Abstract
Dominant mutations in TP63 cause ankyloblepharon ectodermal dysplasia and clefting (AEC), an ectodermal dysplasia characterized by skin fragility. Since ΔNp63α is the predominantly expressed TP63 isoform in postnatal skin, we hypothesized that mutant ΔNp63α proteins are primarily responsible for skin fragility in AEC patients. We found that mutant ΔNp63α proteins expressed in AEC patients function as dominant-negative molecules, suggesting that the human AEC skin phenotype could be mimicked in mouse skin by downregulating ΔNp63α. Indeed, downregulating ΔNp63 expression in mouse epidermis caused severe skin erosions, which resembled lesions that develop in AEC patients. In both cases, lesions were characterized by suprabasal epidermal proliferation, delayed terminal differentiation, and basement membrane abnormalities. By failing to provide structural stability to the epidermis, these defects likely contribute to the observed skin fragility. The development of a mouse model for AEC will allow us to further unravel the genetic pathways that are normally regulated by ΔNp63 and that may be perturbed in AEC patients. Ultimately, these studies will not only contribute to our understanding of the molecular mechanisms that cause skin fragility in AEC patients, but may also result in the identification of targets for novel therapeutic approaches aimed at treating skin erosions.
Keywords: Ankyloblepharon ectodermal dysplasia and clefting, TP63, epidermal differentiation, skin erosions
INTRODUCTION
Ectodermal dysplasias comprise a group of developmental disorders characterized by abnormalities in tissues of ectodermal origin, including the epidermis and epithelial appendages such as teeth, mammary glands, and hair follicles [Priolo et al., 2000]. Dominant mutations in the gene encoding the transcription factor TP63 were found to underlie a subset of ectodermal dysplasias, including ectrodactyly, ectodermal dysplasia and cleft lip (EEC) [Celli et al., 1999], limb-mammary syndrome (LMS) [van Bokhoven et al., 2001], split hand-split foot malformation (SHFM) [Ianakiev et al., 2000], acro-dermato-ungual-lacrimal-tooth syndrome (ADULT) [Duijf et al., 2002], ankyloblepharon ectodermal dysplasia and clefting (AEC or Hay-Wells syndrome) [McGrath et al., 2001], and Rapp-Hodgkin syndrome (RHS) [Kantaputra et al., 2003]. Ectodermal dysplasias caused by TP63 mutations share many manifestations, however, only patients with AEC or RHS exhibit skin fragility, resulting in the formation of erosive skin lesions [Cambiaghi et al., 1994;Siegfried et al., 2005]. In addition to this overlap in clinical symptoms, AEC and RHS are caused by mutations in the same region of the TP63 gene, and identical mutations can give rise to either AEC or RHS [Bertola et al., 2004;Rinne et al., 2007;Sorasio et al., 2006]. Since both the genetic defect and the clinical findings of AEC and RHS overlap, these disorders are now thought to represent the same condition and will be referred to as AEC in this paper.
Current treatment of AEC patients primarily involves prevention of infection and extensive wound care [Siegfried et al., 2005]. To further understand the molecular mechanisms that lead to skin fragility in AEC patients and to facilitate future development of targeted approaches for the treatment of skin erosions in AEC patients, our goal was to develop a mouse model for AEC. The heterozygous TP63 mutations that cause AEC are primarily clustered in the α C-terminus, which is only present in 2 of 6 TP63 isoforms, termed TAp63α and ΔNp63α [McGrath et al., 2001;Rinne et al., 2007]. In addition, a recent study demonstrated that mutations in the ΔNp63 N-terminus occur in a subset of patients with AEC [Rinne et al., 2008]. Together, these data suggest that mutant ΔNp63α proteins are responsible for skin erosions in AEC patients. This is consistent with previous findings that ΔNp63α is the predominantly expressed p63 isoform in postnatal epidermis [Yang et al., 1998]. Within the basal layer of the epidermis, ΔNp63α controls the commitment of keratinocytes to terminal differentiation and contributes to maintaining basement membrane integrity [Koster et al., 2007a;Testoni et al., 2006;Nguyen et al., 2006;Carroll et al., 2006]. In addition, ΔNp63α regulates proliferation of basal keratinocytes as well as the proliferative potential of epidermal stem cells [Senoo et al., 2007;Truong et al., 2006;Koster et al., 2007a].
