Abstract
The JNK inhibitor SP600125 strongly inhibits cell proliferation in many human cancer cells by blocking cell-cycle progression and inducing apoptosis. Despite extensive study, the mechanism by which SP600125 inhibits mitosis-related effects in human leukemia cells remains unclear. We investigated the effects of SP600125 on the inhibition of cell proliferation and the cell cycle, and on microtubule dynamics in vivo and in vitro. Treatment of synchronized leukemia cells with varying concentrations of SP600125 results in significant G2/M cell cycle arrest with elevated p21 levels, phosphorylation of histone H3 within 24 h, and endoreduplication with elevated Cdk2 protein levels after 48 h. SP600125 also induces significant abnormal microtubule dynamics in vivo. High concentrations of SP600125 (200 µM) were required to disorganize microtubule polymerization in vitro. Additionally, SP600125-induced delayed apoptosis and cell death was accompanied by significant poly ADP-ribose polymerase (PARP) cleavage and caspase-3 activity in the late phase (at 72 h). Endoreduplication showed a greater increase in ectopic Bcl-2-expressing U937 cells at 72 h than in wild-type U937 cells without delayed apoptosis. These results indicate that Bcl-2 suppresses apoptosis and SP600125-induced G2/M arrest and endoreduplication. Therefore, we suggest that SP600125 induces mitotic arrest by inducing abnormal spindle microtubule dynamics.
Keywords: anthra(1,9-cd)pyrazol-6(2H)-one; apoptosis; cell cycle; cyclin-dependent kinase inhibitor p21; JNK mitogen-activated protein kinases; microtubule; proto-oncogene proteins c-bcl-2
Introduction
Cell cycle progression to the G1, S, and G2/M phases is controlled by cell cycle checkpoints that ensure the correct order and transition timing (Elledge, 1996). During mitotic phases, the spindle assembly checkpoint monitors the segregation of sister chromatids, inhibiting the onset of anaphase until all of the chromosomes are properly attached to the mitotic spindle apparatus (Shah and Cleveland, 2000). After G2/M arrest, a significant subpopulation of pRb-negative cells demonstrated an excessive amount of 4N DNA, known as endoreduplication (Niculescu et al., 1998). Although endoreduplication is a common event in plants, spontaneous endoreduplication is a phenomenon rarely observed in animals (Weiss et al., 1998). Recently, it has been shown that there are three main types of agent that induce endoreduplication (Cortes et al., 2004). The first type includes agents that interfere with cytoskeleton assembly (i.e. microtubule inhibitors), e.g., colchicines and colcemid. The second type consists of topoisomerase II inhibitors, which includes both enzyme poisons (i.e., etoposide, amsacrine, and adriamycin) and catalytic inhibitors (i.e., merbarone, aclarubicin, and ICRF-193). The third type comprises physical and chemical agents that damage DNA, such as X-rays. Some of these microtubule-interfering agents, such as nocodazole and paclitaxel, induce significant endoreduplication because sister chromatid segregation is interrupted (Andreassen et al., 1996; Jordan et al., 2003). Because disorganized microtubule formation interferes with cell cycle progression and induces apoptosis and endoreduplication, microtubule-targeting drugs have demonstrated broad activity against several human tumors with promising results in clinical trials (Rowinsky, 1993).
