 | -7.0 (4.3) | -21.0, 2.9 |
| Whole brain | -0.5 (0.7) | -2.2, 1.5 | -0.7 (1.0) | -6.0, 1.7 |
| Ventricle | 2.4 (2.0) | -4.6, 6.4 | 3.3 (2.3) | -1.1, 8.9 |
Table 3.
Results from Cox proportional hazards model of time to conversion among normal patients (n=91).
| HR (95% CI) | P | |
|---|---|---|
| Hippocampus APC | 0.86 (0.5, 1.5) | 0.7 |
| ERC APC | 1.12 (0.7, 1.9) | 0.34 |
| Whole brain APC | 1.21 (0.7, 2.2) | 0.27 |
| Ventricle APC | 1.88 (1.0, 3.6) | 0.026 |
Note: There were 13 observed conversions among the normal cohort.
Follow-up starts with second scan.
HR = Hazard Ratio for a 1-standard deviation decrease in annual percent change in volume (except for ventricle estimate which is based on a 1-standard deviation increase).
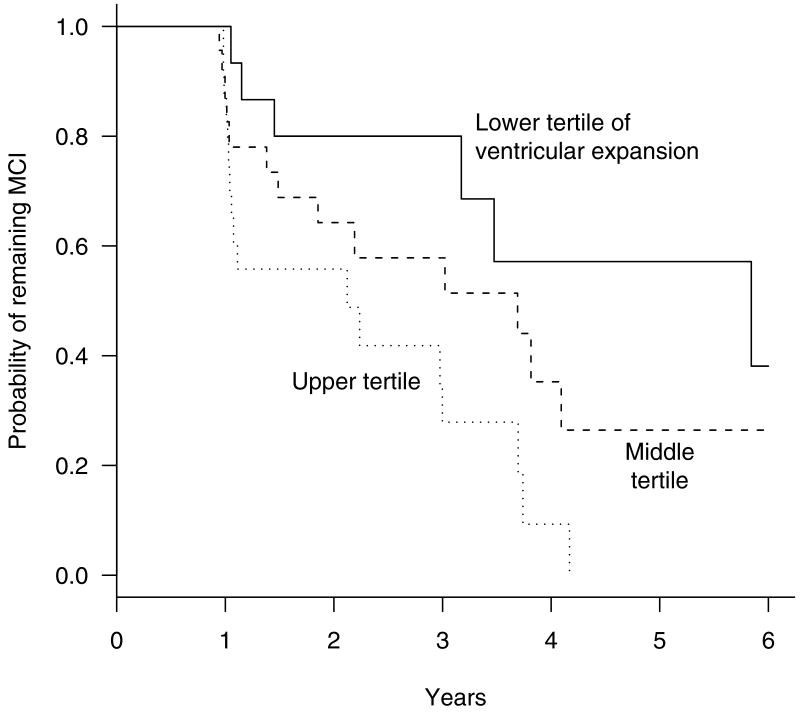
Among the MCI cohort both larger negative whole brain APC (HR of 1.37, 95% CI of 1.1- 1.8, p = 0.007) and larger positive ventricular APC (HR of 1.71, 95% CI of 1.3 - 2.3, p < 0.001) increased the hazard of conversion to AD significantly in univariate analyses (Table 4). To graphically illustrate the ventricular rate results, the MCI cohort was divided into tertiles based on ventricular APC. Separate Kaplan-Meier curves for MCI subjects in each tertile are presented (Figure).
Table 4.
Results from Cox proportional hazards models of time-to-conversion among MCIs (n=72).
| One-variable models | Two-variable model | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted Cross-sectional volume | APC | Adjusted Cross-sectional volume | APC | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Hippocampus | 1.51 (1.1, 2.0) | 0.002 | 1.13 (0.8, 1.5) | 0.23 | 1.51 (1.1, 2.0) | 0.003 | 1.06 (0.8, 1.5) | 0.37 |
| ERC | 1.22 (0.9, 1.7) | 0.12 | 1.13 (0.8, 1.5) | 0.23 | 1.21 (0.9, 1.7) | 0.14 | 1.10 (0.8, 1.5) | 0.28 |
| Whole brain | 1.33 (0.9, 1.9) | 0.05 | 1.37 (1.1, 1.8) | 0.007 | 1.17 (0.8, 1.7) | 0.21 | 1.30 (1.0, 1.7) | 0.034 |
| Ventricle | 1.03 (0.8, 1.4) | 0.43 | 1.71 (1.3, 2.3) | <0.001 | 0.97 (0.7, 1.3) | 0.58 | 1.72 (1.3, 2.4) | <0.001 |
Note: There were 39 observed conversion among the MCI cohort.
