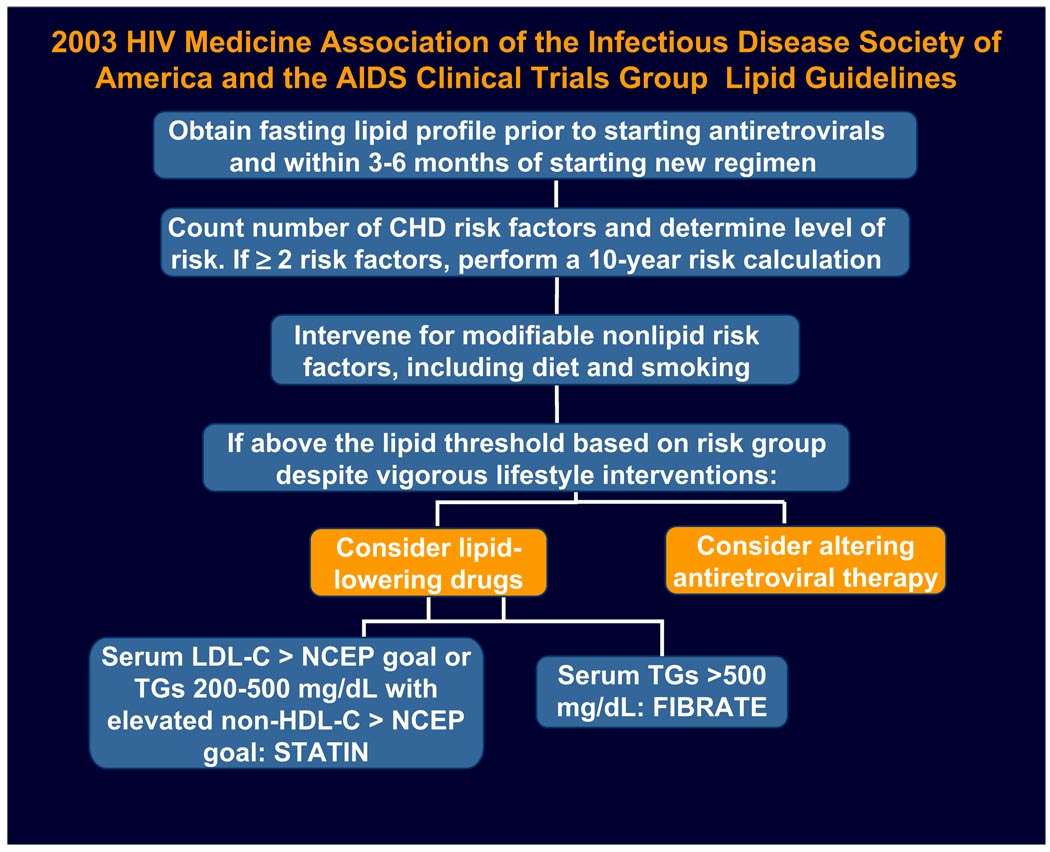
HIV-infected persons may be at increased risk for the development of coronary heart disease because of the chronic inflammatory state associated with the virus itself and the metabolic side effects of the antiretroviral therapies in addition to the known traditional and genetic host factors.1–7 Current guidelines support managing dyslipidemia in HIV-infected persons as in the general population, per the National Cholesterol Education Program (NCEP).8–10 Investigators of the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) observational cohort applied the Framingham equation to individual study participants receiving combination antiretroviral therapy and found that their observed rates of myocardial infarction correlated with the Framingham predicted rates.11 Therefore it has been recommended that HIV-infected patients who have two or more of the traditional cardiac risk factors should have their cardiac risk score calculated by the Framingham equation. Lipid goals should be assessed for every individual. Lifestyle modifications such as smoking cessation, diet, and exercise should be prescribed. If further intervention is needed to achieve lipid goals, lipid-lowering therapy should be initiated, or a switch in antiretroviral therapy should be made (Fig. 1). Special considerations for the HIV-infected person include whether the patient had existing lipid abnormalities before the initiation of antiretroviral therapy, whether specific antiretroviral therapy might contribute to lipid disturbances, and whether there might be drug interactions between antiretroviral therapy and lipid-lowering therapy.
Fig.1.
Guidelines for managing lipid disorders and risk of cardiovascular disease in patients receiving antiretroviral therapy. (Modified from Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:614; with permission.)
DRUG INTERACTIONS BETWEEN ANTIRETROVIRAL AGENTS AND LIPID-LOWERING THERAPY
The Food and Drug Administration (FDA) has approved 25 agents among five classes of antiretroviral therapy for the treatment of HIV (Table 1). Drug–drug interactions between lipid-lowering agents and antiretroviral agents may influence the selection of therapy (Table 2). Not all medications in the statin class can be used safely in the HIV-infected population receiving antiretroviral therapy. The protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) and many of the statins are metabolized by the cytochrome P450 isoenzyme CYP3A4. For example, significant interactions of 30-fold increases in simvastatin area under the curve (AUC) have been demonstrated when given with ritonavir-boosted saquinavir.12 These interactions suggest that simvastatin is contraindicated in the presence of PIs, especially because excessively elevated levels may place patients at risk for rhabdomyolysis.13 Lovastatin would be expected to behave in the same way. In the presence of PIs, moderate increases occur in the levels of atorvastatin; it can be prescribed, but at lower doses than in the general population.12,14 Because pravastatin is eliminated by multiple pathways that do not include CYP3A4, it can be used safely in patients receiving PIs other than darunavir; in the presence of darunavir, pravastatin levels increase by up to fivefold in some subjects through a mechanism that has not yet been described.12,15,16 Hence, the package insert recommends that the lowest possible dose of pravastatin, atorvastatin, or rosuvastatin be prescribed in patients taking darunavir.16 Except in patients taking darunavir, pravastatin may be less effective in lipid lowering in patients receiving PIs, because the induction of enzymes responsible for the metabolism of pravastatin results in decreased levels of pravastatin. Because no information regarding pharmacokinetic interactions with antiretroviral agents was available, the HIV Medicine Association/AIDS Clinical Trials Group guidelines do not include rosuvastatin, a lipid-lowering medication that was approved by the FDA after the publication of those guidelines. Subsequently, two pharmacokinetic studies have suggested that rosuvastatin levels increase similarly to atorvastatin levels in the presence of ritonavir-boosted lopinavir.17,18
Table1.
