Abstract
The development of pulmonary metastasis is the major cause of death in osteosarcoma, and its molecular basis is poorly understood. In this study, we show that β4 integrin is highly expressed in human osteosarcoma cell lines and tumor samples. Furthermore, highly metastatic MNNG-HOS cells have increased levels of β4 integrin. Suppression of β4 integrin expression by shRNA and disruption of β4 integrin function by transfection of dominant negative β4 integrin was sufficient to revert this highly metastatic phenotype in the MNNG-HOS model without significantly affecting primary tumor growth. These findings suggest a role for β4 integrin expression in the metastatic phenotype in human osteosarcoma cells. In addition, we identified a previously uncharacterized interaction between β4 integrin and ezrin, a membrane-cytoskeletal linker protein that is implicated in the metastatic behavior of osteosarcoma. The β4 integrin-ezrin interaction appears to be critical for maintenance of β4 integrin expression. These data begin to integrate ezrin and β4 integrin expression into a model of action for the mechanism of ostesarcoma metastases.
Keywords: β4 integrin, osteosarcoma, metastases, ezrin, interaction
Introduction
Osteosarcoma is the most common primary bone tumor in children and young adults. Over the past two decades, despite significant improvements in the treatment of patients with osteosarcoma, patients with metastatic disease still have very poor prognosis (Meyers et al., 1993; Harris et al., 1998). Compounding the problem is that the molecular basis underlying metastatic disease is poorly understood. Progression of osteosarcoma is thought to result from cells migrating away from the primary tumor, surviving in the circulation, invading into lung tissue and establishing metastatic nodules in the lung. Thus, selectively blocking this migratory and invasive ability, through targeted therapy of key metastatic molecules should be an attractive strategy to inhibit tumor metastasis.
Integrins have been shown to be critical in controlling how tumor cells interact with their microenvironment. Integrins constitute an important family of cell adhesion receptors responsible for mediating interactions between cells and the extracellular matrix (ECM) (Hood and Cheresh, 2002; Hynes, 2002). Integrins consist of non-covalently associated α- and β- subunits. To date, 18 α- and 8 β- subunits have been identified and are known to form at least 25 integrin heterodimers, that regulate key biological processes such as adhesion, signaling, migration, proliferation, survival, angiogenesis, oncogenesis and metastasis (Hood and Cheresh, 2002; Hynes, 2002; Hower et al., 1998; Aplin et al., 1999; Giancotti and Ruoslahti, 1999). An integrin of particular relevance in invasive and metastatic carcinomas is α6β4 (henceforward β4 integrin) which was originally identified as a tumor-associated antigen (Halcioni et al., 1988). In contrast to other β subunits, β4 integrin contains a unique β4 cytoplasmic domain (1017 amino acid). This domain does not share homology with other known β subunits, and has distinctive cytoskeletal and signaling functions (Giancotti and Tarone, 2003). A specific tyrosine residue in the β4 integrin cytoplasmic domain, Y1494 has been identified to be required for the activation of PI3K (Shaw, 2001). Furthermore, the expression of β4 integrin is maintained, or often upregulated, in various types of invasive and metastatic tumors. High levels of this integrin have been linked to poor prognosis in these tumor types (Mercurio and Rabinovitz, 2001). β4 integrin also cooperates with multiple oncogenic receptor tyrosine kinases (RTKs), such as EGF-R, ErbB2/Neu and Met, and enhances cell survival, proliferation, invasion and migration (Mariotti et al., 2001; Guo et al., 2006; Trusolino et al., 2001).
Numerous studies have demonstrated that the expression and function of β4 integrin is implicated in cancer progression (Mercurio and Rabinovitz, 2001; Nikolopoulos et al., 2004; Mariotti et al., 2001; Guo et al., 2006; Trusolino et al., 2001). However, there is a paucity of data on its contribution to tumor metastases in vivo. In this study, we demonstrate that β4 integrin expression and function is required for the metastatic phenotype in an osteosarcoma model. Suppression of β4 integrin expression by shRNA and disruption of β4 integrin function by transfection of dominant negative β4 integrin (Y1494F mutant) resulted in inhibition of metastases in vivo. These data provide evidence that β4 integrin expression promotes tumor metastases and suggest that blockade of β4 integrin may represent a rational approach to inhibit tumor metastases in patients with osteosarcoma.
Results
β4 integrin is highly expressed in osteosarcoma cell lines
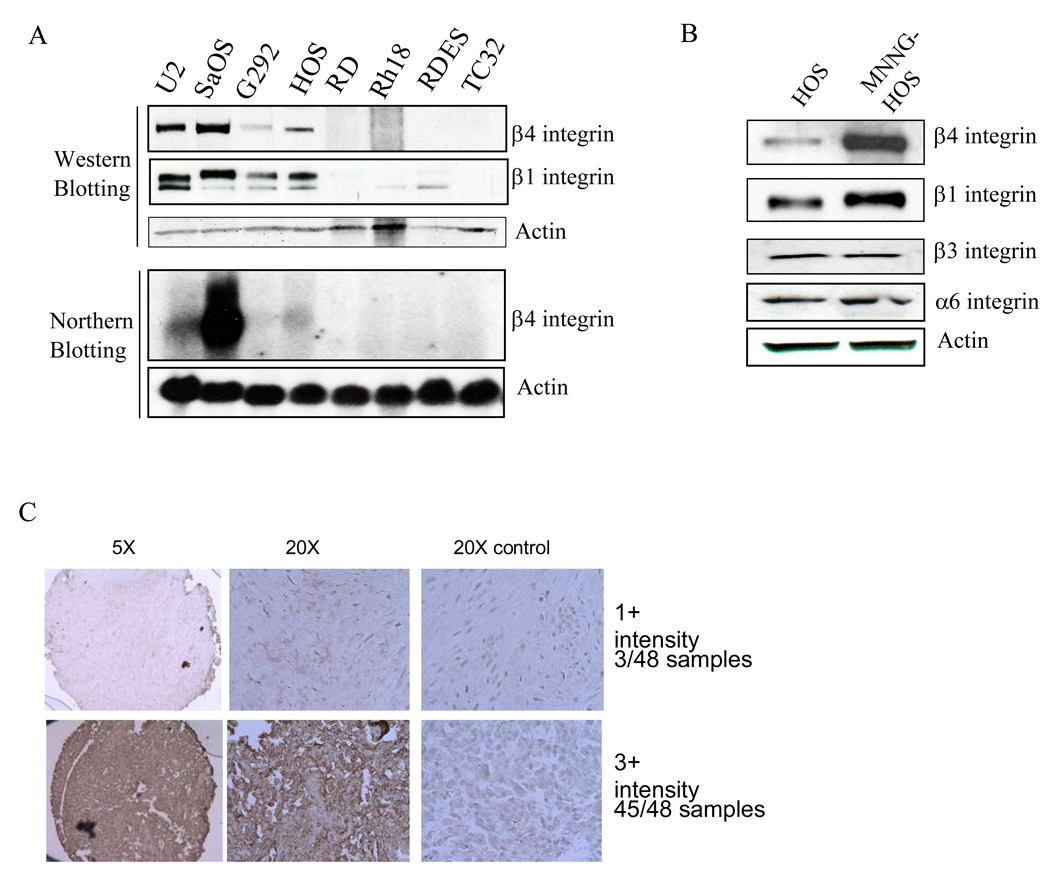
Overexpression of β4 integrin has been linked to cancer progression (Shaw, 2001). We first examined the expression of β4 integrin in 4 osteosarcoma (U2OS, SaOS, G292, and HOS), 2 rhabdomyosarcoma (RD and Rh18), and 2 Ewing’s sarcoma (RDES and TC32) cell lines. β4 integrin RNA and protein were highly expressed only in osteosarcoma cell lines (Figure 1A). In addition, β1 integrin was also highly expressed in osteosarcoma cell lines only (Figure 1A). To further study the role of β4 integrin in osteosarcoma, we utilized the highly metastatic cell line MNNG-HOS. This cell line was previously derived from the non-metastatic HOS human osteosarcoma cell line by chemical transformation with MNNG, a carcinogenic nitrosamine (Rhim et al., 1975). We found that highly metastatic MNNG-HOS cells express higher levels of β4 integrin than non-metastatic parental HOS cells (Figure 1B). Since α6 integrin heterodimerizes with both β4 and β1 integrin subunits, we also examined the expression of β1 integrin and α6 integrin and found that the MNNG-HOS cell line also had an increase in β1 integrin expression, but no changes in either α6 or β3 integrin expression (Figure 1B).
