Abstract
Molecular oxygen (O2) began to accumulate in the atmosphere and surface ocean ca. 2,400 million years ago (Ma), but the persistent oxygenation of water masses throughout the oceans developed much later, perhaps beginning as recently as 580–550 Ma. For much of the intervening interval, moderately oxic surface waters lay above an oxygen minimum zone (OMZ) that tended toward euxinia (anoxic and sulfidic). Here we illustrate how contributions to primary production by anoxygenic photoautotrophs (including physiologically versatile cyanobacteria) influenced biogeochemical cycling during Earth's middle age, helping to perpetuate our planet's intermediate redox state by tempering O2 production. Specifically, the ability to generate organic matter (OM) using sulfide as an electron donor enabled a positive biogeochemical feedback that sustained euxinia in the OMZ. On a geologic time scale, pyrite precipitation and burial governed a second feedback that moderated sulfide availability and water column oxygenation. Thus, we argue that the proportional contribution of anoxygenic photosynthesis to overall primary production would have influenced oceanic redox and the Proterozoic O2 budget. Later Neoproterozoic collapse of widespread euxinia and a concomitant return to ferruginous (anoxic and Fe2+ rich) subsurface waters set in motion Earth's transition from its prokaryote-dominated middle age, removing a physiological barrier to eukaryotic diversification (sulfide) and establishing, for the first time in Earth's history, complete dominance of oxygenic photosynthesis in the oceans. This paved the way for the further oxygenation of the oceans and atmosphere and, ultimately, the evolution of complex multicellular organisms.
Keywords: ocean chemistry, primary production, Proterozoic biosphere
Over the past decade, paleoenvironmental insights from iron speciation (1–7), sulfur isotopes (3, 8–12), Mo systematics (13, 14), and organic geochemistry (15) have converged on a view of Proterozoic oceans (16, 17). With the global cessation of iron formations ≈1,840 million years ago (Ma) (5), euxinic water masses expanded beneath an oxygenated surface mixed layer. We do not know whether the oxygen minimum zone (OMZ) was always and everywhere euxinic (14, 18), but existing data suggest that euxinia was both widespread and persistent for an interval at least 1,000 Ma in duration (14). Deep ocean chemistry is less certain; mid-Proterozoic bottom waters have been modeled variously as sulfidic, anoxic but not sulfidic, and dysoxic (10, 13, 16, 19)—possibly all three existed in varying proportions. What mattered most for Proterozoic life (and, in fact, for the partial pressure of atmospheric oxygen; PO2), however, was the general state of waters within the photic zone and immediately beneath the oxygenated surface ocean. How the world persisted in this seemingly static state, distinct from both Archean and Phanerozoic biospheres, for a billion years (20) remains largely unknown.
Oxygenic photosynthesis provides Earth's only major source of molecular oxygen (O2). In oxygenic cyanobacteria, photosystem I (PSI) strips electrons from chlorophyll to generate energy and reductants (ATP and NADPH), while a second photosystem (PSII), assisted by a Mn-based catalytic complex, replenishes the electron pool by oxidizing H2O to O2 (21). In contrast, green and purple sulfur bacteria (anoxygenic photoautotrophs) commonly use sulfide to drive primary production with PSI- and PSII-like machinery, respectively (22, 23). In this case, the production of oxidized sulfur compounds (S0 or SO4), rather than O2, balances the formation of OM. Similarly, in the presence of sulfide, many cyanobacteria down-regulate PSII and obtain proportionally fewer (or no) electrons from water, instead oxidizing S2− to S0, much like green S bacteria (24). Such versatile cyanobacteria are often observed where sulfide intrudes in the photic zone (25), not surprising insofar as the oxidizing potential required to extract electrons from sulfide is significantly lower than that for water (21, 26). Unlike most anoxygenic photoautotrophs, however, cyanobacteria can quickly reinstate PSII and O2 generation when returned to an oxic environment (27).
In some present day stratified lakes, which often contain shallow chemoclines, anoxygenic photoautotrophs can dominate primary production (up to 83%) (26). Anoxic marine basins commonly have much deeper chemoclines; nonetheless, anoxygenic photosynthesis can still contribute to overall primary production (28). In mid-Proterozoic oceans, lower PO2 and warmer temperatures (reducing O2 solubility) would have made sulfide much more available for anoxygenic photoautotrophy, enhancing their potential contribution to overall primary production. Increasing the relative proportion of anoxygenic photosynthesis would have decreased the direct link between OM burial and O2 generation (Fig. 1). With this in mind, we explore the biogeochemical consequences of mixed oxygenic and anoxygenic photosynthesis in the oceans of Earth's middle age.