JNK was first described as a stress-activated protein kinase that phosphorylates c-Jun at two sites in the NH2-terminal activation domain (Kyriakis and Avruch, 1990; Kyriakis et al., 1994). Phosphorylated c-Jun homodimerizes or heterodimerizes with either Fos or ATF-2 to form AP-1 complexes that serve as transcription factors to alter gene expression (Vogt, 2002). Thus, AP-1 is a downstream effector of JNK activity that negatively affects cell proliferation and apoptosis (Shaulian and Karin, 2001). Recent studies have focused on the effects of JNK in the regulation of cell death (Tournier et al., 2000; Hochedlinger et al., 2002; Min et al., 2008), and it has been reported that the JNK-antisense oligonucleotide inhibits tumor growth and promotes regression in a high proportion of cases (Potapova et al., 2000). Additionally, inhibition of the JNK pathway with the inhibitor SP600125 causes either G2/M arrest or apoptosis in a variety of human cancer cell lines, including multiple myeloma, breast cancer, prostate cancer, and erythroleukemia (Hideshima et al., 2003; Jacobs-Helber and Sawyer, 2003; Du et al., 2004; Mingo-Sion et al., 2004). This research indicates that JNK activity is necessary to promote proliferation and to maintain diploidy in cancer cells. However, the induction mechanisms of G2/M arrest, endoreduplication, and delayed apoptosis due to SP600125 remain unclear.
In this study, we investigated the relationships between polyploid giant cells and delayed apoptosis in human leukemia cells treated with SP600125. Our results strongly implicate the effects of SP600125 in G2/M phase arrest, endoreduplication, and delayed apoptosis via disorganization of microtubule polymerization.
Results
SP600125 induces a dose-dependent reduction in cell growth and expansion of cell size in human leukemia cells
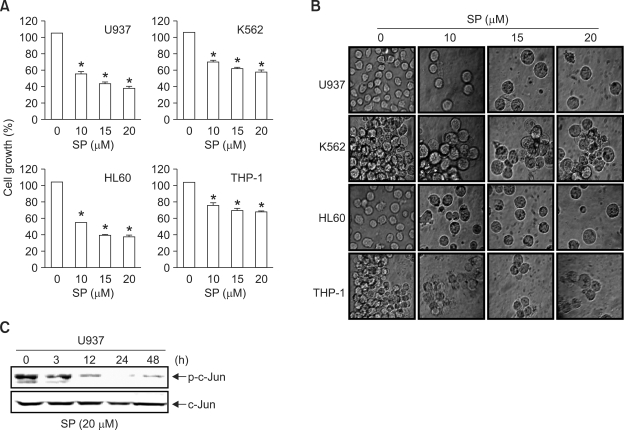
Recent studies have shown that SP600125, a pharmacological inhibitor of JNK, causes cell growth inhibition in certain cell types, including breast cancer (Mingo-Sion et al., 2004), multiple myeloma (Hideshima et al., 2003), and B-lymphoma (Jacobs-Helber and Sawyer, 2004). To verify this effect on cell growth, four different leukemia cell lines (U937, K562, HL60, and THP-1) were treated with varying concentrations of SP600125 for 48 h. Cell growth and morphological changes were assessed using MTT assays and phase contrast microscopy, respectively. As shown in Figure 1A, significant inhibition of cell growth in a dose-dependent manner was detected in the four leukemia cell lines. DMSO (0.1%), used as a vehicle control, did not affect cell viability or morphology. When cells were examined under phase contrast microscopy, cells treated with up to 10 µM SP600125 presented with swelling and modest apoptotic shrinkage at 48 h, compared to vehicle control cells (Figure 1B). To confirm that SP600125 inhibited JNK activity, Western blot analysis was conducted using p-c-Jun antibodies in U937 cells. As shown in Figure 1C, treatment with 20 µM SP600125 almost completely abolished c-Jun phosphorylation after 12 h, but total c-Jun protein levels had no influence on the expression status. These results indicate that SP600125 causes anti-proliferative effects with an enlarged cell morphology in leukemia cells through suppression of JNK activity.
Figure 1.
Inhibition of JNK activity using SP600125 reduces cell proliferation in human leukemia cells and enlarges the cell size. (A) Cells were plated at 5 × 104 cells/ml and incubated for 12 h. Cells were treated with the indicated concentrations of SP600125 for 48 h and the cell viability was measured using a metabolic-dye-based MTT assay. Each point represents the mean ± SD of three independent experiments. Significance was determined using Student's t-test (*P < 0.05 vs. vehicle control). (B) Cells were incubated with the indicated concentrations of SP600125. After a 48 h-incubation, cells were sampled and examined under light microscopy. Magnification × 200. (C) After U937 leukemia cells were treated with SP600125 for the indicated times, expression of c-Jun and phosphorylated c-Jun was detected using Western blot analysis.