FDA-approved antiretroviral therapies
| GenericName | TradeName |
|---|---|
| Nucleoside reverse transcriptase inhibitors | |
| Abacavir (ABC) | Ziagen |
| Didanosine (ddI) | Videx |
| Emtricitabine (FTC) | Emtriva |
| Lamivudine (3TC) | Epivir |
| Stavudine (d4T) | Zerit |
| Tenofovir (TDF) | Viread |
| Zalcitabine (ddC) withdrawn 2005 | Hivid |
| Zidovudine (ZDV or AZT) | Retrovir |
| 3TC/ABC | Epzicom |
| 3TC/ABC/ZDV | Trizivir |
| 3TC/ZDV | Combivir |
| FTC/TDF | Truvada |
| Non-nucleoside reverse transcriptase inhibitors | |
| Delavirdine (DLV) | Rescriptor |
| Efavirenz (EFV) | Sustiva |
| Nevirapine (NVP) | Viramune |
| Etravirine (ETV) | Intelence |
| Multiple Class Fixed Dose Combination | |
| Tenofovir/Emtricitabine/Efavirenz (TDF/FTC/EFV) Atripla | |
| Protease inhibitors | |
| Amprenavir (APV) discontinued 2004 | Agenerase |
| Atazanavir (ATV) | Reyataz |
| Darunavir (DRV) | Prezista |
| Fosamprenavir (FPV) | Lexiva |
| Indinavir (IDV) | Crixivan |
| Lopinavir/ritonavir (LPV/RTV) | Kaletra |
| Nelfinavir (NFV) | Viracept |
| Ritonavir (RTV) | Norvir |
| Saquinavir (SQV hgc) | Invirase |
| Tipranavir (TPV) | Aptivus |
| Fusion inhibitors | |
| Enfuvirtide (ENF or T-20) | Fuzeon |
| CCR5 antagonists | |
| Maraviroc (MRV) | Selzentry |
| Integrase inhibitors | |
| Raltegravir (RAL) | Isentress |
Table 2.
Dosing recommendations based on drug–drug interactions between commonly used lipid-lowering agents and antiretroviral agents
| Lipid-Lowering Drug | Protease Inhibitors | Efavirenz | Nevirapine |
|---|---|---|---|
| Simvastatin | ↑ AUC 506%–3059%: do not coadminister12 |
↓ AUC 58%: may need increased dose simvastatin19 |
No data |
| Atorvastatin | ↑ AUC 71%–488%: use lowest starting dose atorvastatin12,14 |
↓ AUC 43%: may need increased dose atorvastatin19 |
No data |
| Pravastatin | Minimal change: no dose adjustment12,14,15 except use lowest dose with darunavir16 |
– | No data |
| Rosuvastatin | ↑ AUC 210%–470%: use lowest starting dose17,18 |
No data | No data |
| Fibrates | No dose adjustment | No dose adjustment | No dose adjustment |
| Bile acid sequestrants |
May interfere with absorption of antiretroviral therapy: do not co-administer |
May interfere with absorption of antiretroviral therapy: do not co-administer |
May interfere with absorption of antiretroviral therapy: do not co-administer |
| Niacin | No dose adjustment | No dose adjustment | No dose adjustment |
| Ezetimibe | No dose adjustment | No dose adjustment | No dose adjustment |
| Fish Oil | No dose adjustment | No dose adjustment | No dose adjustment |
Drug–drug interactions also occur between statins and the antiretroviral class of NNRTIs. Efavirenz has been shown to decrease the AUC of atorvastatin by 43% and of simvastatin by 58%, suggesting that higher doses of atorvastatin and simvastatin may be needed to reduce low-density lipoprotein (LDL) cholesterol effectively in patients taking efavirenz.19 Raltegravir, an integrase inhibitor, is eliminated mainly by UGT1A1 glucouronidation and not by a substrate of cytochrome P450 enzymes.20 Although significant interactions with lipid-lowering drugs would not be expected, there are no published data regarding interactions between raltegravir and the lipid-lowering agents. Alternatively, elvitegravir, an integrase inhibitor in development, is a moderate inducer of CYP3A; drug interaction studies have not been reported as of the time of this publication.21 Maraviroc, a CCR5 entry inhibitor, is a substrate of the cytochrome P450 enzyme system (CYP3A) and p-glycoprotein and as such may have significant interactions with lipid-lowering therapies.21 Caution always should be used in the absence of data regarding drug interactions between lipid-lowering drugs and the newer classes of antiretroviral therapy. Patients should be monitored for any signs or symptoms of toxicity or less-than-expected responses.
DATA FROM INTERVENTIONAL STUDIES OF LIPID-LOWERING DRUGS
In accordance with the guidelines, the type of lipid abnormality should be determined first to guide the choice of lipid-lowering therapy. Some of studies of the lipid-management therapy in HIV-infection persons included all types of dyslipidemias within the trial, and this practice may have affected outcomes of these trials. A common belief among providers is that lipid-lowering therapy may not work as well among persons who have HIV infection, but the percent reductions in lipids achieved by the individual agents are similar to those reported in the general population. Given the safety of pravastatin with most PIs and NNRTIs, pravastatin was the statin most often studied in clinical trials.
Moyle and colleagues22 conducted a randomized, open-label comparative 24-week trial of dietary advice alone or dietary modification plus pravastatin in 31 male patients taking PIs who had total cholesterol levels greater than 252 mg/dL (6.5 mmol/L). The mean total cholesterol level at baseline was 286 mg/dL (7.4 mmol/L) in the dietary advice arm and 290 mg/dL (7.5 mmol/L) in the pravastatin arm. At week 24, total cholesterol fell significantly in the pravastatin arm (46 mg/dL; 1.2 mmol/L; 17.3%; P < .05) but not in the dietary advice arm (12 mg/dL; 0.3 mmol/L; 4%). The difference between the two groups approached significance at week 24 (P = .051). The reduction in LDL cholesterol was 48 mg/dL (1.24 mmol/L; 19%) in the pravastatin arm and 3 mg/dL (0.07 mmol/L; 5.5%) in the dietary advice arm. The high-density lipoprotein (HDL) cholesterol level rose non-significantly by 23 mg/dL (0.6 mmol/L) in both groups. As expected, there was no significant change in triglycerides (TG).
Calza and colleagues23 conducted an open-label, randomized, prospective study of the efficacy and safety of bezafibrate, gemfibrozil, fenofibrate, pravastatin, and fluvastatin as pharmacologic treatment for PI-related dyslipidemia. Of the 106 evaluable subjects, bezafibrate was used in 25 cases, gemfibrozil in 22, fenofibrate in 22, pravastatin in 19, and fluvastatin in 18. The investigators reported that the use of fibrates (n = 69) resulted in reductions in TG of 41% and of 23% for LDL cholesterol while increasing HDL cholesterol by 20%. Statins (n = 37) reduced TG by 35% and LDL cholesterol levels by 26%, and the HDL cholesterol level increased by 24%.