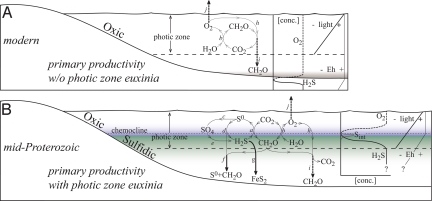
Fig. 1.
A schematic representation of marine primary productivity during the modern (A) and postulated Proterozoic (B). Geochemical cycles not directly related to primary productivity are neglected for simplicity. Dark heavy arrows reflect exit channels. Letters associated with fluxes are as follows: a, anoxygenic photosynthesis; b, oxygenic photosynthesis; c, sulfur oxidation by disproportionation or (non)phototrophic S-oxidizers; d, S0 respiration; e, sulfate reduction; f, S0-OM export/ballasting; g, pyrite formation; h, aerobic respiration; i, OM export; and j, O2 export. (A) Primary productivity performed by oxygenic photosynthesis and where O2 and CH2O (OM) are produced in stoichiometric proportions. Light penetrates to a given depth, and due to available O2, Eh remains high throughout the water column. Only within the sediments is O2 exhausted and sulfide allowed to accumulate, both encapsulated by a decrease in Eh. (B) A mixed community of primary producers, with surface oxygenic photosynthesis dominated by cyanobacteria (rather than algae) and subchemocline anoxygenic photosynthesis driven by sulfide. Here, OM production reflects the sum of all primary production pathways, which is balanced by the production of O2 in surface waters and S0 (or other oxidative intermediates: All represented as Sint) below the chemocline. As O2 is depleted and sulfide concentrations increase, appreciable pools of Sint could accumulate. Light again decreases with depth, and as O2 decreases across the chemocline, Eh would drop sharply. The specific chemistry of deep water will contribute to burial efficiencies, but is not central to our argument.
In Proterozoic surface waters underlain by an anoxic OMZ, fixed N may have exerted fundamental control on total primary productivity (17, 29). Thus, when considering the aggregate of oxygenic and anoxygenic photosynthesis, the Proterozoic nitrogen (N) cycle must be taken into account simultaneously. In the modern oligotrophic ocean, upwelling of remineralized inorganic NO3− provides most of the nutrient N (30) for photoautotrophs. In OMZs, however, N:P often falls well below the Redfield ratio of 16:1, suggesting net N loss via biological denitrification and anammox (31). In mid-Proterozoic oceans with a strong redoxcline, a microbial nutrient gauntlet would have developed as nutrient N (NO3− and/or NH4+) ascended from deep waters toward the photic zone (29). Collectively, denitrification and anammox reactions would have removed upwardly advecting bioavailable N (31–33), and when the OMZ fell within the photic zone, anoxygenic photoautotrophs would have consumed much or all of remaining fixed N before it reached obligately oxygenic photoautotrophs in surface waters. Thus, a persistent fixed-N deficiency throughout the OMZ and photic zone (17, 31) becomes likely and would have conferred ecological advantage on photoautotrophs able to fix N2. In the Proterozoic ocean, then, both impinging sulfide and a scarcity of fixed-N would have favored diazotrophic (N2-fixing) photoautotrophic bacteria over eukaryotic algae. Molecular fossils of pigments derived from anoxygenic phototrophs provide direct evidence for photic zone euxinia in Proterozoic oceans (15); such data, however, remain limited.
Widespread OMZ euxinia thus would have exerted a strong influence on the nature of primary producers in mid-Proterozoic oceans. Trace metal scarcity in Proterozoic oceans might further have limited the amount of primary production, via its effects on certain key enzymes (17, 34). The effect of trace metal limitation on primary production in Proterozoic seas remains an area of active debate and experimentation (35–37), but regardless of its resolution, the predominant influence on marine redox conditions in mid-Proterozoic oceans was the proportional contribution of anoxygenic photosynthesis to overall primary production.
Regardless of the source of OM, for energetic reasons, oxygen would still be the favored oxidant for OM remineralization; and as is true today, the propensity toward water column anoxia would scale with the amount of exported OM. Importantly, however, it is the fraction of OM escaping aerobic respiration that would set limits on anoxygenic photosynthesis (38). In Proterozoic oceans, with low overall NO3− and Fe3+ availability, SO42− reduction would have been the principal reductive metabolism after aerobic respiration, generating sulfide within oxygen-depleted OMZs. An increase in photosynthetic electron donation from this sulfide source (rather than from H2O) would depress surface O2 concentrations further, simultaneously enhancing the potential for N2-fixation. This would increase both primary and, presumably, export production—an overall positive feedback on OMZ euxinia (39) (Fig. 2A) that would limit rather than foster PO2 accumulation. That is, when primary production includes a nontrivial contribution from anoxygenic photoautotrophy, the generation of organic matter, in principle, exceeds the generation of oxygen available to complete the carbon cycle. This would increase the probability that the OMZ will become euxinic and, in consequence, that sulfidic conditions will encroach on the photic zone. This photic zone sulfide is available for further anoxygenic photosynthesis—establishing the feedback loop.