Follow-up starts with second scan.
HR = Hazard Ratio for a 1-standard deviation decrease in cross-sectional volume or annual percent change in volume (except for ventricle estimate which is based on a 1-standard deviation increases).
Figure.
Kaplan-Meier estimates of the probability of remaining amnestic MCI by tertiles of ventricle APC. The lower tertile includes patients with ventricle APC ≤ 2.07, the middle tertile includes patients with ventricle APCs > 2.07 and ≤ 3.79, while the upper tertile includes patients with ventricle APC > 3.79. Each tertile had 24 patients.
Although our primary interest was in rate measures, we did calculate adjusted baseline cross sectional volumes and their association with time to conversion in order to perform bivariate modeling with APC values. This was done in MCI subjects only. Adjusted cross-sectional hippocampal volume (HR (CI) of 1.51 (1.1, 2.0), p = 0.002) and whole brain (HR (CI) of 1.33 (0.9, 1.9), p = 0.05) volumes were significantly associated with conversion from MCI to AD. Adjusted baseline line cross sectional ERC and ventricle volume were not (Table 4).
For each of the four brain structures, we examined the joint effects of cross-sectional adjusted volume and APC in bivariate analyses in the MCI cohort (Table4). For hippocampus, cross-sectional volume was and APC was not significant. For ERC neither cross-sectional volume nor APC were significant. For whole brain and ventricle APC was and cross-sectional volume was not significant.
Finally, we examined the joint effects of cross-sectional hippocampal volume plus ventricle and the joint effects cross-sectional hippocampal volume plus whole brain APC in bivariate models in the MCI cohort. Both baseline cross sectional hippocampus (HR (CI) of 1.53 (1.1, 2.1), p = 0.003) and whole brain APC (HR (CI) of 1.32 (1.0, 1.7), p = 0.009) were significant. Both baseline cross sectional hippocampus (HR (CI) of 1.49 (1.1, 2.), p = 0.008) and ventricle APC (HR (CI) of 1.59 (1.2, 2.2), p = 0.001) were significant.
Discussion
Among subjects with MCI, higher rates of atrophy of both the whole brain and the ventricle measured from serial MRI scans over a 1-2 year interval were associated with greater relative hazard of subsequent conversion to AD. In cognitively normal subjects, higher rates of ventricular enlargement were associated with greater relative hazard of subsequent conversion to MCI or AD.
Neither hippocampal nor ERC rates were significantly associated with conversion in either the normal or MCI cohorts. While this may simply reflect small sample size, the observed discrepancy in predictive power could have a technical explanation. Both the whole brain and ventricular measures were performed using the boundary shift integral method. While the BSI required operator dependant pre-processing, the actual calculation of volume change between serial MRI studies is fully automated. In contrast, the hippocampal and ERC volumes were hand traced. The operator was blinded to chronologic ordering of the scans as well as clinical classification of subjects in all cases. And, the images were restricted to (cropped) only the temporal lobe structures of interest thus removing anatomic cues about the temporal ordering of the scans to the operator. Despite these methods to remove subjectivity, manual tracing of boundaries does involve a significant degree of interaction and interpretation by the trained research associate. Test re-test precision of the whole brain and ventricular APC rates from serial MRI is significantly better than test re-test precision of ERC APC rates (5). It is possible therefore, that the greater degree of automation of the whole brain and ventricle rates accounted for their superior performance. However, small sample size may also account for a lack of statistical significance of hippocampal and ERC rate measures in this study.
Although use of structural MRI in the evaluation of dementia is gaining acceptance, few studies have been published that explicitly address the issue of rates of atrophy predicting subsequent conversion. We are aware of none that employs time-to-event statistical methods or that evaluates this association in MCI subjects. It is also difficult to directly compare the few publications on this topic due to differences in patient populations, MRI acquisition, and image processing methods. One group (11-13) found accelerating rates of both hippocampal atrophy and hemispheric atrophy in pre-symptomatic individuals who subsequently developed AD. However, these were subjects with familial AD and it is likely that disease progression is more aggressive in younger familial patients than in the elderly subjects with late onset disease in our study. A different group (14) found that atrophy rates of the medial temporal lobe did not predict which elderly non-demented subjects would subsequently decline to AD, but measures of the entire temporal lobe did. The ages of patients, MRI acquisition methods, and MRI measurement methods were all different in that study, however, than in ours.