The AIDS Clinical Trials Group (ACTG) A508724 was a randomized, open-label trial for subjects who had mixed dyslipidemia as defined by elevated LDL cholesterol, elevated TG, and low HDL cholesterol by NCEP criteria. Subjects were assigned to fenofibrate, 200 mg/d (n = 88), or pravastatin, 40 mg/d (n = 86). Subjects who did not reach the NCEP composite goal on monotherapy by week 12 received both drugs. Although the composite goal at week 12 was achieved by only 1% of fenofibrate and 5% of pravastatin subjects, 36% of the randomly assigned subjects achieved LDL cholesterol goals, 49% achieved HDL cholesterol goals, and 18% achieved TG goals with pravastatin monotherapy. Treatment with fenofibrate monotherapy led to 9%, 66%, and 48% of subjects achieving LDL, HDL, and TG goals, respectively. The percent reductions in lipid parameters were similar to the expected results in the general population. Furthermore, combination therapy with fenofibrate and pravastatin for HIV-related dyslipidemia provided substantial improvements in all lipid parameters and seemed to be safe, even if it was unlikely to achieve all NCEP targets for lipid levels.
In a pharmacokinetic study of healthy, HIV-seronegative subjects, the rosuvastatin AUC and maximal drug concentration were increased 2.1- and 4.7-fold, respectively, in combination with lopinavir/ritonavir, but the LDL reduction was attenuated when the subjects were given both drugs. This finding led to questions about the clinical utility of rosuvastatin in HIV-infected persons taking PIs.18 Van der Lee and colleagues17 also explored the lipid-lowering effect of rosuvastatin and assessed the effect of lopinavir/ritonavir on the pharmacokinetics of rosuvastatin in a small (n = 22) HIV-infected population. They found that the minimal dosage levels of rosuvastatin were about 1.6-fold higher than the levels reported among the general population. The mean reductions in total cholesterol and LDL cholesterol from baseline to week 4 in subjects taking rosuvastatin, 10 mg once daily, were 27.6% and 31.8%, respectively.
The French Agence Nationale de Recherche sur le Sida25 conducted ANRS 126, a randomized, open-label trial comparing pravastatin, 40 mg/d (n = 42), with rosuvastatin, 10 mg/d (n = 42). The median baseline total cholesterol level was 292 mg/dL (7.48 mmol/L), the median baseline LDL cholesterol level was 192 mg/dL (4.93 mmol/L), the median baseline TG level was 204 mg/dL (2.29 mmol/L), and the median baseline HDL level was 50 mg/dL (1.27 mmol/L). Rosuvastatin reduced LDL cholesterol by 37%, compared with a 19% reduction by pravastatin (P < .001). TG levels were reduced by 19% in persons taking rosuvastatin and by 7% in persons taking pravastatin (P = .035). HDL levels did not differ between arms. This study suggests that rosuvastatin may be superior to pravastatin for the management of elevated LDL in HIV-infected persons taking PIs.
No studies have been conducted evaluating long-term cardiac outcomes of different statins prescribed to HIV-infected persons, so statin preference usually is based on safety and tolerability as well as on inferences from data from the general population. Similarly, the use of ezetimibe either alone or in combination is based largely on its benefits reported in the general population. The results of the simvastatin/ezetimibe combination in the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) study26 were disappointing, because the study failed to show an additive effect on the surrogate clinical marker, carotid intima media thickness (IMT), even after producing a further lowering of LDL cholesterol of approximately 50 mg/dL. Eighty percent of the subjects had been treated with statins and had very thin baseline carotid IMT, suggesting that previous treatment already may have lowered the risk and thinned the intima media and may have precluded finding differential IMT changes in just 2 years. Therefore this study should not be used to preclude the use of ezetimibe in who have HIV, because the combination of ezetimibe with any statin is less likely to produce significant drug interactions with HIV therapy than the use of very high-dose statins alone. Nevertheless, maximizing statin therapy should be the first goal in all situations.
Negredo and colleagues27 reported the first prospective, open-label study evaluating the addition of ezetimibe, 10 mg/d, in 19 HIV-infected subjects who had LDL levels higher than 130 mg/dL and who were taking pravastatin, 20 mg/d. At week 24, 61.5% of patients achieved the end point of the study (LDL cholesterol level < 130 mg/dL). Significant declines in mean total and LDL cholesterol levels were observed between baseline and weeks 6, 12, and 24, irrespective of the type of antiretroviral agent (PI or NNRTI). Also of importance, no differences were observed in the minimal drug levels of lopinavir or nevirapine levels measured just before and 12 weeks after ezetimibe introduction.
Bennett and colleagues28 retrospectively analyzed lipid parameters in 33 HIV-infected patients who were prescribed ezetimibe, 10 mg/d. The mean total cholesterol level was reduced from 269 mg/dL (6.95 mmol/L) to 213 mg/dL (5.51 mmol/L; 21% reduction; P < .001). The mean LDL cholesterol level was reduced from 157 mg/dL (4.05 mmol/L) to 102 mg/dL (2.63 mmol/L; 35% reduction; P < .001), and the mean TG level was reduced from 551 mg/dL (6.22 mmol/L) to 341 mg/dL (3.85 mmol/L; 34% reduction; P = .006). The mean HDL level increased from 41 mg/dL (1.07 mmol/L) to 45 mg/dL (1.16 mmol/L; 8% increase; P = .038).
Furthermore a double-blind, placebo-controlled, crossover design study evaluated ezetimibe, 10 mg/d, on the lipid levels of 48 HIV-infected subjects not taking any other lipid-lowering therapy.29 The investigators reported a small but significant change in the LDL cholesterol level (mean baseline level, 128 mg/dL), with 35% of subjects having at least a 17% reduction in LDL cholesterol, as has been reported in the general population. Unlike the previously mentioned studies, there were no significant changes in TG or HDL cholesterol levels.