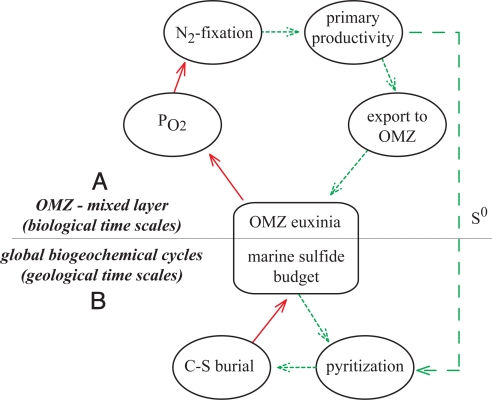
Fig. 2.
A schematic view of feedbacks that acted to sustain Proterozoic environments on both short and long geologic time scales (A and B, respectively). The point of entrance into this cycle is the establishment of sulfidic conditions at ≈1,840 Ma (5) and possibly earlier. Dashed green and solid red arrows note the direction of the feedback. If an increase in one quantity is followed by a decrease in the next, the connecting arrow is red (a negative feedback). If an increase in one quantity leads to an increase in the next, then the connecting arrow is green (a positive feedback). For example, if we begin in A with an increase in OMZ sulfide, PO2 correspondingly decreases (thus a red arrow preceding the PO2 ellipse), propagating responses through the remainder of the system. The presence of sulfide increases the likelihood of anoxygenic (by cyanobacteria, purple S bacteria, and/or green S bacteria) contributions to primary productivity, which would then produce less overall O2, encourage N2 fixation, increase primary production and carbon export, and increase the degree of euxinia (a positive feedback). (B) A sulfide-rich ocean in which S0 is an oxidant byproduct of primary producers and provides sedimentary conditions conducive to burial of both pyrite and carbon, although the burial of anoxygenically produced carbon is not strictly coupled to residual O2 (no O2 left behind). The loss of sulfide through pyrite burial dampens the extent of ocean euxinia (a negative feedback). The result is a system that maintains both oxygenic and anoxygenic photosynthesis.
Over geologic time scales, OM burial permits O2 accumulation (40), but only to the extent that primary production is driven by oxygenic photoautotrophs. OM burial in anoxic sediments is usually accompanied by significant pyritization, a net oxidative process relative to sulfide, as it effectively combines H2S with S0. Here, the S0 produced by anoxygenic photoautotrophy (26, 41–43) would pair with H2S and Fe2+, satisfying the electron balance required for pyrite formation (Fig. 2B). Export of OM-associated S0 to sediments, at potentially significant sinking velocities (26), could have served as ballast before fecal pellets came to play this role. Alternatively, iron sulfides may have been produced in the water column, as sinking S0 would react with dissolved sulfide to produce polysulfides, an important precursor to pyrite production (44). Either mechanism would facilitate loss of sulfur to sedimentary burial at a rate no greater than twice Fe delivery, ameliorating the potential for runaway sulfide production (Fig. 2). Finally, as OM burial and pyritization are both electron sinks, they would increase the overall oxidation state of ambient seawater (45). This is not, however, equivalent to increasing the O2 concentration of the ocean-atmosphere system, especially if a fraction of buried carbon derives from anoxygenic species.
In mid-Proterozoic oceans, then, the cycle of primary production and remineralization would have established a system in which two conjoined feedback loops worked to perpetuate OMZ euxinia and maintain moderate, but not high, levels of O2 (Fig. 2). These biologically mediated feedbacks link PO2 and OMZ euxinia, whereas the burial of reducing potential (over geological time scales) allows the accumulation of enough oxidizing capacity to avoid return to a largely anoxic fluid Earth like that of the Archean.
The relative contribution of anoxygenic photoautotrophy to mid-Proterozoic PO2 need not have been large for their presence to be felt. Primary production in the modern ocean is ≈1.5 × 1015 mol C/year, with an organic carbon burial rate of 5.33 × 1012 mol C/year (46, 47). When primary production is 100% oxygenic, the maximum rate at which O2 can accumulate is equivalent to the rate of carbon burial (≈5 × 1012 mol O2/year). Because the theoretical maximum contribution from sulfide-using anoxygenic photoautotrophs is equal to integrated rates of sulfate reduction (38), the modern rate of net sulfide generation (2.62 × 1012 mol S/year) (48) sets an upper limit on the hypothetical contribution from anoxygenic photosynthesis to total modern primary production at ≈0.17% (see SI Text for calculation details).