SP600125 causes G2/M arrest, endoreduplication, and delayed apoptosis in human leukemia cells in a time-dependent manner
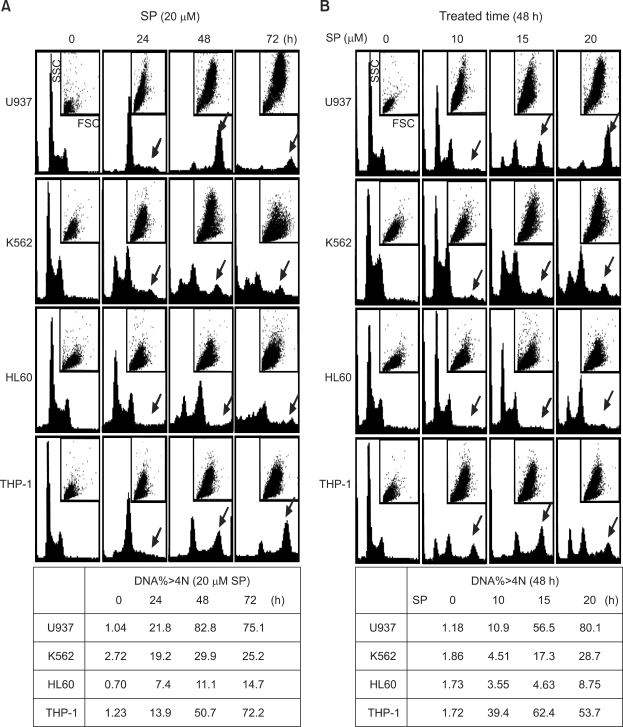
Because SP600125 induces G2/M arrest and apoptosis in breast cancer (Hideshima et al., 2003; Jacobs-Helber and Sawyer, 2004; Du et al., 2004; Mingo-Sion et al., 2004), we investigated these responses in leukemia cells. Cell cycle distributions were analyzed in the four cell lines during asynchronous growth under subconfluent conditions. As shown in Figure 2A, a 20 µM SP600125 treatment strongly induced G2/M arrest in all cell lines at 24 h. A large population of G2/M arrested cells appeared at 24 h and underwent endoreduplication at 48 h. Endoreduplicated cells progressed steadily to delayed apoptosis at 72 h. Apparently, SP600125 leads to G2/M arrest, endoreduplication, and delayed apoptosis in human leukemia cells in a time-dependent manner. SP600125 also increased the cell size (FSC) and the granule content (SSC). Figure 2B shows that SP600125 induces G2/M arrest, endoreduplication, and apoptosis in dose-dependent manner at 48 h. These results demonstrate that SP600125 treatment results in a dose- and a time-dependent G2/M arrest, endoreduplication, and delayed apoptosis in leukemia cells.
Figure 2.
Leukemia cells undergoing SP600125-induced G2/M arrest, endoreduplication, and delayed apoptosis. (A) Cells were plated at 5 × 104 cells/ml and treated with 20 µM SP600125 for 72 h. (B) Exponentially growing cells were grown in different concentrations of SP600125 for 48 h. The cell cycle distribution was analyzed by flow cytometry. Cells were harvested and 10,000 events were analyzed for each sample. The DNA content is represented on the x-axis and the number of cells counted is represented on the y-axis. In the small box, FSC is represented on the x-axis and the SSC count is represented on the y-axis. The arrow indicates endoreduplication stages.