Hypertriglyceridemia remains the most common lipid abnormality among patients who have HIV. Although controversy still exists, many experts believe hypertriglyceridemia is an independent risk for coronary heart disease.30–32 Although statins have mild or moderate effects on TG, the first-line therapy for hypertriglyceridemia is a fibric acid followed by fish oil and niacin in no particular order. Lipid guidelines recommend administration of gemfibrozil or fenofibrate for patients who have hypertriglyceridemia.8–10 Some fibrates (eg, gemfibrozil) are metabolized in the liver via uridine 5′-diphosphate-glucuronosyl transferase enzymes, which are induced by most PIs. Therefore one would expect a decrease in their plasma concentrations, and this decrease might explain the low efficacy reported with the use of gemfibrozil. Miller and colleagues33 conducted a 16-week, randomized, double-blind, comparative study of a low-saturated-fat diet versus a low-saturated-fat diet with gemfibrozil, 600 mg twice daily, in patients who had TG levels of 3 mmol/L (≥266 mg/dL) or higher and who were taking PIs. Subjects were assigned randomly to gemfibrozil or matching placebo following a 4-week period of dietary intervention alone. The primary outcome was the difference between the two groups in mean change in fasting TG at week 16. Seventeen men were assigned to the gemfibrozil arm, and 20 were assigned to the placebo arm; the median fasting TG level was 496 mg/dL (5.6 mmol/L). Mean changes in the TG level from week 4 to week 16 were −108 mg/dL (−1.22 mmol/L) for the gemfibrozil group and +31 mg/dL (+0.35 mmol/L) for placebo groups. The between-group mean difference was 139 mg/dL (1.57 mmol/L) (95% confidence interval, −594–310 mg/dL [−6.7–3.5 mmol/L]; P = 0.08). Only one patient treated met the TG goal of 2.00 mmol/L (≤177 mg/dL) or lower. No significant changes in the other metabolic parameters were observed. The investigators concluded that gemfibrozil is safe and demonstrated, at most, modest efficacy for hypertriglyceridemia in HIV-infected patients taking PIs. They also stated that, given the level of response, it is unclear whether these reductions will confer clinical benefit, at least in the presence of continued PI use.
Because drug–drug interactions between fenofibrate and antiretroviral therapy are unlikely and because data suggest that fenofibrate improves cardiovascular outcomes, fenofibrate has become the fibrate most commonly prescribed for HIV-infected patients who have hypertriglyceridemia. Thomas and colleagues34 initially reported two case reports of significant TG-lowering activity. TG levels decreased from 1450 to 337 mg/dL (76.8%) in one patient and from 1985 to 322 mg/dL (83.8%) in another with the use of fenofibrate. A subsequent case report by de Luis35 reiterated the lipid-lowering benefit and safety among nine patients. In the study described earlier in this article, Calza and colleagues23,36 further demonstrated safety and efficacy in 60 cases. Caramelli and colleagues37 then reported a 45.7% reduction in TG levels (mean baseline, 486 mg/dL) in 13 patients treated with fenofibrate. Palacios and colleagues38 conducted a prospective study of 20 subjects taking antiretroviral therapy who had baseline TG levels higher than 400 mg/dL, with or without high total cholesterol, despite adherence to fitness and dietary measures. The mean TG level of 812 mg/dL at baseline was reduced to 376.6 mg/dL (54% reduction; P = .0001) after 24 weeks of treatment with micronized fenofibrate, 200 mg/d.
Badiou and colleagues39 conducted an open-label, randomized study of the effects of fenofibrate and/or vitamin E on the lipoprotein profile in 36 HIV-infected adults taking antiretroviral therapy who had fasting TG levels of 2 mmol/L (177 mg/dL) or higher. Subjects were assigned randomly to receive either micronized fenofibrate (200 mg/d) or vitamin E (500 mg/d) for 3 months and then to take both for an additional 3-month period. Total cholesterol, HDL cholesterol, LDL cholesterol, TG, apolipoprotein (apo)A1, apoB, apoCIII, lipoprotein composition, LDL particle size, and LDL resistance to copper-induced oxidation were determined before the initiation of fenofibrate or vitamin E and 3 and 6 months thereafter. The mean baseline TG level was 309 mg/dL (3.49 mmol/L). Three months of fenofibrate treatment resulted in a significant decrease in TG (−40%), apoCIII (−21%), total cholesterol (−14%), apoB (−17%), and non-HDL cholesterol levels (−17%) levels and in the TG to apoA1 ratio in HDL cholesterol (−27%) and was associated with an increase in HDL cholesterol (+15%) and apoA1 (+11%) levels. In addition, fenofibrate increased LDL particle size and enhanced LDL resistance to oxidation. The investigators concluded that fenofibrate therapy improves the atherogenic lipid profile in HIV-positive adults who have hypertriglyceridemia. Rao and colleagues40 reported similar reductions in TG levels among 55 patients receiving fenofibrate.
Dual therapy with a statin and a fibrate may be the best approach for achieving NCEP Adult Treatment Panel III lipid targets in patients who have HIV infection; the results of the ACTG 5087 study24 described earlier have demonstrated the effectiveness of this approach. As A5087 and other studies have shown, however, it remains unlikely that HIV-infected patients who have hypertriglyceridemia will reach NCEP goals with fibrate therapy alone, and further intervention is needed. Fish oil is an attractive supplement because of its desirable anti-inflammatory properties, reduction of cardiovascular atherogenic effects, and lack of drug interactions with antiretroviral therapy. The American Heart Association’ dietary guidelines recommend that healthy adults eat at least two servings of fish per week and that people who have elevated TG levels need 2 to 4 g/d of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) as a dietary supplement.41 A few studies evaluating the TG-lowering effects of fish oil have been conducted in HIV-infected subjects.
In a randomized, double-blind, placebo-controlled study by DeTruchis and colleagues,42 administration of fish oil supplement (EPA + DHA, 1.8 g/d) three times daily for 8 weeks to 60 HIV-infected subjects receiving antiretroviral therapy who had a mean baseline TG level of 450 mg/dL resulted in a median 25.5% decrease in TG level, compared with a decrease of only 1% among 62 control subjects receiving a paraffin oil. In the open-label phase of the study, subjects who switched from placebo to fish oil supplement demonstrated a 21.2% decrease in serum TG levels. Wohl and colleagues43 administered EPA plus DHA, 2.9 g/d, to HIV-infected subjects receiving antiretroviral therapy who had a mean baseline TG level of 461 mg/dL and demonstrated a mean decrease in fasting TG levels of 25%.
In the ACTG A518644 phase II open-label study of 100 patients, twice-daily administration of 3 g fish oil supplement (EPA + DHA, 4.86 g/d) or once-daily administration of fenofibrate, 160 mg, reduced median TG levels by 283 mg/dL (46%) and 367 mg/dL (58%), respectively. Patients not achieving the NCEP goal of a TG level below 200 mg/dL on either medication alone and subsequently treated with both agents demonstrated a 65.5% reduction in TG level from baseline. Although this combination therapy achieved TG levels of 200 mg/dL or lower in only 22.7% of study subjects, the median TG level at baseline was 667 mg/dL.