Figure 1.
β4 integrin is highly expressed in human osteosarcoma cell lines and tumor samples. A, β4 and β1 integrin expression were determined by Western blot analysis and β4 integrin was also analyzed by Northern blot analysis in 4 osteosarcoma (U2OS, SaOS, G292, and HOS), 2 rhabdomyosarcoma (RD and Rh18), and 2 Ewing’s sarcoma (RDES and TC32) cell lines. B, expression of β4, β1, β3, and α6 integrins was determined by Western blot analysis in the non-metastatic human osteosarcoma cell line HOS and the highly metastatic cell line MNNG-HOS. C, tissue array analysis of β4 integrin expression was performed on human osteosarcoma samples by immunohistochemistry.
Osteosarcoma patient samples express high levels of β4 Integrin
In order to extend the analysis of β4 integrin expression from cell lines to patient tumor samples we utilized immunohistochemical staining of tissue arrays. The Emory osteosarcoma tissue array consists of 48 samples, 41 of which are high-grade conventional osteosarcoma. All samples expressed β4 integrin. The majority of the samples had high levels of expression (3+ intensity). Three samples had low levels of expression (1+ intensity) and all three were from patients with parosteal osteosarcoma. This subtype of osteosarcoma is a true low-grade lesion with low metastatic potential. Figure. 1C shows representative images of 1+ and 3+ intensities. Although the Emory tissue array is linked with patient outcome, the homogenous high levels of expression present in almost all of the samples precluded stratification of the samples based on histology type, location of the tumor, presence of metastatic disease or outcome.
Knockdown of β4 integrin expression inhibits lung metastases in MNNG-HOS cells
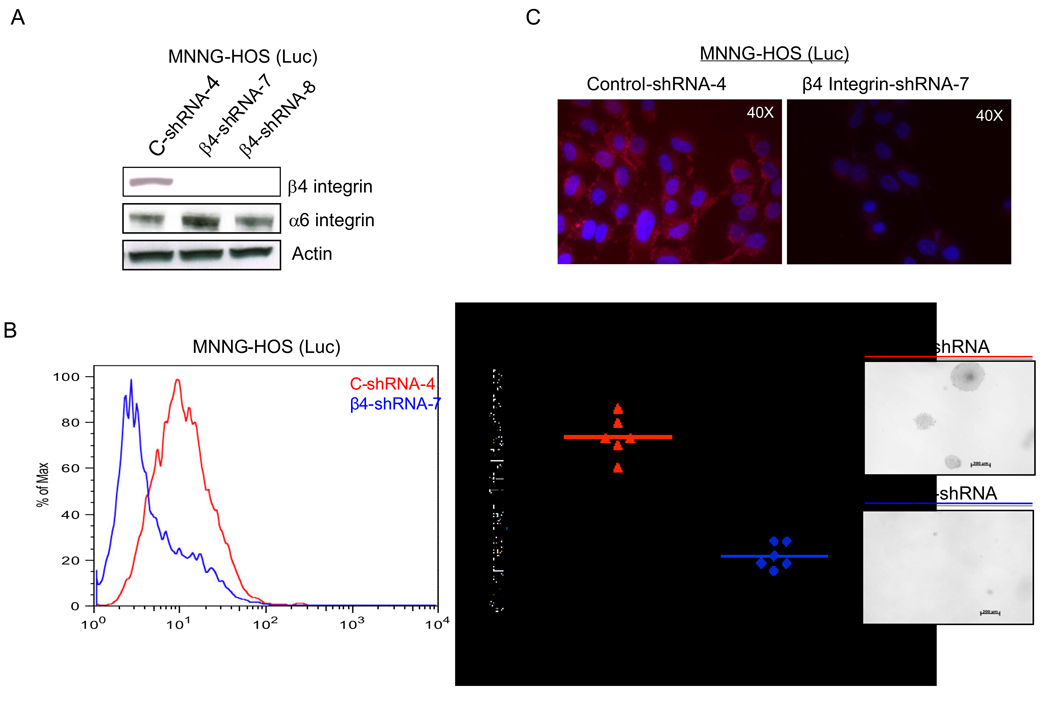
To test the hypothesis that β4 integrin expression correlates with tumor metastases, we used RNA interference to block β4 integrin expression in highly metastatic MNNG-HOS cells. β4 integrin expression was significantly suppressed in MNNG-HOS (Luc) cells infected with a lentivirus that expresses β4 integrin-shRNA in comparison with control-shRNA (Figure 2A). In addition, cell surface expression of β4 integrin was markedly decreased for the β4 integrin-shRNA cell line compared to control-shRNA cell line (Figure 2B). Similar results were seen using different anti-β4 integrin (CD104) antibodies (data not shown). Moreover, immunofluorescent staining for β4 integrin detected a stronger signal in control-shRNA cells compared to β4 integrin knockdown cells. β4 integrin staining signal was mainly localized at the cell surface and the cytoplasmic boundaries at cell-cell interface (Figure 2C).
Figure 2.
Knockdown of β4 integrin expression results in a clumpy cell morphology in 3D co-cultures. A, MNNG-HOS (Luc) cells were infected with lentivirus expressing control-shRNA or β4 integrin-shRNA. Expression β4 and α6 integrins was analyzed by Western blot analysis in control-shRNA or β4 integrin-shRNA infected cells. B, the cell surface expression of β4 integrin was evaluated by FACS in control and β4 integrin knockdown cells. Normal IgG was used as a negative control (figure not shown). C, the surface expression and localization of β4 integrin were examined by immunofluorescent staining using same antibody as above in control and knockdown cells. D, MNNG-HOS (Luc) cells infected with lentivirus of control-shRNA (left panel) or β4 integrin-shRNA (right panel) are shown on top as traditional 2D images. Below, are ostoesarcoma cells that were labeled with CellTracker Green CMTPX dye (green). NIH3T3 fibroblasts were labeled separately with CellTracker Orange CMRA dye (red). All cells were then labeled with Hoechst 33342 nuclear dye (blue) and co-cultured in a nanofiber-based matrix labeled with Cy5 dye (white) containing Cultrex basement membrane extract. The lower right panels show merged views.
