Abstract
The mixing behavior of an exchangeable phospholipid (A) with an exchangeable sterol (B) has been examined in host bilayers made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), containing varying concentrations of cholesterol, via the nearest-neighbor recognition (NNR) method. At low sterol concentrations (i.e., 2.5 mol%), the mixing between A and B is close to ideal. Incremental increases in sterol concentrations to 40 mol% led to net increases in the affinity between A and B. Similar mixing experiments that were carried out in the presence of chloroform showed a leveling effect, where moderate sterol-phospholipid affinity was observed in all cases. These results, together with the fact that the number of chloroform molecules that are absorbed per phospholipid is essentially constant and independent of the sterol content, supports a model in which chloroform favors solvation of the phospholipids and a common membrane state is produced. Fluorescence measurements and Raman spectra have also shown that chloroform significantly loosens both cholesterol-poor and cholesterol-rich membranes made from DPPC. In a broader context, these results suggest a fundamentally new mechanim of anesthesia, where the anesthetic, by solvating the lipid components, profoundly changes the lateral organization of the lipid framework.
The mechanism of action of general anesthetics is still open to debate.1 One long-standing hypothesis is that they loosen cell membranes, resulting in “relaxed” and dysfunctional transmembrane proteins.2 A second hypothesis is that they bind to receptors in transmembrane proteins.2 Here, we show that chloroform not only loosens cholesterol-rich and cholesterol-poor phospholipid membranes, but it also rearranges them. This finding suggests a fundamentally new mechanism of anesthesia, where the anesthetic, by solvating the lipid components, profoundly changes the lateral organization of the lipid framework.
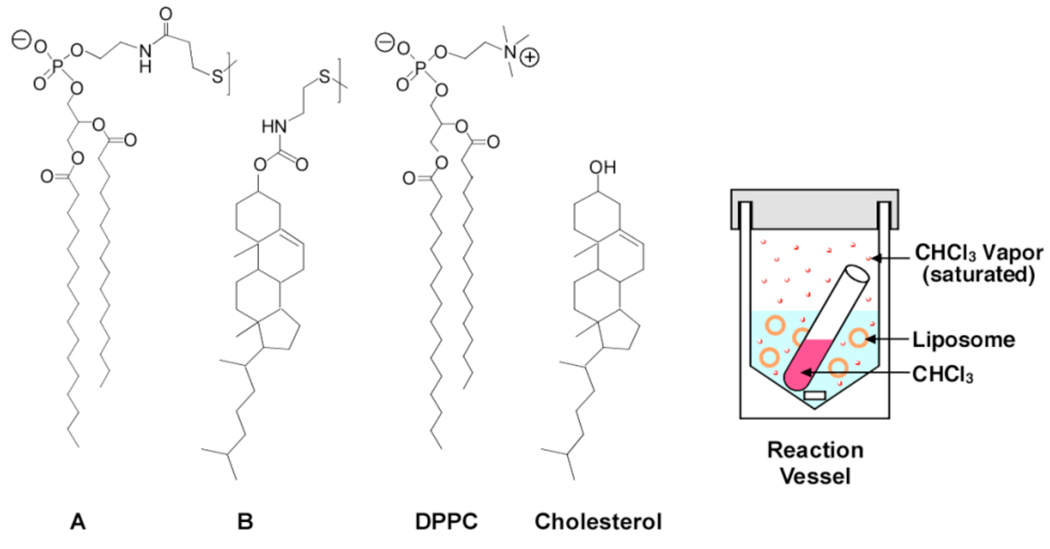
To probe the influence of an anesthetic on lipid mixing, we employed the nearest-neighbor recognition (NNR) technique.3,4 By virtue of its high sensitivity toward lipid-lipid associations, this method is ideally suited for quantifying such effects. As a first anesthetic, we selected chloroform because it is a moderately lipophilic molecule that should readily insert itself into the lipid bilayer. Given the prevalence of cholesterol and high-melting lipids in mammalian cell membranes, and the strong effects that cholesterol is known to have on the structure of phospholipid bilayers, we examined the mixing of A with B in host bilayers made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and cholesterol (Figure 1).3,5,6
Figure 1.