Baril and colleagues45 conducted a study with an open-label, parallel and crossover design to evaluate the effects of 1 g salmon oil (EPA + DHA, 0.9 g/d) administered three times daily. The salmon oil group (n = 26) received treatment for 24 weeks, and the no-intervention arm (n = 32) received salmon oil during weeks 12 through 24. At week 12, the salmon oil group experienced a nonsignificant decrease in TG level of 97 mg/dL (1.1 mmol/L), and the no-intervention arm had a nonsignificant increase in TG level of 27 mg/dL (0.3 mmol/L). The limitations of this study were the absence of a dietary intervention before the study, the low dose of EPA plus DHA prescribed, and concomitant lipid-lowering therapies taken by 58.6% of the subjects.
Bile acid sequestrants and niacin are alternative therapies recommended in the lipid guidelines. There are no published studies regarding bile sequestrants, probably reflecting the concern that these drugs may decrease the absorption and therefore the virologic efficacy of antiretroviral therapy. Investigators at Washington University46 conducted an open-label pilot study evaluating the safety of extended-release niacin in 14 HIV-infected subjects taking antiretroviral therapy who had TG levels higher than 200 mg/dL at baseline. Niacin was initiated at a dose of 500 mg/d and was increased to a maximum of 2000 mg/d. Although the investigators reported that the median TG level decreased by 34%, 7 of 11 evaluable subjects were glucose intolerant (3 of these subjects developed glucose intolerance during the study).
ACTG 5148 was a similar study evaluating extended-release niacin in 33 HIV-infected subjects taking antiretroviral therapy who had TG levels of 200 mg/dL or higher and non-HDL levels of 180 mg/dL or higher.47 Forty-two percent of the subjects had prediabetes at entry. Overall niacin was well tolerated, with only four subjects discontinuing therapy. Twenty-three subjects (70%) received the full 2000-mg dose of niacin. At 48 weeks, the median percent reduction in TG level was 38% and in non-HDL level was 8%. Although no subjects developed diabetes by fasting glucose definition, one subject did meet criteria by 2-hour glucose tolerance testing, and other subjects had mild glucose elevations, as anticipated with niacin.
Although there are limited data regarding the effect of lipid-lowering therapies on the long-term cardiovascular outcomes, the HIV Outpatient Study,48 composed of more than 8000 patients followed since 1993, reported that the incidence of myocardial infarction has been decreasing since a peak in the year 2000. Investigators attribute the decrease to the increasing use of lipid-lowering therapy. They reported hazard ratios of 2.38 for being over the age 40 years, 2.45 for having diabetes, 2.22 for smoking, and 0.34 for the use of lipid-lowering therapy.
SWITCHING ANTIRETROVIRAL THERAPIES
Given the extent and severity of lipid abnormalities reported among persons who have HIV, it is not surprising that single- or dual-agent lipid-lowering therapies may not meet the NCEP goal. Another strategy that has been considered is changing specific anti-retroviral agents. Readers are referred to an extensive review by Barragan and colleagues.49 The HIV Medicine Association/AIDS Clinical Trials Group guidelines emphasize that altering a treatment regimen to improve the lipid profile may not produce the anticipated result because of the multifactorial nature of dyslipidemia in patients receiving treatment for HIV infection.9 The dyslipidemia that is labeled “HIV related” is complex. The lipid abnormalities may be caused by HIV itself, by antiretroviral therapy, or by host factors. Results of the Strategies for Management of Antiretroviral Therapy study5 demonstrated that discontinuation of antiretroviral therapy resulted in increased cardiovascular deaths compared with continued antiretroviral therapy. The D:A:D observational cohort study4 suggests that long-term exposure to PIs is associated with an increased risk for myocardial infarction but that the risk is not as high as that of traditional risk factors such as male gender, advanced age, and smoking. An analysis of the D:A:D cohort50 suggested that recent use didanosine and abacavir was associated with the risk of coronary heart disease, but in the past the thymidine analogues (zidovudine and stavudine) were associated with mitochondrial toxicity and alterations in the sterol regulatory–binding proteins leading to permutations of metabolic pathways and resulting in insulin resistance and dyslipidemia.51 The association of didanosine and abacavir with coronary heart disease has not been confirmed by prospective, randomized studies, and no mechanism for such an association has been elucidated.
One cannot attribute an abnormal lipid parameter simply to one specific agent, because studies now show that these abnormalities may not be the effect of an individual drug or even a class effect. Instead, the abnormalities may be caused by a particular drug or by combinations of antiretroviral therapy in specific individuals who have particular drug metabolism polymorphisms or genetic predispositions toward the development of dyslipidemia.52,53 Not everyone given a specific agent develops abnormal lipid values, suggesting that genomics do in fact play a role. Also, there never has been a study demonstrating that pre-existing lipid abnormalities before HIV infection and antiretroviral therapy that worsen on antiretroviral therapy will normalize after a switch. Switching medications should be reserved for those who have developed lipid abnormalities on a specific regimen and for whom such a switch will not adversely affect virologic orimmunologic control. Also, results from clinical studies suggest that a change in antiretroviral therapy may have only limited effects on overall cardiovascular risk.
Although the PIs were the class of antiretroviral therapy most commonly associated with the development of dyslipidemia, combination studies demonstrated that certain combinations of drugs were most often associated with lipid abnormalities. The PI ritonavir and the nucleoside reverse transcriptase inhibitor stavudine (d4T) are more commonly associated with elevated total cholesterol, LDL cholesterol, and TG levels than other agents. The ESS40002 study54 comparing zidovudine/lamivudine/abacavir, zidovudine/lamivudine/nelfinavir, and stavudine/lamivudine/nelfinavir highlighted the varying effects of combinations and suggested that race/ethnicity and gender also play important roles. The investigators noted that among nelfinavir recipients, women were more likely than men to develop increased LDL cholesterol levels, and the association between female sex and LDL elevations was even stronger in the stavudine-containing arm than in the zidovudine-containing arm. Also, blacks were more likely than whites and Hispanics to develop increased LDL levels. Overall, subjects in the three-nucleoside arm had the most favorable lipid parameters, but this combination has lower virologic efficacy than the efavirenz-containing regimens and no longer is listed as a preferred regimen by national guidelines. Tenofovir (TDF), a nucleotide, seems to be one of the more “lipid-friendly” of the nucleoside reverse transcriptase inhibitors.55,56 At week 24 in the TDF intensification study, there was a decrease in total cholesterol and TG levels of 17.5 mg/dL and 24 mg/dL, respectively, in the TDF group compared with a decrease in 3.8 mg/dL and 3.4 mg/dL in the placebo group.56 When this placebo group was rolled over to receive TDF from weeks 24 to 48, the total cholesterol level decreased by 12.1 mg/dL, and the TG level decreased by 22.0 mg/dL. In the Gilead 903 extension study57 comparing TDF with d4T on an efavirenz-containing regimen, subjects taking d4T experienced a mean increase in fasting TG level of 102 mg/dL and a mean increase in fasting total cholesterol level of 59 mg/dL by year 3 in the original study; after switching from d4T to tenofovir, TG levels decreased by a mean of 61 mg/dL, and total cholesterol levels declined by a mean 21 mg/dL by the end of year 5 (both, P < .001).