In this formulation, O2 production is sensitive to: (i) The magnitude of overall primary production and burial fluxes and (ii) their respective ratios to overall sulfate reduction rates modified by the efficiency with which sulfide becomes available to autotrophs. Much of the sulfide produced in today's ocean will not be available for photoautotrophic oxidation, because the sulfide is produced deep within the marine realm, most commonly within sediments. However, in mid-Proterozoic oceans, although the magnitude of primary production may have been smaller (17), sulfate reduction rates would have been similar or higher, as a greater fraction of primary organic matter was not aerobically respired (49). When combined with increased sulfide availability near or within the photic zone (15, 50), these conditions enhanced the likelihood that photosynthetic sulfide oxidizers would moderate oxygen levels due to positive feedbacks (Fig. 2). Simply, as the ratio of export production to sulfate reduction approaches unity, the potential for anoxygenic photoautotrophy to buffer PO2 increases. Thus, using approximations for Proterozoic sulfate reduction (reference 49 and SI Text) and keeping a modern burial efficiency (which likely underestimates Proterozoic burial due to low deep-water O2), we can estimate a rate of diminished O2 production of 0.4% PO2 per 10 million years, even with the relative contribution of anoxygenic photosynthesis at only ≈1% of the total production. When considering that Proterozoic O2 was likely much lower today, perhaps 1–10% of modern O2 (or PO2 ≈0.2–2%), it is clear that even these humble contributions from anoxygenic photosynthesis, integrated across geologic time scales, would have impacted Earth's surface oxygen budget. Although our arguments are framed in terms of water column budgets, they also apply to microbial mat systems, which were widespread on Proterozoic seafloors (51).
In contrast to the Proterozoic Eon, when euxinia was persistent, more recent episodes of euxinia in the Phanerozoic oceans have been transient, presumably because euxinia cannot not be sustained over multimillion year time scales in the face of the greater PO2 and, thus, the buffering capacity of the Phanerozoic atmosphere (SI Text).
If Earth's middle age was self-sustaining, what drove its demise? Canfield and colleagues (4) recently reported that anoxic subsurface waters of later Neoproterozoic oceans returned to an iron-rich state more characteristic of Archean seas. This reversion resulted from the long-term removal of sulfur by the subduction of pyrite-rich Proterozoic marine sediments (52) (Fig. 2) and an increase in the proportional input of Fe to S into the ocean (53, 54). The loss of photic zone euxinia terminated quantitatively important contributions from sulfide-driven anoxygenic photosynthesis, thus ending control of the “sulfur world” on the oxidation state of the ocean-atmosphere system (Fig. 3). This switch removed two direct inhibitors of eukaryotic evolution: Sulfide, which is toxic to most eukaryotes, and low available N, as eukaryotic photoautotrophs cannot fix N2 (55–57). More work is required to determine whether N cycling (assimilatory and dissimilatory) and availability (balance of sources and sinks) would increase in a ferruginous ocean, relaxing N stress and favoring algal diversification, or whether continued anoxia, despite the loss of euxinia, would keep available N low.
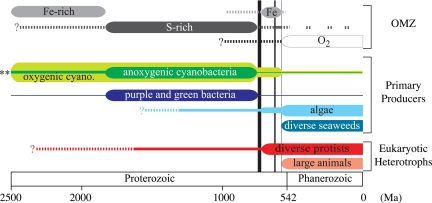
Fig. 3.
A timeline showing OMZ chemistry (1–4, 7–10, 13, 14, 17), the relative contributions from different primary producers (17, 55, 69), and the evolution of eukaryotic heterotrophs (55–59, 70, 71). Band thicknesses approximate the importance of each feature through time. Dashed lines represent postulated or uncertain histories. The specific evolutionary sequence of oxygenic and anoxygenic photoautotrophs (including both cyanobacteria and purple/green S bacteria), marked here by **, rests in the Archean rock record (>2,500 Ma). As both processes had evolved by 1,800 Ma (23) (when our story begins), we make, nor require, any distinct sequence. The two thicker vertical lines represent the major Neoproterozoic glaciations (72), and the thinner line to the right marks the Ediacaran Gaskiers glaciation. The precise timing of Neoproterozoic climatic and biogeochemical events is the subject of ongoing research. We highlight the mixed contributions to primary productivity through the Proterozoic, a transition in OMZ chemistry at 800–700 Ma, and the coincident change in cyanobacteria, algal, protist, and animal abundances, based on body and molecular fossils.
Overall, these observations are consistent with the geologic record, as the oldest well-characterized eukaryotic microfossils occur in near-shore environments (58), where OMZ sulfide incursion was least likely. Similarly, the oldest eukaryotic fossils attributable to an extant phylum, bangiophyte red algae in ca. 1200 Ma rocks from Canada, lived on a tidal flat (59). Broadly coincident with the late Neoproterozoic geochemical transition, microfossils associated with N2-fixing cyanobacteria (Nostocales) decline strongly (60), diverse protists appear (56, 61, 62), and organic-rich sediments begin to record increases in sterane abundances (63) that herald the rise of green algae to ecological prominence.