Using reporter gene assays, we found that mutant ΔNp63α proteins expressed in AEC patients (ΔNp63α-AEC) have a dominant-negative function towards wild type (wt) ΔNp63α, suggesting that impaired ΔNp63α function underlies skin erosions in AEC patients. Consistent with these findings, we found that downregulating ΔNp63 expression in mouse epidermis caused a skin fragility phenotype that was indistinguishable from that of AEC patients. In both cases, skin erosions were characterized by basement membrane abnormalities, suprabasal proliferation, and delayed terminal differentiation. In addition, these defects were also observed in non-lesional skin of AEC patients. Together, these defects likely contribute to skin fragility by failing to provide structural stability to the epidermis.
MATERIALS AND METHODS
Reporter gene assays
A 1.2 kb fragment of the human IKKα promoter was cloned into pGL3-basic (Promega). HaCaT immortalized keratinocytes were cotransfected with the IKKα reporter (0.5 μg), ΔNp63α (1 μg) and ΔNp63α-Q536L or ΔNp63α-L514V (0.5-1 μg; gifts from Dr L. Guerrini (University of Milano)) [Ghioni et al., 2002], and pRL-null (0.1 μg) using Lipofectamine Plus (Invitrogen). Cells were lysed 24 hrs later and luciferase assays were performed using the Promega Dual-Luciferase Reporter® Assay (Promega). Average reporter gene activities and standard deviations were determined based on three independent experiments.
Transgenic mouse lines
ΔNp63 i-kd mice were previously generated and characterized [Koster et al., 2007a]. To downregulate ΔNp63, ΔNp63 i-kd mice were topically treated with 1 mg/ml RU486. All experiments involving mice were performed under IACUC approval.
Patient samples
Biopsies from lesional skin were obtained from three previously described AEC patients [Payne et al., 2005;Kantaputra et al., 2003]. Biopsies from non-lesional skin were obtained from Dr. Alanna Bree (Texas Children's Hospital, Houston, TX). These biopsies were obtained under IRB approval #H-20716 through Baylor College of Medicine, Houston, TX.
In vivo BrdU incorporation and immunofluorescence
Mice were injected i.p. with 250 μg/g BrdU (Sigma) in 0.9% sterile saline solution. After 1 hr, skin was fixed in 10% neutral buffered formalin. Primary antibodies used for immunofluorescence were FITC anti-BrdU (Becton Dickinson), guinea pig anti-K14 [Yuspa et al., 1989], rabbit anti-K1 [Yuspa et al., 1989], mouse anti-Ki67 (Novacastra), and rabbit anti-collagen IV (Progen). Secondary antibody conjugates used were Alexa-conjugated fluorochromes 594 goat anti-guinea pig, 594 goat anti-rabbit, 488 goat anti-mouse, and 488 goat anti-rabbit (Invitrogen).
RESULTS AND DISCUSSION
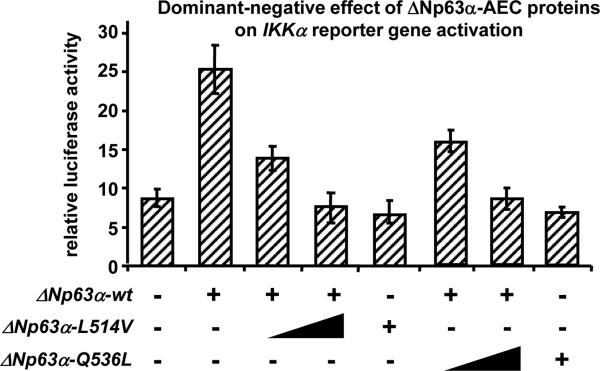
Within the basal layer of the epidermis, ΔNp63α induces the expression of numerous target genes which control keratinocyte proliferation, keratinocyte differentiation, and basement membrane integrity [Koster et al., 2008]. We previously demonstrated that in mice and humans, IKKα is a direct transcriptional target of ΔNp63α [Koster et al., 2007a;Marinari et al., 2008]. Therefore, to determine the molecular function of ΔNp63α-AEC proteins, we performed in vitro reporter gene assays using a reporter gene based on the promoter of IKKα. Two ΔNp63α-AEC proteins (ΔNp63α-Q536L [formerly referred to as Q540L] and ΔNp63α-L514V [formerly referred to as L518V]) were used to ensure that the observed effects were not unique to one specific mutation. The Q536L mutation was identified in a patient with erosions on the scalp and palmo-plantar epithelia, while the L514V mutation was identified in a patient with erosions on the scalp and trunk [Hay et al., 1976;McGrath et al., 2001]. As predicted, we found that wt ΔNp63α could activate the IKKα promoter in HaCaT cells, a human keratinocyte cell line (Fig. 1). However, ΔNp63α-AEC proteins had a dominant-negative function as demonstrated by the greatly reduced ability of wt ΔNp63α to activate the IKKα promoter in the presence of ΔNp63α-Q536L or ΔNp63α-L514V (Fig. 1).