A study of 88 HIV-infected patients assessed the metabolic effects of switching from a ritonavir-boosted, lopinavir-containing regimen to ritonavir-boosted atazanavir. 58 This switch resulted in a 13% decrease in total cholesterol levels and a 30% decrease in TG levels but resulted in only slight reductions, from 12% to 10%, in the Framingham-calculated 10-year risk of coronary heart disease. The Switching to Atazanavir study (n = 419) was a 2:1 randomization of subjects taking a PI-based regimen to switch to atazanavir or continue the current PI-containing regimen.59 Of note, only 9% of subjects who switched to atazanavir took ritonavir for boosting. Significant decreases in total cholesterol levels (15% versus 3%) and TG levels (33% versus an increase of 9%) were reported upon switching to primarily unboosted atazanavir compared with staying on the current PI regimen. There were no significant changes in HDL cholesterol or LDL cholesterol levels, however, and the impact of the switch on Framingham risk was not assessed.
Another important aspect of switching antiretroviral therapy is that one may be able to discontinue lipid-lowering therapies after the switch and subsequent improvements in lipid parameters. Martinez and colleagues60 reported 162 subjects who switched to ritonavir-boosted atazanavir as part of the Bristol Myers Squibb early access program. Thirty-four percent of subjects were taking boosted lopinavir at time of the switch. Six months after the switch they reported mean decreases of 12%, 10%, and 18% in total cholesterol, LDL cholesterol, and TG levels, respectively. Almost one third of the subjects were able to discontinue lipid-lowering therapy.
Switching Antiretroviral Therapy Versus Lipid-Lowering Therapy
There are limited data comparing the benefits and risks of switching or modifying anti-retroviral therapy compared with lipid-lowering therapy. One randomized, prospective study61 in HIV-infected patients who had mixed hyperlipidemia and who were being treated with their first antiretroviral therapy regimen compared the lipid-lowering effects of switching from a PI to a NNRTI (either nevirapine or efavirenz) or of treatment with either pravastatin or bezafibrate added to the current unchanged antiretroviral therapy regimen for up to 12 months. These treatment strategies resulted in reductions in mean TG levels of 25.2%, 9.4%, 41.2%, and 46.6%, respectively, with statistically significant differences noted between the NNRTI arms and the lipid-lowering agents (P < .01). In addition, treatment with pravastatin or bezafibrate resulted in significantly greater decreases in mean plasma total and LDL cholesterol than in the nevirapine- and efavirenz -treated patients. This study was powered to compare the strategies of switching versus lipid-lowering therapy and could not detect differences among the four arms. Although this strategy was commonly used at the time the study was conducted, data today suggest that efavirenz is associated with lipid abnormalities more commonly than nevirapine, so it is not surprising that the switch to efavirenz was suboptimal compared with lipid-lowering agents.
Another analysis from the D:A:D cohort62 compared the effects of lipid-lowering treatments with switching from PI- to NNRTI-based antiretroviral therapy. The results showed significant reductions in total cholesterol with both lipid-lowering treatments and antiretroviral therapy switching, compared the absence of either intervention. Intervention with lipid-lowering treatments resulted in greater mean reductions in total and LDL cholesterol levels (P = not significant), whereas switching to NNRTI-based antiretroviral therapy resulted in a greater mean reduction in the HDL cholesterol level. Both strategies had similar reductions in serum TG levels and total cholesterol to HDL ratios.
SUMMARY
HIV-infected patients may have lipid abnormalities caused by the HIV infection itself, by antiretroviral therapy, or by host factors (genetic and lifestyle). The current management guidelines recommend that patients who have HIV infection be managed similarly to the general population with the exception that drug-induced hyperlipidemia may be modifiable by a switch in antiretroviral therapy. Switching should be done only when there are antiretroviral therapy options that may result in a more favorable lipid profile and will maintain virologic suppression. Patients who already are taking antiretroviral therapy should not discontinue it except under the guidance of an HIV expert, because as discontinuation of antiretroviral therapy may lead to increased risk of coronary heart disease. Patients who have two or more cardiovascular risk factors should have their absolute risk of coronary heart disease assessed with the Framingham score calculations used to provide guidance for management and treatment. The HIV-infected population may have other characteristics dissimilar to the general population that warrant additional strategies. For instance, the HIV-infected community is reported to have higher rates of smoking. Smoking cessation programs should be readily available. HIV-infected patients also may have other comorbidities such as wasting, and the typical American Heart Association diet may be contraindicated. It is strongly encouraged that patients who have HIV be referred to a nutritionist who is knowledgeable about HIV disease. Finally, the drug interactions between lipid-lowering agents and antiretroviral therapy pose additional challenges, and caution should be used whenever prescribing additional medications to an already complex disease and its therapies. Future studies probably will explore the strategy of switching from current PIs and NNRTIs to the newly approved classes of integrase inhibitors and entry inhibitors as a means for improving lipid parameters and reducing cardiovascular risk.
Acknowledgments
This work was supported in part by National Institute of Allergy and Infectious Diseases grant AI-068636 to the AIDS Clinical Trials Group and grant AI-069532 to the New York University AIDS Clinical Trials Unit.
REFERENCES
- 1.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 4.DAD Study Group. Friis-Moller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 5.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 6.Cespedes MS, Aberg JA. Cardiovascular and endothelial disease in HIV infection. Curr Infect Dis Rep. 2005;7(4):309–315. doi: 10.1007/s11908-005-0064-3. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein K, Armon C, Buchacz K. Analysis of cardiovascular risk factors in the HIV outpatient study (HOPS) cohort. Presented at the Thirteenth Conference on Retroviruses and Opportunistic Infections (CROI); February 5–8; Denver, Colorado. 2006. [Google Scholar]
- 8.National Cholesterol Education Program N. [Accessed May 23, 2008];Third report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2008 Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm.