We do not discount the possibility that tectonic circumstances also contributed to the perpetuation of Earth's middle age, and we note that factors such as the low bioavailability of trace metals (14, 17, 34, 64, 65) may further have dampened the responsiveness of the Proterozoic biosphere to biogeochemical forcings. Our model, however, strongly implicates the sulfide-driven contribution of anoxygenic photoautotrophy to overall primary production in sustaining Earth's “boring billion” years (20). The eventual exhaustion of this sulfide reservoir in the Neoproterozoic (4) allowed the strict biogeochemical coupling of O2 accumulation to OM burial: The “carbon world.” Alone, this might not have driven an immediate increase in PO2, but it would have established a framework within which high rates of sedimentation and, hence, OM burial in late Neoproterozoic basins would have a significant and direct effect on global O2 (55, 66).
Our model can be tested in a number of ways. Consistent with Canfield and colleagues (4), we predict that the loss of widespread OMZ euxinia will be resolved as an event separate from and earlier than the widespread oxygenation of these water masses. If our model is correct, then as organic geochemical research proceeds, biomarkers for anoxygenic photoautotrophs will prove to be prominent in Proterozoic basins marked by OMZ euxinia (such as in reference 15), but rare thereafter. And, consistent with this, our model predicts that the timing of Neoproterozoic eukaryotic diversification, as recorded in both paleontological and biomarker records, will be linked stratigraphically to the demise of euxinic OMZs. These geological predictions should be addressed in light of experimental research aimed at constraining contributions from Fe2+ using anoxygenic photoautotrophs (67) and, possibly, cyanobacteria (68) to Neoproterozoic primary production, an avenue yet to be explored.
Together, these perspectives issue a challenge: How do we quantify the interplay between rates of mixed primary production, summing oxygenic and anoxygenic photosynthesis, and remineralization efficiencies (presuming differing availabilities of O2, Fe3+, and SO42− through time) in a world where OM burial is not strictly linked to PO2, the ultimate source of electron acceptors? In the end, we may find that the three long lasting states of Earth's biosphere —broadly, the anoxic Archean, intermediate Proterozoic, and fully oxygenated Phanerozoic—will find relatively straightforward explanation in primary production that was largely anoxygenic in the Archean, oxygenic in the Phanerozoic, and mixed in between.
Supplementary Material
Acknowledgments.
We thank P. Cohen, N. Tosca, P. Girguis, A. Anbar, P. Fromme, J. Golbeck, L. Jahnke, L. Miller, and R. Oremland for conversations and comments on early drafts; T. Lyons, D. Canfield, and M. Follows for thoughtful reviews; and W. Vernaas for stimulating early conversations on the flexibility of cyanobacterial metabolism. This work was supported by the Microbial Sciences Initiative at Harvard (to D.T.J.), National Aeronautics and Space Administration (NASA) Exobiology Grant NNX07AV51G (to D.T.J. and A.H.K.), National Science Foundation Minority Postdoctoral Fellowship DBI-0511972 (to F.W.S.) and the NASA Astrobiology Institute (to A.H.K. and F.W.S.), and the David and Lucille Packard Foundation (to A.P.).
Footnotes
The authors declare no conflicts of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909248106/DCSupplemental.