Lipids and reaction vessel used for NNR experiments.
As discussed elsewhere, NNR experiments take molecularlevel snapshots of bilayer organization by detecting and quantifying the thermodynamic tendency of exchangeable monomers to become nearest-neighbors of one another.4 Typically, two lipids of interest (A and B) are converted into exchangeable dimers (AA, AB and BB), which are then allowed to undergo monomer interchange via thiolate-disulfide exchange. The resulting equilibrium that is established, whereby one molecule of AA reacts with one molecule of BB to give two molecules of AB, is then governed by an equilibrium constant, K = [AB]2/([AA][BB]). When monomers A and B mix ideally, this is reflected by an equilibrium constant that equals 4.0. When homoassociations are favored, the equilibrium constant is less than 4.0; favored hetero-associations are indicated by a value that is greater than 4.0. As shown previously, nearest-neighbor association between A and B in host membranes made from DPPC and cholesterol reflect the phase of the bilayer, and becomes increasingly favored as one moves from the liquid-disordered (ld) to the liquid-ordered (lo) phase.2a
Mechanistic studies involving general anesthetics and lipid membranes are inherently complex due to partitioning of the anesthetic among the solution phase, the gas phase and the membrane phase. To simplify our experiments, NNR exchange reactions were carried out under saturation conditions using sealed reaction vessels that contained an open tube of chloroform (Figure 1).
Using procedures similar to those previously described, NNR reactions were carried out at 45°C in the absence and in the presence of chloroform. All host liposomes (200 nm, extrusion) included 2.5 mol% A, 2.5 mol% B with varying concentrations of cholesterol. Those bilayers having low (2.5 mol%) and high (32 mol% or 40 mol%) sterol concentrations are in the ld and lo states, respectively; at intermediate concentrations (e.g., 20 mol%), they are in the lo/ld coexistence region.7 To ensure that equilibrium product mixtures were obtained in each case, one set of experiments was carried out using pure heterodimer, AB, as the source of the exchangeable lipids, and a second set used liposomes containing an equimolar mixture of the corresponding homodimers, AA and BB.8 Values of K were then calculated from the average dimer composition from both sets of experiments. Examination of particle sizes by dynamic light scattering (Nicomp) confirmed that these liposomes were stable under the conditions used for thiolate-disulfide interchange (Supporting Information.