- 9.Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 10.Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. [Accessed May 23, 2008];HIV Med. 2008 Feb;9(2):72–81. doi: 10.1111/j.1468-1293.2007.00534.x. Available at: http://www.eacs.eu/guide/index.htm. [DOI] [PubMed] [Google Scholar]
- 11.Law MG, Friis-Moller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D study. HIV Med. 2006;7(4):218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 12.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 13.Aboulafia DM, Johnston R. Simvastatin-induced rhabdomyolysis in an HIV-infected patient with coronary artery disease. AIDS Patient Care STDS. 2000;14:13–18. doi: 10.1089/108729100318091. [DOI] [PubMed] [Google Scholar]
- 14.Carr R, Andre A, Bertz R. Concomitant administration of ABT-378/ritonavir (ABT-378/r). Results in a clinically important pharmacokinetic (PK) interaction with atorvastatin but not pravastatin [abstract 1644]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 17–20; Toronto. 2000. [Google Scholar]
- 15.Aberg JA, Rosenkranz S, Fichtenbaum CJ, et al. Pharmacokinetic interaction between nelfinavir and pravastatin in HIV-seronegative volunteers: ACTG study A5108. AIDS. 2006;20:725–729. doi: 10.1097/01.aids.0000216373.53819.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raritan NJ. Prezista (Darunavir) package insert. [Accessed April 27, 2008];Tibotec Therapuetics. 2008 February; Available at: http://www.tibotectherapeutics.com/tibotectherapeutics/documents/us_package_insert.pdf. [Google Scholar]
- 17.van der Lee M, Sankatsing R, Schippers E, et al. Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patients. Antivir Ther. 2007;12(7):1127–1132. [PubMed] [Google Scholar]
- 18.Kiser JJ, Gerber JG, Predhomme JA, et al. Drug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. J Acquir Immune Defic Syndr. 2008;47(5):570–578. doi: 10.1097/QAI.0b013e318160a542. [DOI] [PubMed] [Google Scholar]
- 19.Gerber JG, Rosenkranz S, Fichtenbaum CJ, et al. The effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of ACTG 5108 study. J Acquir Immune Defic Syndr. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 20.Croxtall JD, Lyseng-Williamson KA, Perry CM. Raltegravir. Drugs. 2008;68(1):131–138. doi: 10.2165/00003495-200868010-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kiser JJ. Pharmacologic characteristics of investigational and recently approved agents for the treatment of HIV. Curr Opin HIV AIDS. 2008;3(3):330–341. doi: 10.1097/COH.0b013e3282fbaa6b. [DOI] [PubMed] [Google Scholar]
- 22.Moyle GJ, Lloyd M, Reynolds B, et al. Dietary advice with or without pravastatin for the management of hypercholesterolaemia associated with protease inhibitor therapy. AIDS. 2005;15(12):1503–1508. doi: 10.1097/00002030-200108170-00007. [DOI] [PubMed] [Google Scholar]
- 23.Calza L, Manfredi R, Chiodo F. Statins and fibrates for the treatment of hyperlipidaemia in HIV-infected patients receiving HAART. AIDS. 2003;17(6):851–859. doi: 10.1097/00002030-200304110-00010. [DOI] [PubMed] [Google Scholar]
- 24.Aberg JA, Zackin RA, Brobst SW, et al. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses. 2005;21:757–767. doi: 10.1089/aid.2005.21.757. [DOI] [PubMed] [Google Scholar]
- 25.Aslangul E, Assoumou L, Bittar R, et al. ANRS 126, a prospective, randomized, open label trial comparing the efficacy and safety of rosuvastatin versus pravastatin in HIV-infected subjects receiving ritonavir boosted PI with lipid abnormalities [abstract LBPS7/2]. Eleventh European AIDS Conference; October 24–27; Madrid. 2007. [Google Scholar]
- 26.Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 27.Negredo E, Molto J, Puig J, et al. Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins. AIDS. 2006;20:2159–2164. doi: 10.1097/01.aids.0000247573.95880.db. [DOI] [PubMed] [Google Scholar]
- 28.Bennett MT, Johns KW, Bondy GP. Ezetimibe is effective when added to maximally tolerated lipid lowering therapy in patients with HIV. Lipids Health Dis. 2007;6:15. doi: 10.1186/1476-511X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohl D, Hsue P, Richard S, et al. Ezetimibe’ effects on the LDL cholesterol levels of HIV-infected patients receiving HAART [abstract 39]. 14th Conference on Retroviruses and Opportunistic Infections; February 25–28; Los Angeles. 2007. [Google Scholar]
- 30.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 31.Cullen P. Evidence that triglycerides are an independent coronary artery disease risk factor. Am J Cardiol. 2000;86:943–949. doi: 10.1016/s0002-9149(00)01127-9. [DOI] [PubMed] [Google Scholar]
- 32.McBride PE. Triglycerides and risk factors for coronary heart disease: editorial. JAMA. 2007;298:236–238. doi: 10.1001/jama.298.3.336. [DOI] [PubMed] [Google Scholar]
- 33.Miller J, Brown D, Amin J, et al. A randomized, double-blind study of gemfibrozil for the treatment of protease inhibitor-associated hypertriglyceridaemia. AIDS. 2002;16:2195–2200. doi: 10.1097/00002030-200211080-00012. [DOI] [PubMed] [Google Scholar]
- 34.Thomas JC, Lopes-Virella MF, Del Bene VE, et al. Use of fenofibrate in the management of protease inhibitor-associated lipid abnormalities. Pharmacotherapy. 2000;20(6):727–734. doi: 10.1592/phco.20.7.727.35179. [DOI] [PubMed] [Google Scholar]
- 35.de Luis DA, Bachiller P, Aller R. Fenofibrate in hyperlipidaemia secondary to HIV protease inhibitors. Fenofibrate and HIV protease inhibitor. Nutrition. 2001;17(5):414–415. doi: 10.1016/s0899-9007(01)00582-2. [DOI] [PubMed] [Google Scholar]
- 36.Calza L, Manfredi R, Chiodo F. Use of fibrates in the management of hyperlipidemia in HIV-infected patients receiving HAART. Infection. 2002;30(1):26–31. doi: 10.1007/s15010-001-2052-3. [DOI] [PubMed] [Google Scholar]
- 37.Caramelli B, de Bernoche CY, Sartori AM, et al. Hyperlipidemia related to the use of HIV-protease inhibitors: natural history and results of treatment with fenofibrate. Braz J Infect Dis. 2001;5(6):332–338. doi: 10.1590/s1413-86702001000600007. [DOI] [PubMed] [Google Scholar]