Knockdown of β4 integrin did not influence the proliferative capability of theses cells in vitro (data not shown) and did not cause morphological changes in these cells. However, there was a marked decrease in anchorage independent growth of the β4 integrin shRNA cell line versus the control shRNA cell line (Figure 2D). Migration through a porous membrane and invasion through a matrigel coated porous membrane resulted in small differences in the majority of experiments, but these were not always consistent, thus precluding an interpretation.
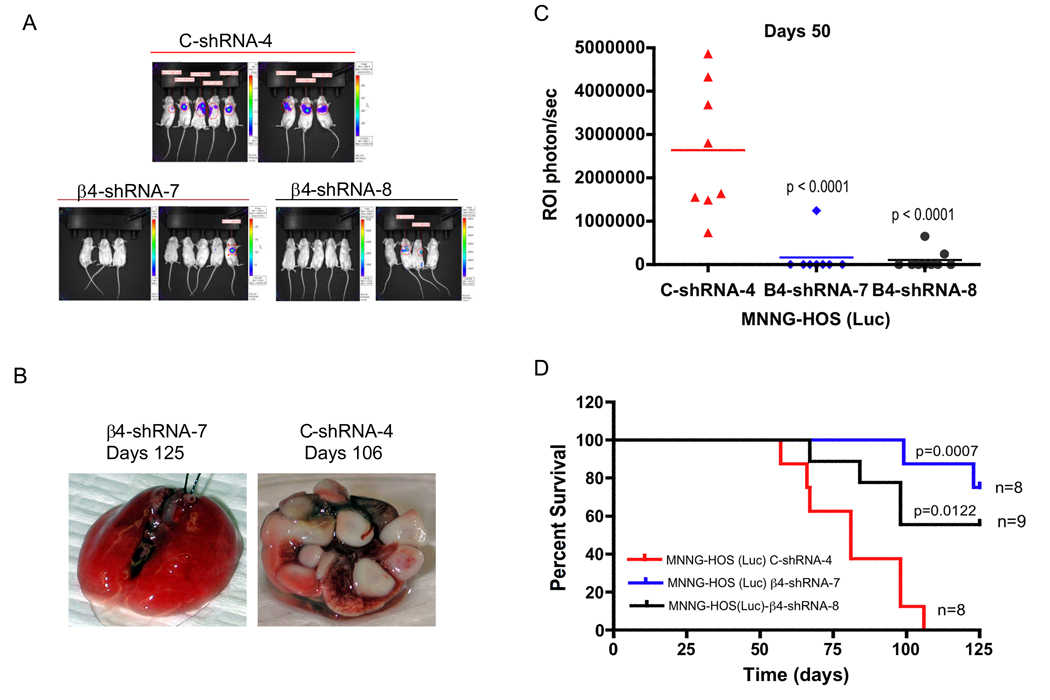
To further test the hypothesis that β4 integrin is an important contributor to tumor metastases, we injected control-shRNA or β4 integrin-shRNA cells into the tail vein of RAG2 knockout mice[JB1]. After injection of these cells, lung metastases in the mice were detected and monitored by bioluminescent imaging of luciferase activity (Figure 3A). β4 integrin-shRNA-7 and −8 groups showed a significant decrease in luminescent intensity compared to the control-shRNA group at day 50 (Figure 3A and B). All eight mice in the control-shRNA group had a luminescent signal 50 days after injection of the cells, but only one of eight (12.5%) mice in the β4 integrin-shRNA-7 group and two of nine (22%) mice in the β4 integrin-shRNA-8 group had a luminescent signal within the lung (Figure 3A and B). We continued to monitor survival of the mice for 125 days. Mice that had suppression of β4 integrin had significantly prolonged survival compared to control mice (Figure 3C). In the control-shRNA group, there were no long-term survivors and all 8 mice died prior to day 106 (Figure 3D). In contrast, 70% of mice with knockdown of β4 integrin were alive on day 125, when the experiment was stopped (6/8 mice in the shRNA-7 group and 6/9 mice in the shRNA-8 group). To determine whether β4 integrin is still suppressed in the metastatic tumors of mice that were injected with β4 integrin knockdown cells, we examined β4 integrin in the metastatic tumors of lung by immunohistochemistry. β4 integrin is highly re-expressed in these tumor samples at 125 days injection of β4 integrin knockdown cells (data not shown). The mechanism by which the tumors re-express β4 integrin remains unclear, but further work on the time course to re-expression may help elucidate at which point in the metastatic cascade β4 integrin functions. In addition, we also examined the effects of β4 integrin on primary tumor growth in vivo and spontaneous metastases. Knockdown of β4 integrin by shRNA failed to decrease primary tumor growth in vivo, but did inhibit spontaneous metastases (p=0.03).
Figure 3.
Control-shRNA and β4 integrin-shRNA infected MNNG-HOS (Luc) cells were injected into the tail vein of RAG knockout mice. These mice then underwent luminescent imaging. Suppression of β4 integrin expression by shRNA led to significant reduction of luminescent intensity in the lungs of mice 50 days after injection of cells, shown graphically (A) with representative lung images at time of sacrifice (B). C, quantitation of luminescence for animals in (A). D, Kaplan-Meier survival curves for MNNG-HOS control-shRNA or β4-shRNA cells.
Experimental metastasis is reduced by expressing mutant β4 integrin (Y1494F) in MNNG-HOS cells
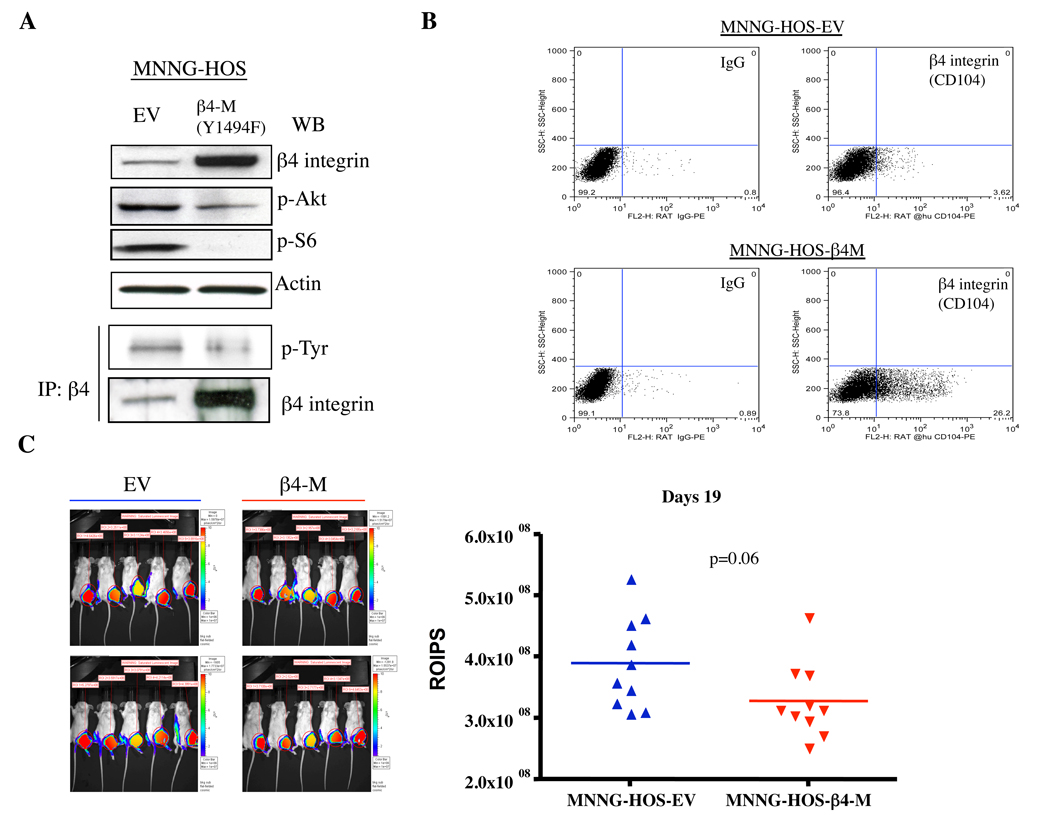
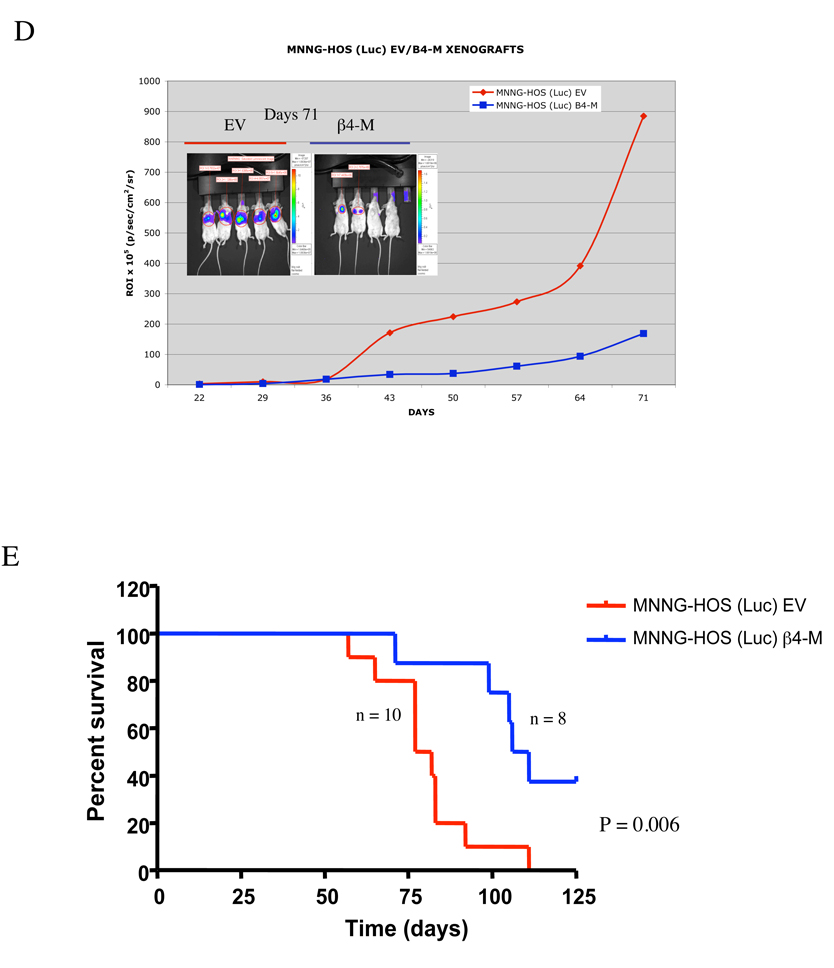