SP600125 treatment causes induction of the p21 and Cdk2 proteins, and induces histone H3 phosphorylation at different times
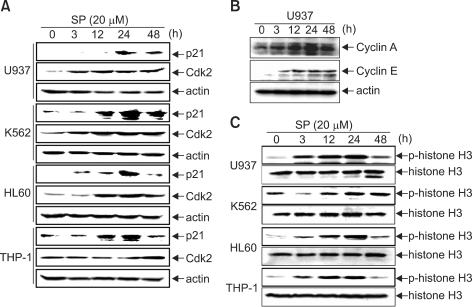
Recent research has shown that p21-induced growth arrest is associated with depletion of mitosis-control proteins leading to abnormal G2/M arrest (Chang et al., 2000). Additionally, inducible overexpression of dominant-negative Cdk2 significantly inhibited endoreduplication through suppression of the interaction between Cdk2 and cyclin E (Gui et al., 2007). For confirmation, we investigated the expressions of p21 and Cdk2. As shown in Figure 3A, p21 expression was minimally detectable in vehicle control cells, while SP600125 treatment significantly increased p21 levels from 12 h to 24 h, when G2/M arrest occurred, which then gradually began to decrease at 48 h. However, Cdk2 expression continuously increased to 48 h, and reached a maximum at 48 h when endoreduplication was strongly induced. Cyclin A and cyclin E levels were increased in SP600125-treated U937 cells in a time-dependent manner (Figure 3B). Additionally, SP600125-induced G2/M arrest and endoreduplication were confirmed by analysis of Ser10 phosphorylation of histone H3, which has emerged as a sensitive marker for mitotic cells (Hendzel et al., 1997). As shown in Figure 3B, the Ser10 phosphorylation of histone H3 displayed low levels in control cells, but was clearly evident in SP600125-treated cells at 12 h and 24 h, and then began to decrease at 48 h. However, Ser10 phosphorylation of histone H3 was retained in K562 cells at 48 h. As seen in Figure 2A, SP600125 time-specifically induced G2/M phase arrest at 24 h with p21 expression and histone H3 phosphorylation on Ser10 as a G2/M arrest marker, and then induced endoreduplication at 48 h with a high level of Cdk2 expression. This indicates that p21 and Cdk2 can be expressed at different times between G2/M arrest and endoreduplication because endoreduplication occurs after G2/M arrest. However, K562 cells suffered significant apoptosis and strongly endoreduplication, indicating that Bcl-2 induces weak endoreduplication through suppression of apoptosis because K562 cells are Bcl-2-null cells.
Figure 3.
SP600125 treatment causes induction of the p21 and Cdk2 proteins and causes histone H3 phosphorylation at different times. Cells were treated with SP600125 for the indicated times and lysed for protein extraction. Samples (50 µg protein/lane) were subjected to 12% SDS-PAGE and Western blot analysis for the detection of specific proteins [anti-rabbit p21 and Cdk2 polyclonal antibodies (A), cyclin A and cyclin E (B) and histone H3 and anti-rabbit p-Ser10-histone H3 monoclonal antibody (C)]. Actin was used as a loading control.