Figure 1.
ΔNp63α-AEC proteins have a dominant-negative effect towards wt ΔNp63α. A reporter construct containing 1.2 kb of the human IKKα promoter (0.5 μg) was transfected with or without a wt ΔNp63α expression vector (1 μg) in the presence of increasing amounts of ΔNp63α-AEC (Q536L or L514V) expression vectors (0, 0.5, 1 μg). Note that both ΔNp63α-Q536L and ΔNp63α-L514V have a dominant-negative function towards wt ΔNp63α.
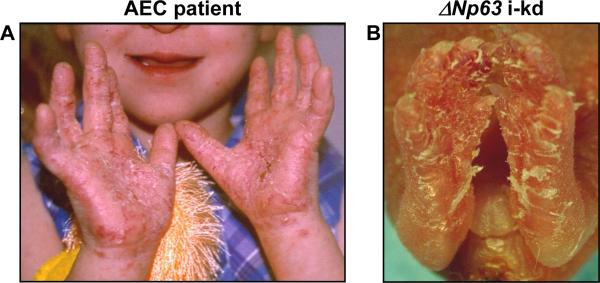
These data suggested that, in AEC patients, ΔNp63α-AEC proteins could compromise the function of wt ΔNp63α proteins that are expressed from the remaining wt TPp63 allele. Therefore, we hypothesized that the human AEC skin phenotype could be mimicked in mouse skin by downregulating ΔNp63α. To test this hypothesis, we recently generated mice in which ΔNp63 expression can be downregulated specifically in the epidermis by topical application of RU486 (ΔNp63 inducible knockdown (i-kd) mice; [Koster et al., 2007a]). Because of the inducibility of ΔNp63 downregulation, this mouse model allowed us to selectively downregulate ΔNp63 by applying RU486 to restricted areas of the epidermis, for example back or footpad skin. When we treated footpad epithelia of newborn mice with RU486 for six consecutive days, the resulting skin lesions closely resembled lesions that develop on the palmo-plantar epithelia of a subset of AEC patients (Fig. 2). Similar skin erosions were observed when ΔNp63 was downregulated in the dorsal epidermis of newborn or adult mice [Koster et al., 2007a]. To determine if these lesions were also similar on the molecular level, we obtained lesional skin biopsies from three AEC patients [Payne et al., 2005;Kantaputra et al., 2003] and non-lesional skin biopsies from eighteen AEC patients, also reported by Dishop et al in this series. We compared the molecular characteristics of these skin samples with those of skin samples obtained from adult ΔNp63 i-kd mice that were topically treated with RU486 for seven consecutive days.
Figure 2.
Skin erosions reminiscent of those that develop in AEC patients develop in ΔNp63 i-kd mice. Skin erosions on (A) the palms of an AEC patient and (B) the paws of a ΔNp63 i-kd mouse. To downregulate ΔNp63, the ΔNp63 i-kd mouse in (B) was treated with RU486 for 6 days on the footpad epithelia. Image of AEC patient in (A) was provided by the National Foundation for Ectodermal Dysplasias (NFED).