The focus of this report was on the ability of measures of rates of change to predict time to subsequent clinical conversion in cognitively normal and MCI subjects. However, among the MCI cohort we also assessed the possibility that cross-sectional volume measurements derived from the MRI at the clinical start point (the time of MRI scan 2) might already contain most of the predictive information found in the APCs. We and others have previously shown that greater atrophy in the hippocampus or other medial temporal lobe structures on a single scan is associated with a higher risk of subsequent conversion to AD from MCI (or other clinical prodromal AD syndromes) (15-20). The current study reinforces this finding for the hippocampus and suggests a 50% increase in risk for a 1-SD decrease in hippocampal volume. Similarly, a single cross-sectional measure of whole brain volume at the time of scan 2 was univariately associated with the hazard of conversion from MCI to AD although this association was not as strong as that of the hippocampus.
Of greater interest perhaps are results of bivariate modeling. In none of the bivariate analyses were both cross-sectional and APC measures significant. The cross-sectional adjusted hippocampus was significant while hippocampal APC was not. For both whole brain and ventricle the APC was significant while cross-sectional adjusted volumes were not. We conclude that in terms of associated risk of conversion in MCI subjects, a single cross-sectional hippocampal measure contains most of the useful information for that structure, while atrophy rate measures contain most of the useful information for whole brain and ventricle. This conclusion is reinforced by the results of bivariate analyses combining cross-sectional adjusted hippocampus with whole brain APC, and cross-sectional hippocampus with ventricle APC. In both of these models both the cross-sectional adjusted hippocampus and the rate measure provide complementary predictive information. That is, even among patients at the same stage of hippocampal decline, those whose whole brain or ventricle have atrophied at a greater rate in the last 1-2 years are at higher risk of conversion.
In this study, we followed cohorts forward and observed variable times to conversion. Thus, time-to-event methods are appropriate while direct comparisons of atrophy rates among converter vs. non converter subjects are not because of variable follow-up periods. It is likewise not appropriate to form two groups, converters and non converters, and test the diagnostic accuracy of specific cut points. However, overlap in baseline adjusted volumes as well as atrophy rates among those who did vs. those who did not convert indicate that these measures will not provide absolute prognostic information for individual patients.
Elderly patients with a memory complaint are often evaluated with serial clinical visits at fairly short intervals in order to assess clinical progression. MRI studies are often obtained in conjunction with clinical visits. Our results confirm that cross sectional hippocampal volume is an important predictor of time to conversion among MCI subjects. In addition, atrophy rates of either whole brain or ventricle from serial scans provide predictive information about the hazard of subsequent conversion from MCI to AD which is complimentary to that provided by a cross sectional hippocampal measure. Among MCI patients, the key MRI predictors of conversion appear to be how much the hippocampus has atrophied at the baseline time point as well as how fast the brain has been atrophying for the 1 to 2 years prior to baseline.
Acknowledgments
The National Institute on Aging - AG16574, AG06786, AG11378, and The Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program
References
- 1.Petersen RC, Kokmen E, Tangalos EG, et al. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 2.Petersen R, Morris J. Clinical Features of Mild Cognitive Impairment. In: Petersen RC, editor. Mild Cognitive Impairment. Oxford Press; 2003. pp. 15–39. [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Smith GE, Petersen RC, Parisi JE, et al. Definition, course, and outcome of mild cognitive impairment. Aging, Cognition, and Neuropsychology. 1996;3:141–147. [Google Scholar]
- 5.Jack CR, Jr, Shiung MM, Mintzer J, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Bentley M, Twomey CK, et al. MR-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology. 1990;176:205–209. doi: 10.1148/radiology.176.1.2353093. [DOI] [PubMed] [Google Scholar]
- 7.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans on Medical Imaging. 1997;15:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 8.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. Journal of Magnetic Resonance Imaging. 1997;7:1069–75. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 9.Gunter JL, Shiung MM, Manduca A, et al. Methodological considerations for measuring rates of brain atrophy. JMRI. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer's disease. The Lancet. 1999;353:2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 12.Fox NC, Crum WR, Scahill RI, et al. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 13.Schott JM, Fox NC, Frost C, et al. Assessing the onset of structural change in familial Alzheimer's disease. Ann Neurol. 2003;53:181–188. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 14.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 15.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Petersen RC, Xu Y, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser PJ, Scheltens P, Verhey FRJ, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurology. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 18.Killiany R, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 19.Killiany RJ, Hyman BT, Gopmez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 20.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiology of Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]