Topoisomerase II activity is not involved in SP600125-induced endoreduplication
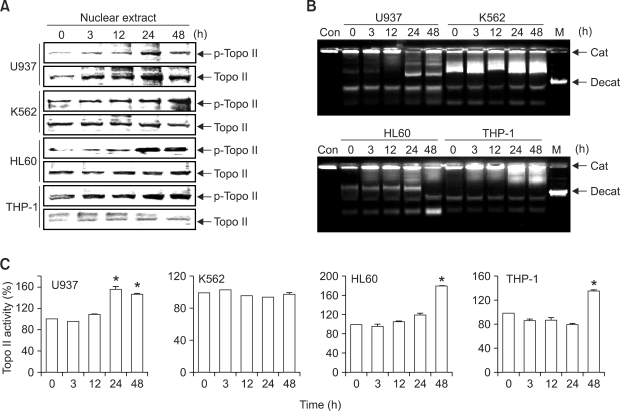
The essential nuclear enzymes DNA topoisomerase I and II regulate DNA topology during many cellular processes. topoisomerase I breaks and rejoins one DNA strand at a time while topoisomerase II is able to decatenate intertwined DNA molecules (Wang, 1996). This enzyme is hyperphosphorylated at mitosis for its activity (Isaacs et al., 1998). With regard to the relationship between the DNA nucleotide sequence and topoisomerase II, recent studies have demonstrated that reduction in topoisomerase II activity can induce endoreduplication in some cell types (Mateos et al., 2005; Cantero et al., 2006). To determine whether SP600125 affects the topoisomerase II activity that controls endoreduplication in leukemia cells, we carried out topoisomerase II Western blot analysis and an in vitro topoisomerase II catalytic assay in nuclear extracts treated with SP600125. As shown in Figure 4A, SP600125 induced phosphorylation of topoisomerase II in a time-dependent manner at 24 h. However, SP600125 partially decatenated the DNA substrate (Figure 4B and 4C). SP600125 induced total phosphorylation of topoisomerase II in the nucleus, but not topoisomerase II activity in vivo. On the basis of these results, since endoreduplication has been linked to inhibition of topoisomerase II activity, the induction of endoreduplication by SP600125 does not appear to be associated with topoisomerase II activity, and there may be another mechanism responsible.
Figure 4.
SP600125 differently regulates topoisomerase II activity during G2/M arrest and endoreduplication. (A) Nuclear extracts were obtained as described in Methods, and Western blot analysis was conducted. (B) The ability of topoisomerase II to decatenate kinetoplast DNA after incubation for different times with SP600125 (20 µM) ranging from 3 h to 48 h was assayed using DNA gel electrophoresis. (C) Respective densitometric profiles are shown in Figure 4B. Each point represents the mean ± SD of three independent experiments. Significance was determined using student's t-test (*P < 0.05 vs. vehicle control).
SP600125 induces formation of tubulin polymerization
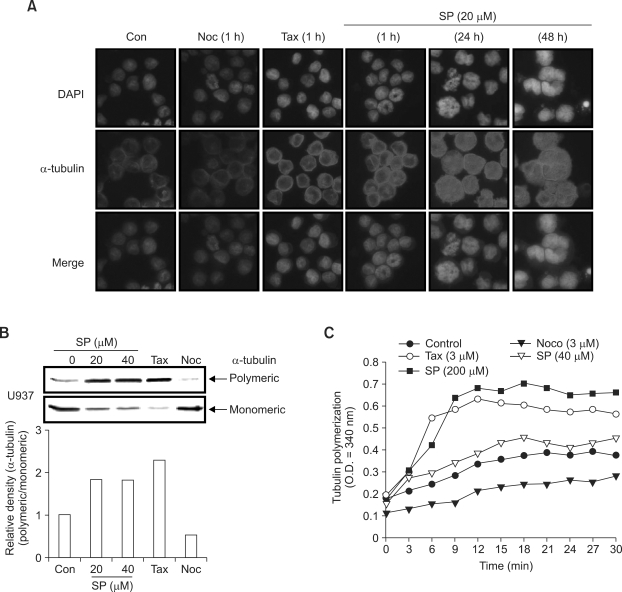
Microtubules play an important role in cell replication and division, maintenance of cell shape, and cellular movement. Microtubules are composed of α-, β-tubulin, and microtubule-associated proteins (MAPs). They are in an unstable steady state of a highly dynamic process of polymerization and depolymerization, and disrupting the dynamics of microtubules leads to endoreduplication (Cortes et al., 2004). In order to examine the functioning of MTs in SP600125- mediated endoreduplication, we reasoned that the microtubule network itself might be a target of SP600125 action. To address this question, we examined whether cells treated with SP600125 displayed measurable changes in tubulin polymerization. Treatment with SP600125 increased the nuclear structure size and promoted an increased intensity of α-tubulin staining, measured by indirect immunofluorescence (Figure 5A). Immunofluorescence analysis does not readily provide a quantitative measure of tubulin polymerization in the cell. To quantify the effect observed by immunofluorescence, we took advantage of the differential solubilities of monomeric and polymeric tubulin in nonionic detergents. For the purposes of quantification, we established the extreme limits of 100% monomeric tubulin and 100% polymeric tubulin using nocodazol and paclitaxel treatments, respectively (Figure 5B). Western blot analysis indicated that SP600125 results in an increase in polymeric α-tubulin and a decrease in monomeric α-tubulin (Figure 5B). To determine whether SP600125 has a direct effect on tubulin polymerization/depolymerization, we performed in vitro tubulin polymerization assays. The addition of paclitaxel (3 µM) caused increased tubulin polymerization and the addition of nocodazol (3 µM) caused decreased tubulin polymerization. Compared with vehicle controls, high concentrations of SP600125 (200 µM) are required to increase tubulin polymerization in vitro. In these in vitro assays with MAP-rich-tubulin, SP600125 had an effect on tubulin polymerization similar to paclitaxel.