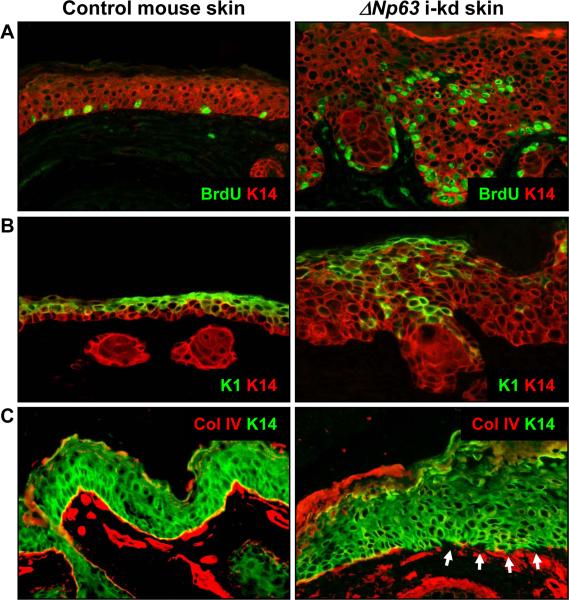
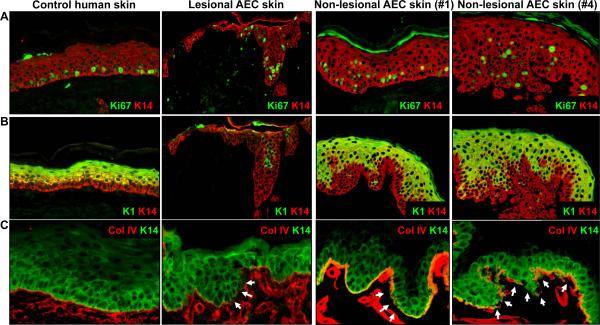
We found that like ΔNp63 i-kd mice, AEC patients failed to properly develop a spinous layer, the first suprabasal cell layer of normal mouse and human epidermis, which consists of keratinocytes that have permanently withdrawn from the cell cycle and that express keratin 1 (K1) [Koster et al., 2007b]. First, unlike in control epidermis, proliferating keratinocytes were observed in suprabasal cell layers in ΔNp63 i-kd mice (detected by staining with an anti-BrdU antibody), lesional skin of AEC patients (3/3) (detected by staining with an anti-Ki67 antibody), and non-lesional skin of AEC patients (18/18) (detected by staining with an anti-Ki67 antibody) (Fig. 3A and 4A). In addition, K1 expression was delayed in the epidermis of ΔNp63 i-kd mice, lesional skin of AEC patients (3/3), and non-lesional skin of AEC patients (17/18) (Fig. 3B and 4B). In contrast to non-lesional AEC epidermis, we found that K1 expression was completely absent from some areas of lesional AEC epidermis (Fig. 4B). In addition to defects in keratinocyte differentiation, we observed discontinuous staining for the basement membrane component collagen IV in skin of ΔNp63 i-kd mice, lesional skin of AEC patients (3/3), and non-lesional skin of AEC patients (18/18), indicating basement membrane abnormalities (Fig. 3C and 4C). At least one component of the basement membrane, Fras1, is directly induced by ΔNp63α in mouse epidermis [Koster et al., 2007a]. However, whether reduced expression of Fras1 is also a contributing factor in skin lesions in AEC patients remains to be determined.
Figure 3.
Epidermis of ΔNp63 i-kd mice is characterized by suprabasal proliferation, delayed terminal differentiation, and basement membrane abnormalities. Mice were topically treated with 1 mg/ml RU486 for 7 days starting at three weeks of age. (A) Immunofluorescence analysis using an antibody against BrdU (green), a marker for proliferating cells, on skin of ΔNp63 i-kd mice. (B) Immunofluorescence analysis using an antibody against K1 (green), a marker of keratinocyte terminal differentiation, on skin of ΔNp63 i-kd mice. (C) Immunofluorescence analysis using an antibody against collagen IV (red), a marker of the basement membrane, on skin of ΔNp63 i-kd mice. Arrows in (C) indicate areas of the basement membrane where collagen IV staining is reduced. Staining with a K14 antibody (red in (A) and (B), green in (C)) was used to highlight the epidermis.
Figure 4.
Lesional and non-lesional epidermis of AEC patients is characterized by suprabasal proliferation, delayed terminal differentiation, and basement membrane abnormalities. (A) Immunofluorescence analysis using an antibody against Ki67 (green), a marker for proliferating cells, on lesional or non-lesional skin obtained from AEC patients. (B) Immunofluorescence analysis using an antibody against K1 (green), a marker of keratinocyte terminal differentiation, on lesional or non-lesional skin obtained from AEC patients. (C) Immunofluorescence analysis using an antibody against collagen IV (red), a marker of the basement membrane, on lesional or non-lesional skin obtained from AEC patients. Arrows in (C) indicate areas of the basement membrane where collagen IV staining is reduced. Staining with a K14 antibody (red in (A) and (B), green in (C)) was used to highlight the epidermis. AEC patients #1 and #4 correspond to these same numbers in the Dishop et al paper in this series.