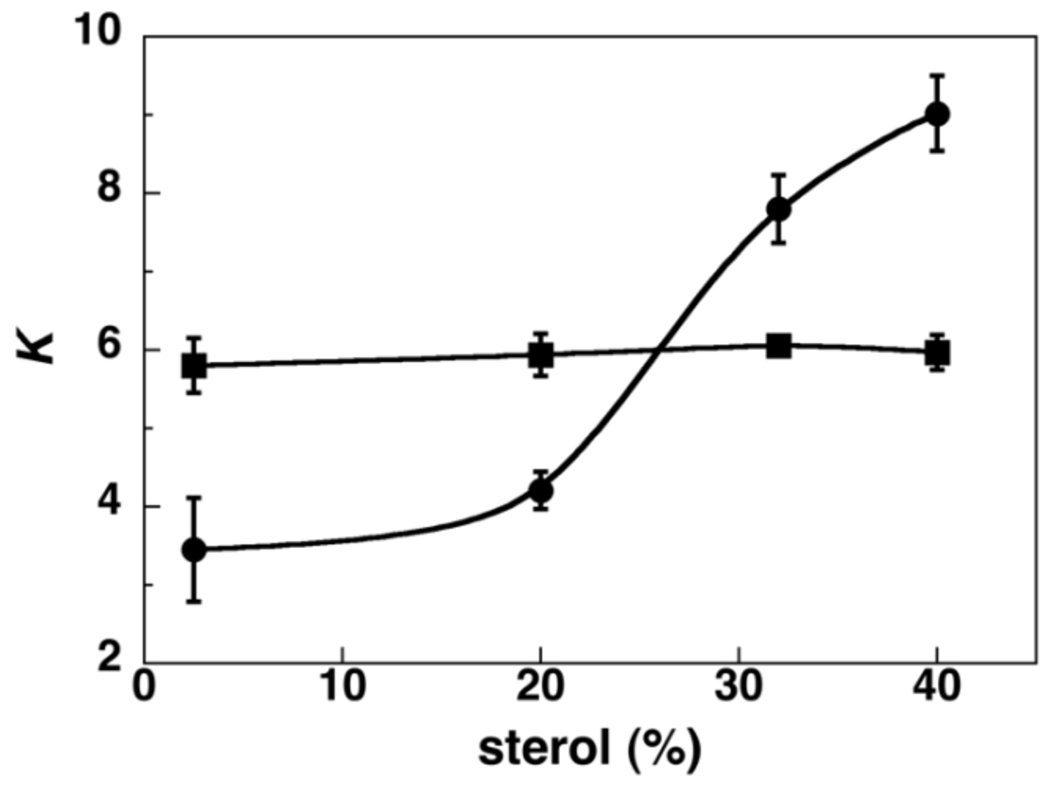
Values of K for the four types of membranes, in the absence and in the presence of chloroform, are plotted in Figure 2. As is apparent, the presence of chloroform has a leveling effect on sterol—phospholipid mixing; that is, it increases K in cholesterol-poor membranes and decreases K in cholesterol-rich membranes. These results imply that the presence of chlorofom creates a common state that is characterized by moderate sterolphospholipid affinity.
Figure 2.
Plot of K versus sterol concentration in (●) the absence and (■) presence of a saturated chloroform atmosphere.
Further evidence for a common state was obtained by measuring the amount of chloroform that was absorbed by the liposomes under our experimental conditions. Thus, whereas 3.06, 2.42 and 1.48 molecules of chloroform were absorbed per lipid (DPPC + cholesterol) in liposomes containing 2.5, 20 and 40 mol% cholesterol respectively, the number per DPPC molecule was very similar for all three membranes; that is, 3.09, 3.03 and 2.47 chloroform molecules per phospholipid. Since this number is practically constant, it implies that chloroform favors the solvation of DPPC and that this state of solvation is independent of the concentration of cholesterol that is present.9
To gain insight into the effects of chloroform on the compactness of these membranes, we measured the generalized polarization (GP) of a bound fluorescent probe (i.e., Laurdan) as a function of temperature.10 As shown previously, Laurdan detects changes in membrane phase properties by sensing the polarity of its microenvironment in the bilayer.10 Specifically, variations in membrane water content induce shifts in Laurdan’s emission spectrum, which can be quantified by calculating its generalized polarization, GP = (I440−I490)/(I440+I490), where I440 and I490 are emission intensities at these wavelengths (λex = 350 nm).
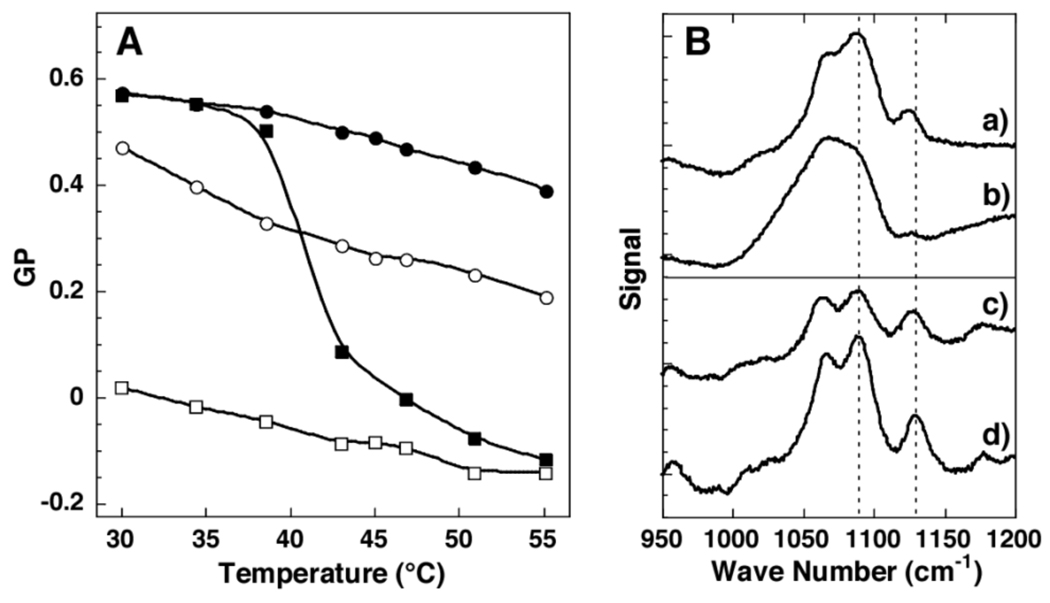