References
- 1.Canfield DE, Poulton SW, Narbonne GM. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science. 2007;315:92–95. doi: 10.1126/science.1135013. [DOI] [PubMed] [Google Scholar]
- 2.Shen YN, Canfield DE, Knoll AH. Middle Proterozoic ocean chemistry: Evidence from the McArthur Basin, northern Australia. Am J Sci. 2002;302:81–109. [Google Scholar]
- 3.Shen Y, Knoll AH, Walter MR. Evidence for low sulphate and anoxia in a mid-Proterozoic marine basin. Nature. 2003;423:632–635. doi: 10.1038/nature01651. [DOI] [PubMed] [Google Scholar]
- 4.Canfield DE, et al. Ferruginous conditions dominated later Neoproterozoic deep-water chemistry. Science. 2008;321:949–952. doi: 10.1126/science.1154499. [DOI] [PubMed] [Google Scholar]
- 5.Poulton SW, Fralick PW, Canfield DE. The transition to a sulphidic ocean similar to 1.84 billion years ago. Nature. 2004;431:173–177. doi: 10.1038/nature02912. [DOI] [PubMed] [Google Scholar]
- 6.Jackson MJ, Raiswell R. Sedimentology and carbon sulfur geochemistry of the Velkerri formation, a mid-Proterozoic potential oil source in northern Australia. Precambrian Res. 1991;54:81–108. [Google Scholar]
- 7.Shen YN, Zhang TG, Hoffman PF. On the coevolution of Ediacaran oceans and animals. Proc Natl Acad Sci USA. 2008;105:7376–7381. doi: 10.1073/pnas.0802168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fike DA, Grotzinger JP, Pratt LM, Summons RE. Oxidation of the Ediacaran ocean. Nature. 2006;444:744–747. doi: 10.1038/nature05345. [DOI] [PubMed] [Google Scholar]
- 9.McFadden KA, et al. Pulsed oxidation and biological evolution in the Ediacaran Doushantuo formation. Proc Natl Acad Sci USA. 2008;105:3197–3202. doi: 10.1073/pnas.0708336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canfield DE. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–453. [Google Scholar]
- 11.Kah LC, Lyons TW, Frank TD. Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature. 2004;431:834–838. doi: 10.1038/nature02974. [DOI] [PubMed] [Google Scholar]
- 12.Johnston DT, et al. Active microbial sulfur disproportionation in the Mesoproterozoic. Science. 2005;310:1477–1479. doi: 10.1126/science.1117824. [DOI] [PubMed] [Google Scholar]
- 13.Arnold GL, Anbar AD, Barling J, Lyons TW. Molybdenum isotope evidence for widespread anoxia in mid-Proterozoic oceans. Science. 2004;304:87–90. doi: 10.1126/science.1091785. [DOI] [PubMed] [Google Scholar]
- 14.Scott C, et al. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature. 2008;452:456–458. doi: 10.1038/nature06811. [DOI] [PubMed] [Google Scholar]
- 15.Brocks JJ, et al. Biomarker evidence for green and purple sulphur bacteria in a stratified Palaeoproterozoic sea. Nature. 2005;437:866–870. doi: 10.1038/nature04068. [DOI] [PubMed] [Google Scholar]
- 16.Lyons TW, Anbar AD, Severmann S, Scott C, Gill BC. Tracking euxinia in the ancient ocean: A multiproxy perspective and Proterozoic case study. Annu Rev Earth Planet Sci. 2009;37:507–534. [Google Scholar]
- 17.Anbar AD, Knoll AH. Proterozoic ocean chemistry and evolution: A bioinorganic bridge? Science. 2002;297:1137–1142. doi: 10.1126/science.1069651. [DOI] [PubMed] [Google Scholar]
- 18.Meyer KM, Kump LR. Oceanic euxinia in Earth history: Causes and consequences. Annu Rev Earth Planet Sci. 2008;36:251–288. [Google Scholar]
- 19.Slack JF, Grenne T, Bekker A, Rouxel OJ, Lindberg PA. Suboxic deep seawater in the late Paleoproterozoic: Evidence from hematitic chert and iron formation related to seafloor-hydrothermal sulfide deposits, central Arizona. USA Earth Planet Sci Lett. 2007;255:243–256. [Google Scholar]
- 20.Buick R, Marais DJD, Knoll AH. Stable isotopic compositions of carbonates from the Mesoproterozoic Bangemall Group. Northwestern Australia Chem Geol. 1995;123:153–171. doi: 10.1016/0009-2541(95)00049-r. [DOI] [PubMed] [Google Scholar]
- 21.Blackenship RE, Sadekaar S, Raymond J. In: Evolution of Primary Producers in the Sea. Falkowski PG, Knoll AH, editors. New York, NY: Academic; 2007. pp. 22–33. [Google Scholar]
- 22.Mulkidjanian AY, et al. The cyanobacterial genome core and the origin of photosynthesis. Proc Natl Acad Sci USA. 2006;103:13126–13131. doi: 10.1073/pnas.0605709103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blankenship RE. Origin and early evolution of photosynthesis. Photosynth Res. 1992;33:91–111. [PubMed] [Google Scholar]