To evaluate the role of functional β4 integrin during metastasis, MNNG-HOS (Luc) cells were stably transfected with mutant dominant negative β4 integrin (Y1494F) (Figure 4A). To determine the extent to which transfection of the mutant construct inhibits β4 integrin function, we assayed the tyrosine phosphorylation of the mutant β4 integrin after immunoprecipitating with β4 integrin-specific antibodies followed by anti-phospho-tyrosine Western blot analysis. The Y1494 mutation construct resulted in a significant decrease in levels of β4 integrin-tyrosine, Akt and S6 kinase phosphorylation (Figure 4A). Moreover, the cell surface expression of β4 integrin was significantly increased in mutant β4 integrin (Y1494F)-transfected cells compared to empty vector-transfected cells (Figure 4B). The Y1494F–expressing MNNG-HOS (Luc) cells were then injected either orthotopically into the gastrocnemius muscle or via tail vein to assess the consequences of the Y1494F β4 integrin mutant on primary tumor growth and tumor metastasis. Mutant β4 integrin (Y1494F) had limited effects on the suppression of primary tumor growth as measured by bioluminescent imaging of luciferase activity (p=0.06)(Figure 4C). In addition, there was also slight increase in survival in a spontaneous metastasis model (88 days versus 76 days). However, these results did not reach statistical significance (p=0.07). This trend to increased survival mimics the results we saw using shRNA knockdown but the lack of statistical significance may reflect differences between RAG2 and SCID/BEIGE mice. Expression of mutant β4 integrin (Y1494F) significantly inhibited experimental pulmonary metastasis. Mice injected with mutant β4 integrin (Y1494F) containing cells had significantly lower luminescent intensity in the lungs compared to mice injected with cells expressing empty vector (Figure 4D). Disruption of β4 integrin also resulted in significantly prolonged survival (p=0.006) (Figure 4E). These data suggest that blockade of β4 integrin function had a more significant impact on experimental metastasis.
Figure 4.
Effect of dominant negative β4 integrin expression on primary tumor growth and experimental metastases in vivo. A, disruption of β4 integrin function by transfection of mutant dominant negative β4 integrin. MNNG-HOS cells were transfected with empty vector (EV) and dominant-negative β4 integrin (Y1494F). After batch selection with medium containing G418, cells were lysed and subjected to Western blot analysis of β4 integrin expression (top panel), and immunoprecipititated with β4 integrin antibody overnight followed by Western blot for phospho-tyrosine and β4 integrin (lower panel). B, the cell surface expression of mutant β4 integrin was measured by FACS in mutant β4 integrin (Y1494F) transfected cells. C, empty vector (EV)- and dominant negative β4 integrin (β4-M)-transfected MNNG-HOS (luc) cells were injected into the gastrocnemius muscle of SCID/BEIGE mice and assayed for growth of primary tumors by luminescent imaging (left panel) and quantitation of luminescence signal (right panel). D, empty vector (EV)- and dominant negative β4 integrin (β4M)-transfected MNNG-HOS (luc) cells were injected into the tail vein of RAG knockout mice. Luminescence was quantified weekly with mean intensity for each group plotted over time. E, Kaplan-Meier survival curves showing percent survival for each cell line over time.