As shown in Figure 3, in the absence of chloroform, cholesterol-poor membranes show a well-defined gel to fluid phase transition, with a transition temperature, Tm, of 41°C. In sharp contrast, exposure to chloroform results in a fluid-like state that extends from above to below its Tm. It should be noted that the GP for Laurdan dissolved in chloroform is 0.66. For cholesterol-rich membranes, a relatively high level of compactness was found between 30°C and 55°C. When exposed to chloroform, the membrane’s compactness shifted towards an intermediate level at all temperatures.
Figure 3.
(A) Plot of GP versus temperature in liposomes made from DPPC/DPPG/cholesterol (97.5/2.5/2.5, mol%) without (■) and with (□) CHCl3; DPPC/DPPG/cholesterol (57.5/2.5/40, mol%) without (●) and with (○) CHCl3. (B) Raman spectra at 45°C for liposomes made from (top frame): DPPC/DPPG/cholesterol (97.5/2.5/2.5, mol%) and (bottom frame): DPPC/DPPG/cholesterol (57.5/2.5/40, mol%) without (a and c) and with (b and d) CHCl3.
Additional evidence for chloroform’s strong fluidizing effect on the gel phase was obtained by NNR measurements. Thus, the mixing of A with B in gel phase liposomes (35°C) derived from DPPC strongly favored homolipid associations (i.e., K=0.78). Upon exposure to chloroform, however, A and B now became favored nearest-neighbors at this same temperature (K=5.3).
As a final confirmation that chloroform fludizes cholesterol-rich and cholesterol-poor membranes, we measured the Raman spectrum of both types of membranes in the absence and presence of chloroform at 48°C (Figure 3). Here, the ratio of the 1130 cm−1/1090 cm−1 band intensities reflect the conformation of the acyl chains of the phospholipids.11 Specifically, the lower the ratio, the greater the number of gauche conformers. As is apparent, the presence of chloroform increases the number of gauche conformers in both types of membranes.
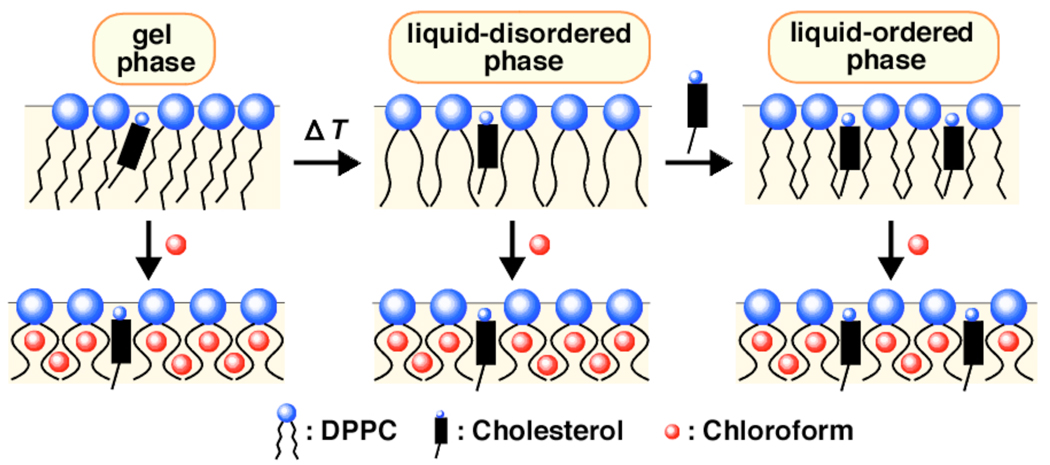
A simple model that takes all of these observations into account is illustrated in Scheme 1. Thus, when chloroform is absorbed either by the ld or the lo phase, the extent of solvation of the phospholipids is essentially the same. This constancy then leads to moderate sterol-phospholipid affinity, which is also constant and independent of the concentration of cholesterol in the membrane. The insertion of spherical-like chloroform molecules into the lipid bilayers loosens the assembly by disrupting chain packing and introducing additional “kinks” into the acyl chains of the phospholipids.