- 38.Palacios R. Efficacy and safety of fenofibrate for the treatment of hypertriglyceridemia associated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(2):251–253. doi: 10.1097/00126334-200210010-00018. [DOI] [PubMed] [Google Scholar]
- 39.Badiou S. Fenofibrate improves the atherogenic lipid profile and enhances LDL resistance to oxidation in HIV-positive adults. Atherosclerosis. 2004;172(2):273–279. doi: 10.1016/j.atherosclerosis.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Rao A, D’Amico S, Balasubramanyam A, et al. Fenofibrate is effective in treating hypertriglyceridemia associated with HIV lipodystrophy. Am J Med Sci. 2004;327(6):315–318. doi: 10.1097/00000441-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Kris-Etherton PM, Harris WS, Appel LJ for the AHA Nutrition Committee. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 42.De Truchis P, Kirstetter M, Perier A, et al. for the VIH Study Group. Reduction in triglyceride levels with N-3 polyunsaturated fatty acids in HIV-infected patients taking potent antiretroviral therapy: a randomized prospective study. J Acquir Immune Defic Syndr. 2007;44:278–285. doi: 10.1097/QAI.0b013e31802c2f3d. [DOI] [PubMed] [Google Scholar]
- 43.Wohl DA, Tien H-C, Busby M, et al. Randomized study of the safety and efficacy of fish oil (omega-3 fatty acids) supplementation with dietary and exercise counseling for the treatment of antiretroviral therapy-associated hypertriglyceridemia. Clin Infect Dis. 2005;41:1498–1504. doi: 10.1086/497273. [DOI] [PubMed] [Google Scholar]
- 44.Gerber JG, Kitch DW, Fichtenbaum CJ, et al. Fish oil and fenofibrate for the treatment of hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: results of ACTG A5186. J Acquir Immune Defic Syndr. 2008;47(4):459–466. doi: 10.1097/QAI.0b013e31815bace2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baril JG, Kovacs CM, Trottier S, et al. Effectiveness and tolerability of oral administration of low-dose salmon oil to HIV patients with HAART-associated dyslipidemia. HIV Clin Trials. 2007;8(6):400–411. doi: 10.1310/hct0806-400. [DOI] [PubMed] [Google Scholar]
- 46.Gerber MY, Mondy KE, Yarasheski KE, et al. Niacin in HIV-infected individuals with hyperlipidemia receiving potent antiretroviral therapy. Clin Infect Dis. 2004;39(3):419–425. doi: 10.1086/422144. [DOI] [PubMed] [Google Scholar]
- 47.Dubé MP, Wu JW, Aberg JA, et al. for the AIDS Clinical Trials Group Study A5148. Safety and efficacy of extended-release niacin for the treatment of dyslipidemia in patients with HIV infection: AIDS Clinical Trials Group study A5148. Antivir Ther. 2006;11:1081–1089. [PMC free article] [PubMed] [Google Scholar]
- 48.Lichtenstein K, Armon C, Buchacz K, et al. Analysis of cardiovascular risk factors in the HIV outpatient study cohort [abstract 735]. Program and abstracts of the 13th conference on retroviruses and opportunistic infections; February 5–8, 2006; Denver. 2006. [Google Scholar]
- 49.Barragan P, Fisac C, Podzamczer D. Switching strategies to improve lipid profile and morphologic changes. AIDS Rev. 2006;8(4):191–203. [PubMed] [Google Scholar]
- 50.D:A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones SP, Qazi N, Morelese J, et al. Assessment of adipokine expression and mitochondrial toxicity in HIV patients with lipoatrophy on stavudine- and zidovudine-containing regimens. J Acquir Immune Defic Syndr. 2005;40(5):565–572. doi: 10.1097/01.qai.0000187443.30838.3e. [DOI] [PubMed] [Google Scholar]
- 52.Tarr PE, Taffe P, Bleiber G, et al. Modeling the influence of APOC3, APOE, and TNF polymorphisms on the risk of antiretroviral therapy–associated lipid disorders. J Infect Dis. 2005;191:1419–1426. doi: 10.1086/429295. [DOI] [PubMed] [Google Scholar]
- 53.Foulkes AS, Wohl DA, Frank I, et al. Associations among race/ethnicity, ApoC-III genotypes, and lipids in HIV-1-infected individuals on antiretroviral therapy. PLoS Med. 2006;3(3):e52. doi: 10.1371/journal.pmed.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar PN, Rodriguez-French A, Thompson MA, et al. A prospective, 96-week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral-naive patients: effect of sex and ethnicity. HIV Med. 2006;7:85–98. doi: 10.1111/j.1468-1293.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 55.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 56.Squires K, Pozniak AL, Pierone G, Jr, et al. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann Intern Med. 2003;139:313–320. doi: 10.7326/0003-4819-139-5_part_1-200309020-00006. [DOI] [PubMed] [Google Scholar]
- 57.Madruga JR, Cassetti I, Suleiman JMAH, et al. 903E Study Team. The safety and efficacy of switching stavudine to tenofovir df in combination with lamivudine and efavirenz in HIV-1-infected patients: three-year follow-up after switching therapy. HIV Clin Trials. 2007;8(6):381–390. doi: 10.1310/hct0806-381. [DOI] [PubMed] [Google Scholar]
- 58.Guillemi S, Toulson A, Joy R. Changes in lipid profile upon switching from lopinavir/ritonavir (LPV/r) to atazanavir + ritonavir (ATZ + RTV) based HAART. Paper presented at the XVI International AIDS Conference; August 13–18; Toronto. 2006. [Google Scholar]
- 59.Gatell J, Salmon-Ceron D, Lazzaria A. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN study (AI424-097) 48-week results. Clin Infect Dis. 2007;44(11):1484–1492. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 60.Martinez E, Azuaje C, Antela A. Effects of switching to ritonavir-boosted atazanavir on HIV-infected patients receiving antiretroviral therapy with hyperlipidemia [abstract # 850]. 12th Conference on Retroviruses and Opportunistic Infections; February 22–25; Boston. 2005. [Google Scholar]
- 61.Calza L, Manfredi R, Colangeli V, et al. Substitution of nevirapine or efavirenz for protease inhibitor versus lipid-lowering therapy for the management of dyslipidaemia. AIDS. 2005;19:1051–1058. doi: 10.1097/01.aids.0000174451.78497.8f. [DOI] [PubMed] [Google Scholar]
- 62.Van Der Valk M, Friis-Møller N, Sabin C. Effect of interventions to improve dyslipidaemia. Paper presented at the 8th International Congress on Drug Therapy in HIV Infection; November 12–16; Glasgow, United Kingdom. 2006. [Google Scholar]