Recent data suggested that ΔNp63α is critical for the maintenance of epidermal stem cells [Senoo et al., 2007]. In our experiments, we could not distinguish between effects mediated by epidermal stem cells or by basal keratinocytes. However, our finding that proliferation and differentiation defects in ΔNp63 i-kd epidermis are apparent as early as 5 days after ΔNp63 downregulation [Koster et al., 2007a], suggests that both basal keratinocytes and epidermal stem cells are responsible for these defects. Future experiments using chronic downregulation of ΔNp63 will be required to provide further insights into the role of ΔNp63 in epidermal stem cell maintenance.
Interestingly, we observed suprabasal proliferation, abnormal terminal differentiation and basement membrane abnormalities not only in lesional AEC epidermis, but also in non-lesional AEC epidermis, suggesting that these cellular defects contribute to the development of skin erosions. Together with the data obtained with our mouse model, we conclude that the cellular defects caused by expression of mutant TP63 proteins in the epidermis are responsible for the development of skin erosions in AEC patients.
ACKNOWLEDGEMENTS
We thank Dr. L. Guerrini for providing human p63 expression constructs. This work was supported by NIH grants to MIK (AR054696), DRR (AR052263, CA52607, AR47898) and ASP (AR053505), a research grant from the National Foundation for Ectodermal Dysplasias (NFED) to MIK and DRR, grants from the AIRC and ECFP6 (contract 503576) to AC, a FIRC fellowship to BM, and grants from the European Community's Sixth Framework programme (LSHB-CT-2005-019067) and The Thailand Research Fund (BRG/49/2549) to PNK.
Glossary
Abbreviations
- AEC
Ankyloblepharon ectodermal dysplasia and clefting
- RHS
Rapp-Hodgkin syndrome
- EEC
Ectrodactyly ectodermal dysplasia and cleft lip
- K
Keratin
REFERENCES
- Bertola DR, Kim CA, Albano LM, Scheffer H, Meijer R, van Bokhoven H. Molecular evidence that AEC syndrome and Rapp-Hodgkin syndrome are variable expression of a single genetic disorder. Clin Genet. 2004;66:79–80. doi: 10.1111/j.0009-9163.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Cambiaghi S, Tadini G, Barbareschi M, Menni S, Caputo R. Rapp-Hodgkin syndrome and AEC syndrome: are they the same entity? Br J Dermatol. 1994;130:97–101. doi: 10.1111/j.1365-2133.1994.tb06891.x. [DOI] [PubMed] [Google Scholar]
- Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills A, Brugge JS, Ellisen LW. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, Newbury-Ecob R, Hennekam RC, Van Buggenhout G, van Haeringen A, Woods CG, van Essen AJ, de Waal R, Vriend G, Haber DA, Yang A, McKeon F, Brunner HG, van Bokhoven H. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–153. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- Duijf PH, Vanmolkot KR, Propping P, Friedl W, Krieger E, McKeon F, Dotsch V, Brunner HG, van Bokhoven H. Gain-of-function mutation in ADULT syndrome reveals the presence of a second transactivation domain in p63. Hum Mol Genet. 2002;11:799–804. doi: 10.1093/hmg/11.7.799. [DOI] [PubMed] [Google Scholar]
- Ghioni P, Bolognese F, Duijf PH, van Bokhoven H, Mantovani R, Guerrini L. Complex transcriptional effects of p63 isoforms: identification of novel activation and repression domains. Mol Cell Biol. 2002;22:8659–8668. doi: 10.1128/MCB.22.24.8659-8668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RJ, Wells RS. The syndrome of ankyloblepharon, ectodermal defects and cleft lip and palate: an autosomal dominant condition. Br J Dermatol. 1976;94:277–289. doi: 10.1111/j.1365-2133.1976.tb04384.x. [DOI] [PubMed] [Google Scholar]