- 24.Cohen Y, Jorgensen BB, Revsbech NP, Poplawski R. Adaptation to hydrogen sulfide of oxygenic and anoxygenic photosynthesis among cyanobacteria. Appl Environ Microbiol. 1986;51:398–407. doi: 10.1128/aem.51.2.398-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen BB, Cohen Y, Revsbech NP. Transition from anoxygenic to oxygenic photosynthesis in a microcoleus-chthonoplastes cyanobacterial mat. Appl Environ Microbiol. 1986;51:408–417. doi: 10.1128/aem.51.2.408-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gemenden H, Mas J. In: Anoxygenic Photosynthetic Bacteria. Blankenship RE, Madigan MT, Bauer C, editors. New York, NY: Springer-Verlag; 1995. pp. 49–85. [Google Scholar]
- 27.Oren A, Padan E. Induction of anaerobic, photoautotrophic growth in cyanobacterium oscillatoria-limnetica. J Bacteriol. 1978;133:558–563. doi: 10.1128/jb.133.2.558-563.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manske AK, Glaeser J, Kuypers MM, Overmann J. Physiology and phylogeny of green sulfur bacteria forming a monospecific phototrophic assemblage at a depth of 100 meters in the Black Sea. Appl Environ Microbiol. 2005;71:8049–8060. doi: 10.1128/AEM.71.12.8049-8060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fennel K, Follows M, Falkowski PG. The co-evolution of the nitrogen, carbon and oxygen cycles in the Proterozoic ocean. Am J Sci. 2005;305:526–545. [Google Scholar]
- 30.Lewis MR, Harrison WG, Oakey NS, Hebert D, Platt T. Vertical nitrate fluxes in the oligotrophic ocean. Science. 1986;234:870–873. doi: 10.1126/science.234.4778.870. [DOI] [PubMed] [Google Scholar]
- 31.Kuypers MM, et al. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci USA. 2005;102:6478–6483. doi: 10.1073/pnas.0502088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuypers MM, et al. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature. 2003;422:608–611. doi: 10.1038/nature01472. [DOI] [PubMed] [Google Scholar]
- 33.Lam P, et al. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci USA. 2007;104:7104–7109. doi: 10.1073/pnas.0611081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glass GB, Wolfe-Simon F, Anbar AD. Coevolution of marine metal availability and photoautotrophic nitrogen assimilation. Geobiology. 2009;7:100–123. doi: 10.1111/j.1472-4669.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- 35.Zerkle AL, House CH, Cox RP, Canfield DE. Metal limitation of cyanobacterial N2 fixation and implications for the Precambrian nitrogen cycle. Geobiology. 2006;4:285–297. [Google Scholar]
- 36.Tuit C, Waterbury J, Ravizzaz G. Diel variation of molybdenum and iron in marine diazotrophic cyanobacteria. Limnol Oceanogr. 2004;49:978–990. [Google Scholar]
- 37.Thiel T, Pratte B, Zahalak M. Transport of molybdate in the cyanobacterium Anabaena variabilis ATCC 29413. Arch Microbiol. 2002;179:50–56. doi: 10.1007/s00203-002-0499-y. [DOI] [PubMed] [Google Scholar]
- 38.Canfield DE, Thamdrup B, Kristensen E. Aquatic Geomicrobiology. Advances in Marine Biology. vol 48. San Diego, CA: Elsevier Academic Press; 2006. [DOI] [PubMed] [Google Scholar]
- 39.Canfield DE. Models of oxic respiration, denitrification and sulfate reduction in zones of coastal upwelling. Geochim Cosmochim Acta. 2006;70:5753–5765. [Google Scholar]
- 40.Berner RA. A model for atmospheric CO2 over Phanerozoic time. Am J Sci. 1991;291:339–376. doi: 10.2475/ajs.289.4.333. [DOI] [PubMed] [Google Scholar]
- 41.Oren A, Padan E, Malkin S. Sulfide inhibition of photosystem-II in cyanobacteria (blue-green-algae) and tobacco chloroplasts. Biochim Biophys Acta. 1979;546:270–279. doi: 10.1016/0005-2728(79)90045-8. [DOI] [PubMed] [Google Scholar]
- 42.Moezelaar R, Bijvank SM, Stal LJ. Fermentation and sulfur reduction in the mat-building cyanobacterium Microcoleus chthonoplastes. Appl Environ Microbiol. 1996;62:1752–1758. doi: 10.1128/aem.62.5.1752-1758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stal LJ, Moezelaar R. Fermentation in cyanobacteria. FEMS Microbiol Rev. 1997;21:179–211. [Google Scholar]
- 44.Rickard D, Luther GW. Chemistry of iron sulfides. Chem Rev. 2007;107:514–562. doi: 10.1021/cr0503658. [DOI] [PubMed] [Google Scholar]
- 45.Walker JCG, Brimblecombe P. Iron and sulfur in the pre-biological ocean. Precambrian Res. 1985;28:205–222. doi: 10.1016/0301-9268(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 46.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–206. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 47.Hedges JI, Keil RG. Sedimentary organic matter preservation—an assessment and speculative synthesis. Mar Chem. 1995;49:81–115. [Google Scholar]