β4 integrin interacts with ezrin, which is required for maintenance of β4 integrin expression
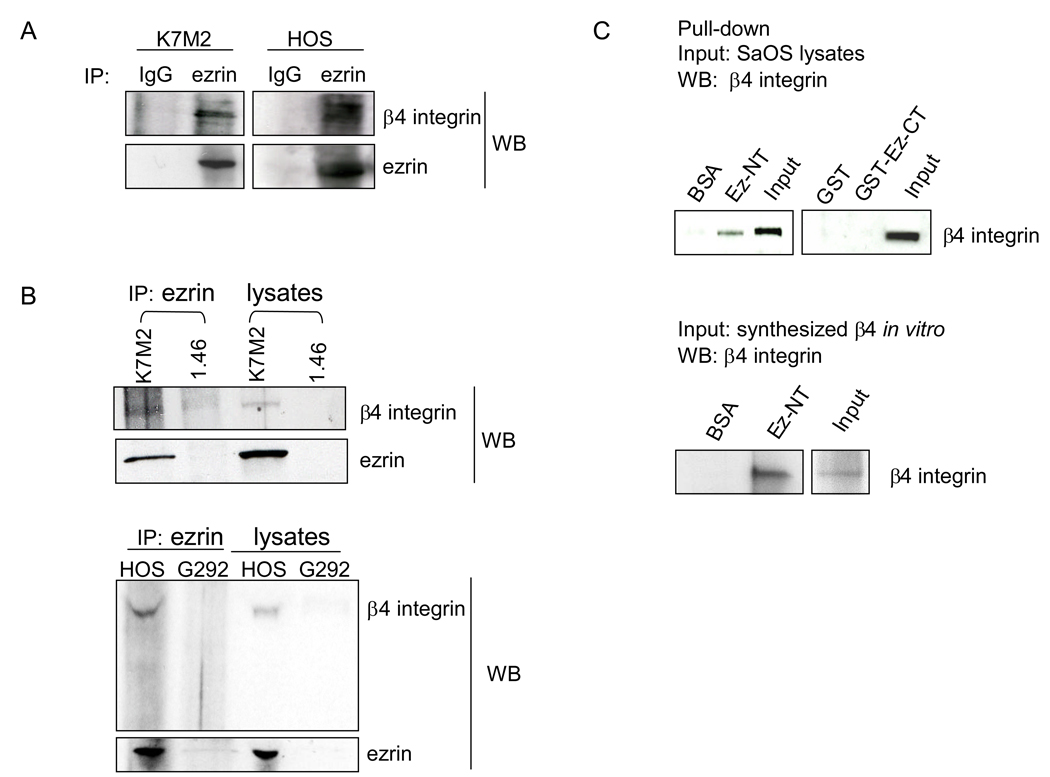
We have previously shown that the cytoskeletal linker protein ezrin is necessary for metastases in a murine model of osteosarcoma (Khanna et al., 2004). However, the mechanism by which ezrin mediates the metastatic process remains unknown. To investigate whether β4 integrin and ezrin interact in cell lysates, coimmunoprecipitation was conducted followed by Western blot analysis. As shown in Figure 5A, β4 integrin was detected in the ezrin immunoprecipitate in both HOS and K7M2 cells which express high levels of ezrin, but was not detected in negative control cells G292 (very low levels of ezrin) and K7M2 ezrin-knockdown 1.46 cells (Figure 5B). Of note[JB2], we could not detect β1 integrin in the ezrin immunoprecipitation in HOS cells (data not shown). We next examined the binding interaction between β4 integrin and ezrin by in vitro pull-down assays. We found that in vitro synthesized β4 integrin or endogenous β4 integrin from SaOS cell lysates were able to bind to the N-terminal region of ezrin, whereas the BSA control and ezrin C-terminal region did not bind β4 integrin (Figure 5C). SaOS cells were selected for this analysis because these cells have the highest level of β4 integrin expression (Figure 1A).
Figure 5.
β4 integrin interacts with ezrin. A and B, cell lysates were immunoprecipitated (IP) with an anti-ezrin antibody overnight followed by Western blot analysis for β4 and ezrin using the indicated antibodies (WB). C, purified recombinant proteins for BSA, ezrin-N-terminal region (residues 1–297)(Ez-NT), GST, and GST-ezrin-C-terminal region (residues 280–585)(GST-Ez-CT) were immobilized onto glutathione-sepharose beads and incubated overnight with lysates (input) from SaOS cells or in vitro synthesized β4 integrin. The bound fractions were washed and subjected to SDS-PAGE, followed by immunoblotting with anti-β4 integrin antibody.
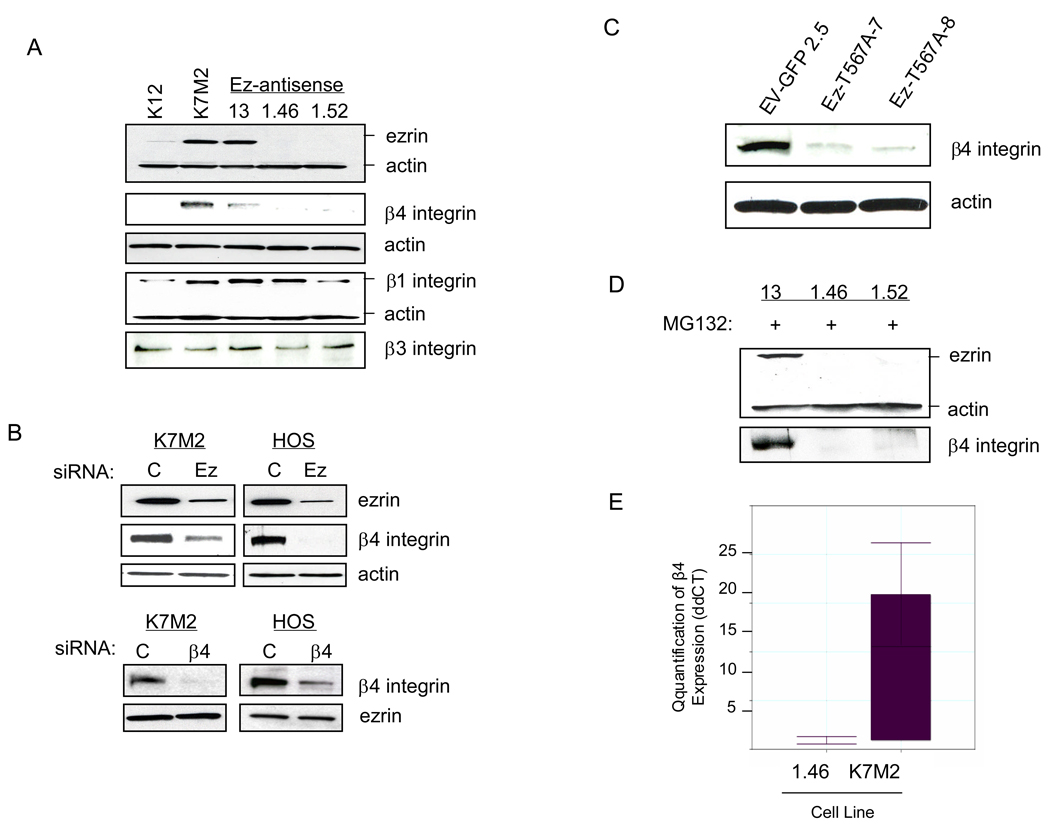
The results shown in Figure 5 reveal that β4 integrin associates with ezrin. To determine the consequences of this association, we examined β4 integrin protein expression in ezrin knockdown cells by Western blot analysis. Suppression of ezrin either by stable transfection of antisense DNA in K7M2 cells or by siRNA in both K7M2 and HOS cells resulted in a marked reduction in β4 integrin protein levels (Figure 6A and B). In contrast, knockdown of β4 integrin by siRNA in both K7M2 and HOS cells failed to alter ezrin expression (Figure 6B). In addition, disruption of ezrin function by transfection of a dominant-negative ezrin-T567A mutant led to decreased β4 integrin expression (Figure 6C). However, transfection of mutant β4 integrin (Y1494F) failed to alter ezrin expression (data not shown). To determine whether the decreased protein level of β4 integrin in ezrin knockdown cells was due to an increased rate of protein turnover, cells were treated with MG132, a proteasome inhibitor, to block protein degradation. As shown in Figure 6D, exposure of cells to MG132 (50 µM) for 6 hours failed to reverse the decreased β4 integrin protein expression induced by ezrin knockdown. This suggested that the ezrin effect on β4 integrin levels might be at the level of transcription. In order to test this hypothesis, K7M2 and AS1.46 cells were analyzed for steady-state levels of β4 integrin mRNA by quantitative real-time PCR (qRT-PCR). As shown in Figure 6E, levels of β4 integrin mRNA are upregulated >15-fold compared to K7M2 ezrin-knockdown 1.46 cells. These findings suggest that ezrin is required for the maintenance of β4 integrin at both the RNA and protein levels.