Scheme 1.
Given cholesterol’s strong condensing and fluidizing effects on phospholipid bilayers, it is reasonable to expect that any perturbation of its time-averaged, lateral distribution could result in altered structure and functioning of neighboring proteins. Thus, the significant influence that chloroform has on sterolphospholipid mixing seen here suggests that inhalation anesthetics could operate by a mechanism involving the rearrangement of the lipid framework; that is, by increasing the concentration of non-phospholipid-complexed cholesterol. In fact, we believe that such a mechanism would be the simplest one that takes the two-dimensional structure of biological membranes into account.
Supplementary Material
Experimental procedures and raw data. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We are grateful to the National Institutes of Health (PHS GM56149) for support of this research and to Julie Molinari and Kamalakanta Routray for valuable technical assistance.
REFERENCES
- 1.Bondarenko V, Yushmanov VE, Xu Y, Tang P. Biophys. J. 2008;94:1681–1688. doi: 10.1529/biophysj.107.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peoples RW, Li C, Weight FW. Annu. Rev. Pharmacol. Toxicol. 1996;36:185–201. doi: 10.1146/annurev.pa.36.040196.001153. [DOI] [PubMed] [Google Scholar]
- 3.(a) Cao H, Zhang J, Jing B, Regen SL. J. Am. Chem. Soc. 2005;127:8813–8816. doi: 10.1021/ja0513988. [DOI] [PubMed] [Google Scholar]; (b) Sugahara M, Uragami M, Regen SL. J. Am. Chem. Soc. 2003;125:13040–13041. doi: 10.1021/ja038102n. [DOI] [PubMed] [Google Scholar]; (c) Sugahara M, Uragami M, Yan X, Regen SL. J. Am. Chem. Soc. 2001;123:7939–7940. doi: 10.1021/ja016199c. [DOI] [PubMed] [Google Scholar]
- 4.Regen SL. Current Opinion in Chem. Biol. 2002;6:729–735. doi: 10.1016/s1367-5931(02)00398-8. [DOI] [PubMed] [Google Scholar]
- 5.(a) McIntosh TJ, editor. Lipid Rafts. New York: Springer-Verlag; 2007. [Google Scholar]; (b) Edidin M. Annu. Rev. Biomol. Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 6.Veatch SL, Keller SL. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Chiang Y-W, Costa-Filho J, Freed JH. J. Phys. Chem. B. 2007;111:11260–11270. doi: 10.1021/jp0732110. [DOI] [PubMed] [Google Scholar]
- 8.Lipids AA, BB and AB were synthesized using methods similar to those previously reported.3a,3c
- 9.Absorption measurements that have been made for isoflurane in bilayers made from egg PC, egg PG and cholesterol strongly suggest that this inhalant also favors solvation of phospholipids over cholesterol: Dickinson R, Franks NP, Lieb WR. Biophys. J. 1994;66:2019–2023. doi: 10.1016/S0006-3495(94)80994-4.
- 10.Parasassi T, DiStefano M, Loiero M, Ravagnan G, Gratton E. Biophys. J. 1994;66:120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaber B, Peticolas WL. Biochim. Biophys. Acta. 1977;465:260–274. doi: 10.1016/0005-2736(77)90078-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and raw data. This material is available free of charge via the Internet at http://pubs.acs.org.