- Ianakiev P, Kilpatrick MW, Toudjarska I, Basel D, Beighton P, Tsipouras P. Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet. 2000;67:59–66. doi: 10.1086/302972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantaputra PN, Hamada T, Kumchai T, McGrath JA. Heterozygous mutation in the SAM domain of p63 underlies Rapp-Hodgkin ectodermal dysplasia. J Dent Res. 2003;82:433–437. doi: 10.1177/154405910308200606. [DOI] [PubMed] [Google Scholar]
- Koster MI, Dai D, Marinari B, Sano Y, Costanzo A, Karin M, Roop DR. p63 induces key target genes required for epidermal morphogenesis. PNAS. 2007a;104:3255–3260. doi: 10.1073/pnas.0611376104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms Regulating Epithelial Stratification. Annual Review of Cell and Developmental Biology. 2007b;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Sorting Out the p63 Signaling Network. J Invest Dermatol. 2008;128:1617–1619. doi: 10.1038/jid.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari B, Ballaro C, Koster MI, Giustizieri ML, Moretti F, Karin M, Alema S, Chimenti S, Roop DR, Costanzo A. IKKα is a p63 transcriptional target involved in the pathogenesis of ectodermal dysplasias. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.202. in press. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Duijf PH, Doetsch V, Irvine AD, de Waal R, Vanmolkot KR, Wessagowit V, Kelly A, Atherton DJ, Griffiths WA, Orlow SJ, van Haeringen A, Ausems MG, Yang A, McKeon F, Bamshad MA, Brunner HG, Hamel BC, van Bokhoven H. Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet. 2001;10:221–229. doi: 10.1093/hmg/10.3.221. [DOI] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della GG, Koster MI, Zhang Z, Wang J, Tommasi d V, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS, Yan AC, Ilyas E, Li W, Seykora JT, Young TL, Pawel BR, Honig PJ, Camacho J, Imaizumi S, Heymann WR, Schnur RE. Two novel TP63 mutations associated with the ankyloblepharon, ectodermal defects, and cleft lip and palate syndrome: a skin fragility phenotype. Arch Dermatol. 2005;141:1567–1573. doi: 10.1001/archderm.141.12.1567. [DOI] [PubMed] [Google Scholar]
- Priolo M, Silengo M, Lerone M, Ravazzolo R. Ectodermal dysplasias: not only 'skin' deep. Clin Genet. 2000;58:415–430. doi: 10.1034/j.1399-0004.2000.580601.x. [DOI] [PubMed] [Google Scholar]
- Rinne T, Brunner HG, van Bokhoven H. p63-associated disorders. Cell Cycle. 2007;6:262–268. doi: 10.4161/cc.6.3.3796. [DOI] [PubMed] [Google Scholar]
- Rinne T, Clements SE, Lamme E, Duijf PHG, Bolat E, Meijer R, Scheffer H, Rosser E, Tan TY, McGrath JA, Schalkwijk J, Brunner HG, Zhou H, van Bokhoven H. A novel translation re-initiation mechanism for the p63 gene revealed by amino-terminal truncating mutations in Rapp-Hodgkin/Hay-Wells-like syndromes. Hum Mol Genet. 2008;17:1968–1977. doi: 10.1093/hmg/ddn094. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Bree A, Fete M, Sybert VP. Skin erosions and wound healing in ankyloblepharon-ectodermal defect-cleft lip and/or palate. Arch Dermatol. 2005;141:1591–1594. doi: 10.1001/archderm.141.12.1591. [DOI] [PubMed] [Google Scholar]
- Sorasio L, Ferrero GB, Garelli E, Brunello G, Martano C, Carando A, Belligni E, Dianzani I, Cirillo Silengo M. AEC syndrome: further evidence of a common genetic etiology with Rapp-Hodgkin syndrome. European Journal of Medical Genetics. 2006;49:520–522. doi: 10.1016/j.ejmg.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Testoni B, Mantovani R. Mechanisms of transcriptional repression of cell-cycle G2/M promoters by p63. Nucl Acids Res. 2006;34:928–938. doi: 10.1093/nar/gkj477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, Duijf PH, Vanmolkot KR, van Beusekom E, van Beersum SE, Celli J, Merkx GF, Tenconi R, Fryns JP, Verloes A, Newbury-Ecob RA, Raas-Rotschild A, Majewski F, Beemer FA, Janecke A, Chitayat D, Crisponi G, Kayserili H, Yates JR, Neri G, Brunner HG. p63 Gene mutations in eec syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69:481–492. doi: 10.1086/323123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]