- 48.Turchyn AV, Schrag DP. Cenozoic evolution of the sulfur cycle: Insight from oxygen isotopes in marine sulfate. Earth Planet Sci Lett. 2006;241:763–779. [Google Scholar]
- 49.Canfield DE, Farquhar J. Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc Natl Acad Sci USA. 2009;106:8123–8127. doi: 10.1073/pnas.0902037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston DT, Poulton SW, Fralick PW, Wing BA, Canfield DE, Farquhar J. Evolution of the oceanic sulfur cycle at the end of the Paleoproterozoic. Geochim Cosmochim Acta. 2006;70:5723–5739. [Google Scholar]
- 51.Grotzinger JP, Knoll AH. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annu Rev Earth Planet Sci. 1999;27:313–358. doi: 10.1146/annurev.earth.27.1.313. [DOI] [PubMed] [Google Scholar]
- 52.Canfield DE. The evolution of the Earth surface sulfur reservoir. Am J Sci. 2004;304:839–861. [Google Scholar]
- 53.Kump LR, Seyfried WE. Hydrothermal Fe fluxes during the Precambrian: Effect of low oceanic sulfate concentrations and low hydrostatic pressure on the composition of black smokers. Earth Planet Sci Lett. 2005;235:654–662. [Google Scholar]
- 54.Johnston DT, et al. Insight into early Neoproterozoic ocean chemistry. Eos. 2008;89(Fall Meet. Suppl) Abstr. PP33D-01. [Google Scholar]
- 55.Knoll AH. Proterozoic and early Cambrian protists—evidence for accelerating evolutionary tempo. Proc Natl Acad Sci USA. 1994;91:6743–6750. doi: 10.1073/pnas.91.15.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butterfield NJ. Macroevolution and macroecology through deep time. Palaeontology. 2007;50:41–55. [Google Scholar]
- 57.Mentel M, Martin W. Energy metabolism among eukaryotic anaerobes in light of Proterozoic ocean chemistry. Philos Trans R Soc Lond B Biol Sci. 2008;363:2717–2729. doi: 10.1098/rstb.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javaux EJ, Knoll AH, Walter MR. Morphological and ecological complexity in early eukaryotic ecosystems. Nature. 2001;412:66–69. doi: 10.1038/35083562. [DOI] [PubMed] [Google Scholar]
- 59.Butterfield NJ. Bangiomorpha pubescens n. gen., n. sp.: Implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology. 2000;26:386–404. [Google Scholar]
- 60.Golubic S, Sergeev VN, Knoll AH. Mesoproterozoic Archaeoellipsoides: Akinetes of heterocystous cyanobacteria. Lethaia. 1995;28:285–298. doi: 10.1111/j.1502-3931.1995.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 61.Allison CW, Hilgert JW. Scale microfossils from the early Cambrian of northwest Canada. J Paleontol. 1986;60:973–1015. [Google Scholar]
- 62.Porter SM, Meisterfeld R, Knoll AH. Vase-shaped microfossils from the Neoproterozoic Chuar Group, Grand Canyon: A classification guided by modern testate amoebae. J Paleontol. 2003;77:409–429. [Google Scholar]
- 63.Grantham PJ, Wakefield LL. Variations in the sterane carbon number distributions of marine source rock derived crude oils through geological time. Org Geochem. 1988;12:61–73. [Google Scholar]
- 64.Saito MA, Sigman DM, Morel FMM. The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary? Inorg Chim Acta. 2003;356:308–318. [Google Scholar]
- 65.Buick R. Did the Proterozoic ‘Canfield Ocean’ cause a laughing gas greenhouse? Geobiology. 2007;5:97–100. [Google Scholar]
- 66.DesMarais DJ, Strauss H, Summons RE, Hayes JM. Carbon isotope evidence for the stepwise oxidation of the Proterozoic environment. Nature. 1992;359:605–609. doi: 10.1038/359605a0. [DOI] [PubMed] [Google Scholar]
- 67.Widdel F, et al. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–836. [Google Scholar]
- 68.Cohen Y. Comparative N and S cycles. In: Klugg MJ, Reddy CA, editors. Current Perspectives in Microbial Ecology. Washington, DC: ASM Press; 1984. pp. 435–441. [Google Scholar]
- 69.Kodner RB, Pearson A, Summons RE, Knoll AH. Sterols in red and green algae: Quantification, phylogeny, and relevance for the interpretation of geologic steranes. Geobiology. 2008;6:411–420. doi: 10.1111/j.1472-4669.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 70.Knoll AH. The early evolution of eukaryotes—a geological perspective. Science. 1992;256:622–627. doi: 10.1126/science.1585174. [DOI] [PubMed] [Google Scholar]
- 71.Knoll AH, Javaux EJ, Hewitt D, Cohen P. Eukaryotic organisms in Proterozoic oceans. Philos Trans R Soc Lond B Biol Sci. 2006;361:1023–1038. doi: 10.1098/rstb.2006.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffman PF, Kaufman AJ, Halverson GP, Schrag DP. A Neoproterozoic snowball earth. Science. 1998;281:1342–1346. doi: 10.1126/science.281.5381.1342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.