Figure 6.
Both ezrin expression and function are required for maintenance of β4 integrin protein expression. A, The expression of ezrin, β1, β3, and β4 integrin, were analyzed by Western blot in K12, K7M2, and K7M2-ezrin antisense cell lines. B, K7M2 and HOS transfected with control (C) or ezrin (Ez) or β4 siRNA. After 3 days, cells were lysed and subjected to Western blot analysis for ezrin and β4 integrin expression. C, β4 integrin expression was analyzed by Western blot in empty vector-GFP and mutant ezrin (T567A) line clones derived from the parental K7M2 cell line. D, K7M2-ezrin antisense cell line clones 13, 1.46 and 1.52 were treated with the proteosome inhibitor, MG132 (50 mM), for 6 h and then analyzed by Western blot for ezrin and β4 integrin expression. E, quantitative RT-PCR analysis for steady-state levels of β4 integrin mRNA in parental K7M2 cells relative to the K7M2-ezrin antisense 1.46 cell line. Samples were normalized to GAPDH mRNA. Mean normalized fold expression is shown from duplicate, independently isolated RNA samples for each cell line.
Discussion
Hallmarks of the metastatic process include increased motility of tumor cells and decreased adhesion to surrounding cells and tissues. Both of these processes are associated with increased and decreased expression of specific integrins (Hood and Cheresh, 2002). The expression of β4 integrin is maintained, and often upregulated, in various types of invasive and metastatic tumors, correlating with poor prognosis (Mercurio and Rabinovitz, 2001; Tagliabue et al., 1998; Grossman et al., 2000; Lu et al., 2008). Consistent with these findings, we found that β4 integrin is highly expressed in osteosarcoma cell lines and patient tumors, in both metastatic sites and primary tumors. The latter is not surprising, since historical data shows that 80% of patients who undergo surgical resection only, will eventually develop metastatic disease, demonstrating the high metastatic potential of this disease (Gaffney et al., 2006). Furthermore, β4 integrin is upregulated in highly metastatic MNNG-HOS cells, compared to non-metastatic parental HOS cells. We tested whether the metastasic behavior of MNNG-HOS may be a reflection of changes in β4 integrin.
We examined the consequences β4 integrin knockdown in MNNG-HOS cells in vitro and found an inability to grow in anchorage independent conditions. Our in vivo data demonstrated that expression of β4 integrin is crucial for the metastatic phenotype in the MNNG-HOS human osteosarcoma model, since knockdown of β4 integrin markedly suppressed experimental pulmonary metastasis. This was strengthened by our findings that sh-RNA mediated knockdown also led to an increase in survival using the more stringent spontaneous model of metastasis.
Integrins influence cell behavior not only by providing a docking site for ECM proteins at the cell surface, but also by acting to relay molecular cues regarding the cellular environment that influence cell shape, growth, survival and migration (Hood and Cheresh, 2002; Gao and Giancotti, 2004). β4 integrin contains a large unique cytoplasmic domain (1017 amino acid), in which a critical tyrosine residue (Y1494) has been identified in the third fibronectin type III repeat of this domain. This residue has been shown to be essential for β4 integrin-mediated activation of PI3K (Shaw, 2001). Introduction of mutant β4 (Y1494F) into MNNG-HOS (Luc) cells resulted in reduced phosphorylation of Akt and S6, both of which are downstream targets of PI3K and mTOR. Furthermore, expression of mutant β4 integrin (Y1494F) resulted in significant reduction of experimental pulmonary metastasis of MNNH-HOS cells, but only a minimal effect on the inhibition of primary tumor growth. Thus, these data suggest that β4 integrin plays a more important role in promoting tumor metastases.
Our previous studies have shown that high expression of ezrin, a membrane-cytoskeletal linker protein, is associated with the metastatic phenotype and activation of Akt/mTOR signaling in osteosarcoma (Khanna et al., 2004; Wan et al., 2005). However, the mechanism by which ezrin mediates metastases remains unclear. In this study, we found that ezrin can interact with β4 integrin both in vitro and in vivo (Figure 5A and B). Moreover, a key finding in the present study is that the association between β4 integrin and ezrin is essential for maintenance of β4 integrin protein expression in osteosarcoma cell lines. We have demonstrated that this occurs at the level of β4 interin transcription but the mechanism by which this occurs is currently unclear.Thus, these data provide a new link in understanding the mechanism of ezrin on the regulation of tumor metastases. Integrins lack enzymatic activity, and are thus dependent on recruitment of adaptor and signaling proteins for effector functions (Liu et al., 2002; Geiger et al., 2001). These proteins include integrin-binding proteins such as talin, adaptors and/or scaffolding proteins that lack intrinsic enzymatic activity such as vinculin, paxillin, tensin and α-actin, and enzymes that modify integrin downstream effectors such as the non-receptor tyrosine kinases FAK (focal adhesion kinase) and Src. Ligand binding to the extracellular integrin domain induces conformational changes and lead to integrin clustering and recruitment of actin-associated proteins and signaling proteins, which link the integrin to the cytoskeleton (Hynes, 2002; Giancotti, 2003). Talin has been demonstrated to be a major cytoskeletal actin-binding protein that binds to the tails of integrins β1, β2, β3, β5 and β7, linking them to the cytoskeleton (Galderwood et al., 1999; Calderwood et al., 2001; Calderwood et al., 2003;Pfaff et al., 1999). In addition, at least two other β integrin tail-binding proteins, β3-endonexin and cytohesin, have also been identified (Kashiwagi et al., 1997; Kolanus et al., 1996). Our study identifies a previously uncharacterized interaction between ezrin and β4 integrin, but not β1 integrin. The interaction between ezrin and β4 integrin appears to be essential for maintenance of β4 integrin expression levels. In this regard, we do not yet know how ezrin regulates β4 integrin but this will be another interesting avenue to investigate in the future.
Materials and Methods
Cell lines
Human osteosarcoma cell lines HOS, MNNG-HOS, U2OS, SaOS and G292 were obtained from the American Type Culture Collection (Rockville, MD). Rhabdomyosarcoma and Ewing’s sarcoma cell lines RD, Rh18, RDES and TC32 have been described previously (Wan and Helman, 2003; Liu et al., 2000). Murine osteosarcoma cell lines K12, K7M2, and ezrin-antisense clones 13, 1.46 and 1.52 cells generated from K7M2 have been previously described (Khanna et al., 2004; Wan et al., 2005). pMSCVpuro-Luciferase was nucleofected into MNNG-HOS cells using the Nucleofector II apparatus (Amaxa Biosystems, Rockville, MD), and were selected following the addition of 2.5 µg/ml of puromycin (Sigma-Aldrich, St. Louis, MO). The MNNG-HOS (Luc) β4 integrin-shRNA and control-shRNA (using a scrambled β4 oligonucleotide) cell lines were generated by infecting these cells with lentivirus of β4 integrin-shRNA or control-shRNA, which were kindly provided by Dr Livio Trusolino (Bertotti et al., 2005; Bertotti et al., 2006) and the virus-containing cell culture supernatants were made by SAIC-Frederick Inc (Frederick, MD). The MNNG-HOS (Luc) cells stably expressing mutant β4 integrin (Y1494F) were generated by nucleofection of these cells with β4 integrin (Y1494F) plasmid or empty vector (a gift from Dr Arthur M. Mercurio)(Shaw, 2001; Chung et al., 2002) following batch section with the addition of 0.5 mg/ml G418.
In vitro Analysis
A base of 0.5% agar in 0.5X media containing 10% FBS was added to 6-well plates. 5,000 cells were added to a 0.33% agar solution in 0.5X media containing 10% FBS and incubated for 20 days. Followed by counting per field using light microscopy. Migration and invasion and migration assays have been described previously (Kim 2008).
Western Blot Analysis
Western blot analysis was done as published previously (Wan et al., 2005). Anti-β4 antibodies were purchased from Cell signaling Technology Inc. (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA). β1, and β3 integrin antibodies were purchased from Santa Cruz Biotechnology. Anti-α6 integrin antibody was purchased from R&D Systems (Minneapolis, MN). Anti-ezrin antibody was purchased from Sigma Chemical Co (St. Louis, MO). Anti-phospho-Tyrosine (P-Tyr-100) was purchased from Cell Signaling Technology Inc. Anti-actin antibody was purchased from Abcam Inc. (Cambridge, MA).
Northern blot analysis
Northern blot analysis was performed as described previously (Wan and Helman, 2003). To generate β4 integrin probe used for hybridization, specific primers (forward, 5’-GACCTGTACATCCTCATGGAC-3’; and reverse, 5’-ATCCAGGTTGCCTGAGATCC-3’) were used to amplify a 300-bp product with a wild-type β4 integrin plasmid DNA (a gift from Arthur M. Mercurio) as a template.
Quantitative RT-PCR
RNA was isolated using Trizol® LS reagent (Invitrogen Corporation, Carlsbad, CA) and purified using the RNeasy spin column clean up protocol according to manufacturers instructions (Qiagen Corporation, Valencia, CA). Samples were quantitated using a Nanodrop apparatus and 1µg of total RNA was reverse transcribed using random hexamers. A Universal Mouse Reference RNA (catalog #740100, Stratagene, La Jolla, CA) was used as a positive control. 2µl of diluted cDNA was used as a template for quantitative PCR using the iQ SYBR Green Supermix and assayed using an iQ5 real time thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA). The following primers were used at a final concentration of 100nM: GAPDH sense primer 5’-CCCCAATGTGTCCGTCGTG-3’, GAPDH antisense primer 5’-GCCTGCTTCACCACCTTCT-3’, β4 integrin sense primer 5’-TGACGATCTGGACAACCTCAAGCA-3’, β4 integrin antisense primer 5’-ATCCAATGGTGTAGTCGCTGGTGA-3’. Melt curve analysis was conducted following each run and a single product was observed for each primer pair and condition. Quantitation was done using the formula 2−ddCT with GAPDH as the reference gene for normalization between conditions and K7M2 values graphed relative to K7M2 ezrin-knockdown 1.46 cells which were set at a value of 1 (Livak and Schmittgen, 2001).
RNA interference
The siRNAs directed against the human ezrin (target sequence: 5’-AAGGAAUCCUUAGCGAUGAGA-3’), mouse ezrin (target sequences of pool: 5’-GGUACAGGACUCUCCGAUAUU-3’; 5’-CCGCACAGGAGGUCCGAAAUU-3’; 5’-GCGCAAGGAGGACGAGGUAUU-3’; 5’-UAUAAGACGCUGCGGCAAAUU-3’), both human and mouse β4 integrin (target sequence: 5’-AAGAACCGGAUGCUGCUUAUU-3’), and a negative control (non-specific target sequence: 5’-AATAGCGACTAAACACATCAA-3’) were purchased from Dharmacon Research Inc. (Lafayette, CO). We applied siRNA duplexes at a final concentration of 100 nM using LipofectAmine 2000 Reagent (Invitrogen, Carlsbad, CA). Lysates were made 72 hours post-transfection.
Immunohistochemistry
Sixty cases of osteosarcoma were retrieved from the pathology files at Emory University. A total of 114 tissue cores were inserted into one recipient paraffin block in duplicate resulting in an osteosarcoma tissue array. Slides were deparaffinized and antigen retrieval was performed using DakoCytomation target retrieval solution (Dako, Carpinteria, CA) in a steam bath for 20 minutes. Samples underwent quenching of endogenous peroxidase using DakoCytomation peroxidase blocking reagent followed by blocking using 10% non-immune normal goat serum (Zymed, Carlsbad, CA). Rabbit polyclonal β4 integrin primary antibody (sc9090, Santa Cruz Biotechnology) at 1:50 dilution was added overnight followed by biotinylated polyclonal goat anti-rabbit immunoglobulin secondary antibody (Dako) at 1:100 dilution for 1 hour. Control slides were treated similarly, without the addition of the primary antibody. Samples underwent streptavidin conjugation using DakoCytomation streptavidin HRP solution, exposure to DakoCytomation DAB chromagen substrate mixture and staining with hematoxylin (Sigma-Aldrich, St. Louis, MO). Lungs from mice injected with MNNG-HOS cells infected with either β4 integrin-shRNA or control-shRNA were harvested, fixed in 10% formalin, sectioned and stained with H+E at American HistoLabs (Gaithersburg, MD). Unstained stained sections were subjected to immunohistochemistry using the conditions above. The antibody recognizes both human and mouse β4 integrin.
Flow Cytometry
Cells were resuspended in fluorescence-activated cell sorting (FACS) buffer (1X BPS, 0.5% FBS and 2 mM EDTA) containing PE-labeled anti-human CD104 or PE-labeled rat IgG as a negative control (BD pharmingen Inc, San Diego, CA). After incubation for 30 min on ice, cells were washed in FACS buffer and analyzed by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA).
Immunofluorescent Staining
Cells growing in 2-well coverslip chambers (Nunc, Rochester NY) at 50% confluence were labeled with Hoechst 33342 nuclear stain for 5 minutes, fixed with a 3.7% formaldehyde solution for 5 minutes and blocked using 10% non-immune goat serum (Zymed/Invitrogen, Carlsbad CA). PE conjugated Rat Anti-Human CD104 antibody at 1/50 dilution was added for 48 hours at 4C (BD Pharmingen, San Jose, CA). Cells were rinsed with PBS three times and viewed using a Zeiss AxioObserver Z1 microscope (Zeiss Inc., Thornwood, NY) using 20X and 40X differential interference contrast objectives and appropriate filter sets. Images were captured with a Zeiss AxioCam MRm monochrome digital camera and analyzed using AxioVision 4.6 software.
In vivo studies
Animal studies were performed in accordance with guidelines of the National Institutes of Health Animal Care and Use Committee. For the experimental metastases model, we injected 1 × 106 cells into the tail vein of RAG2 knockout mice (Taconic, Germantown, NY). For the xenograft tumor model, we injected 2 × 106 cells intramuscularly into the gastrocnemius muscle to generate orthotopic tumors in SCID/beige mice (Charles River Laboratories, Wilmington, MA). The Xenogen IVIS 100 Imaging System (Xenogen, Cranbury, NJ) was utilized to detect luminescence in vivo. Mice were injected intraperitoneally with 100 µl (2.5 mg) of luciferin (Xenogen). After 12 minutes, the mice were anesthetized with isoflurane and imaged.
β4 integrin-ezrin coimmunopercipitations
Cells were lysed in lysis buffer as previously described (Wan et al., 2005). Lysates were centrifuged at maximum speed for 10 min and supernatants were precleared for 1 h with protein-G Sepharose. Precleared supernatants were immunoprecipitated with polyclonal antibody anti-ezrin (a gift from Dr Anthony Bretscher) or anti-β4 integrin overnight followed by anti-β4 integrin or anti-ezrin Western blotting.
Pull-down assays
Bovine serum albumin (BSA) and human ezrin-N terminal (residues 1–297) beads were kindly provided by Dr R Nguyen (Nguyen et al., 2001). GST and GST-ezrin-C terminal constructs (residues 280–585) were kindly provided by Dr M Arpin (Andreoli et al., 1994). Recombinant GST and GST-Ez-CT were produced in Escherichia coli and purified using MagneHis Protein Purification System (Promega, Madison, WI) followed the manufacturer’s instructions. Synthesized β4 integrin in vitro was produced using the TNT T7 Quick Coupled Transcription/Translation system (Promega, Madison, WI) according to manufacturer’s instructions with a wild-type β4 integrin plasmid DNA as the template. For detecting interaction of β4 integrin and ezrin-N terminal, SaOS lysate or synthesized β4 integrin in vitro were incubated on BSA or ezrin N-terminal beads overnight at 4°C. For detecting interaction of β4 integrin and ezrin-C terminal, purified bacterial lysates of GST and GST-Ez-CT were incubated in glutathione-coupled sepharose beads (Amersham Biosciences, Piscatawa, NJ) for 1 h at 4°C. After washing with lysis buffer, the beads were incubated with SaOS lysate overnight at 4°C. These beads were then washed 4 times with lysis buffer, eluted in SDS sample buffer, and subjected to SDS-PAGE. Bound β4 integrin was detected by Western Blot analysis using anti-β4 integrin antibody.
Statistical analysis
Statistical analyses were performed in Prism version 4.0 (GraphPad Software). Statistical analysis of the ROI in the lung metastasis imaging was determined using a Nonparametric Mann-Whitney t-test. Log-rank and log-rank trend statistics were used for survival curves. Statistical significance was defined as P < 0.05.
Acknowledgements
We are thankful to Dr AM Mercurio for providing empty vector and β4 mutant (Y1494F), Dr L Trusolino for providing control-shRNA and β4-shRNA, Dr M Arpin for providing GST and GST-ezrin-CT, Dr R Nguyen for providing BSA and ezrin-NT. This Research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Andreoli C, Martin M, Le Borgne R, Reggio H, Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J Cell Sci. 1994;107:2509–2521. doi: 10.1242/jcs.107.9.2509. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res. 2005;65:10674–10679. doi: 10.1158/0008-5472.CAN-05-2827. [DOI] [PubMed] [Google Scholar]
- Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol. 2006;175:993–1003. doi: 10.1083/jcb.200605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Huttenlocher A, Kiosses WB, Rose DM, Woodside DG, Schwartz MA, et al. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol. 2001;3:1060–1068. doi: 10.1038/ncb1201-1060. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Fujioka Y, de Pereda JM, Garcia-lvarez B, Nakamoto T, Margolis B, et al. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165–174. doi: 10.1083/jcb.200112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nigris F, Rossiello R, Schiano C, Arra C, Williams-Ignarror S, Barbieri A, et al. Deletion of Yin Yang 1 protein in osteosarcoma cells on cell invasion and CXCR4/angiogenesis and metastasis. Cancer Res. 2008;68:1797–1808. doi: 10.1158/0008-5472.CAN-07-5582. [DOI] [PubMed] [Google Scholar]
- Falcioni R, Sacchi A, Resau J, Kennel SJ. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res. 1988;48:816–821. [PubMed] [Google Scholar]
- Gaffney R, Unni KK, Sim FH, Slezak JM, Esther RJ, Bolander ME. Follow-up study of long-term survivors of osteosarcoma in the prechemotherapy era. Hum Pathol. 2006;244:799–804. doi: 10.1016/j.humpath.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Grossman HB, Lee C, Bromberg J, Liebert M. Expression of the alpha6beta4 integrin provides prognostic information in bladder cancer. Oncol Rep. 2000;7:13–16. [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Harris MB, Gieser P, Goorin AM, Ayala A, Shochat SJ, Ferguson WS, et al. Treatment of metastatic osteosarcoma at diagnosis: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16:3641–3648. doi: 10.1200/JCO.1998.16.11.3641. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nature Reviews Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kashiwagi H, Schwartz MA, Eigenthaler M, Davis KA, Ginsberg MH, Shattil SJ. Affinity modulation of platelet integrin alphaIIbbeta3 by beta3-endonexin, a selective binding partner of the beta3 integrin cytoplasmic tail. J Cell Biol. 1997;137:1433–1443. doi: 10.1083/jcb.137.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee CH, Midura BV, Yeung C, Mendoza A, Hong SH, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008;25:201–211. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, et al. Alpha L beta 2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- Lipscomb EA, Simpson KJ, Lyle SR, Ring JE, Dugan AS, Mercurio AM, et al. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 2005;65:10970–10976. doi: 10.1158/0008-5472.CAN-05-2327. [DOI] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2002;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- Liu XF, Helman LJ, Yeung C, Bera TK, Lee B, Pastan I. XAGE-1, a new gene that is frequently expressed in Ewing’s sarcoma. Cancer Res. 2000;60:4752–4755. [PubMed] [Google Scholar]
- Livak KG, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu S, Simin K, Khan A, Mercurio AM. Analysis of Integrin {beta}4 Expression in Human Breast Cancer: Association with Basal-like Tumors and Prognostic Significance. Clin Cancer Res. 2008;14:1050–1058. doi: 10.1158/1078-0432.CCR-07-4116. [DOI] [PubMed] [Google Scholar]
- MacEwen EG, Kutzke J, Carew J. c-Met tyrosine kinase receptor expression and function in human and canine osteosarcoma cells. Clin Exp Metastasis. 2003;20:421–430. doi: 10.1023/a:1025404603315. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion—lessons from the α6β4integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- Meyers PS, Heller G, Healey G, Huvos A, Applewhite A, Sun M, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11:449–453. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- Nguyen R, Reczek D, Bretscher A. Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem. 2001;276:7621–7629. doi: 10.1074/jbc.M006708200. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Pfaff M, Du X, Ginsberg MH. Calpain cleavage of integrin beta cytoplasmic domains. FEBS Lett. 1999;460:17–22. doi: 10.1016/s0014-5793(99)01250-8. [DOI] [PubMed] [Google Scholar]
- Rhim JS, Park DK, Arnstein P, Huebner RJ, Weisburgern EK, Nelson-Rees WA. Transformation of human cells in culture by N-methyl-N’-nitro-N-nitrosoguanidine. Nature. 1975;256:751–753. doi: 10.1038/256751a0. [DOI] [PubMed] [Google Scholar]
- Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol. 2001;21:5082–5093. doi: 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene. 2003;22:8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–2411. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]