Figure 5.
SP600125 increases G2/M arrest and endoreduplication through tubulin polymerization. (A) Tubulin polymerization was analyzed using immunofluorescent staining in U937 cells treated with 20 µM SP600125 for the indicated times and with 5 nM nocodazole (Noc) or 5 nM paclitaxel (Tax) at 1 h. Cells were fixed, permeabilized, and stained with anti-α-tubulin monoclonal antibody. Monoclonal antibody was detected using an anti-mouse secondary antibody conjugated with Texas Red. Cells were analyzed using fluorescence microscopy (× 400). DAPI was used for nuclear staining. (B) Polymeric tubulin and monomeric tubulin were extracted from U937 leukemia cells treated with SP600125 (20 and 40 µM) for 48 h. Polymerized tubulin was also extracted from cells treated for 48 h with either 5 nM Noc or with 5 nM Tax to indicate the range from less than 5% polymeric tubulin to greater than 95% polymerized tubulin. The quantification of polymeric tubulin from three independent experiments is shown. (C) Effects of SP600125 on in vitro tubulin polymerization. MAP-rich tubulin (1 mg/ml) was incubated at 37℃ for 0-30 min, then treated with SP600125 (40 µM and 200 µM), 3 µM Noc, and 3 µM Tax. The indicated compounds were then added to the indicated concentrations. A340 values were recorded once every 3 min. The results are from one representative experiment of three performed that showed similar patterns.
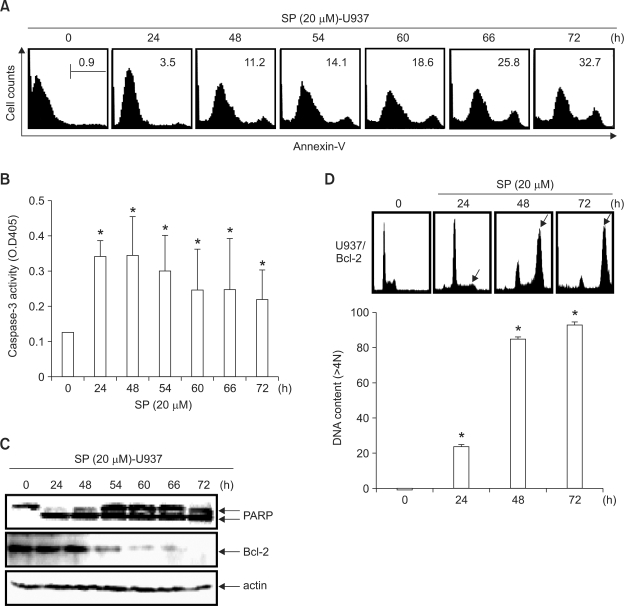
SP600125 induces delayed apoptosis in leukemia cells after endoreduplication and ectopic Bcl-2 expression increases SP600125-induced endoreduplication but protects apoptosis
To assess whether delayed apoptosis contributed to the growth inhibitory effects of SP600125, we assayed the effects of SP600125 on apoptosis. In U937 cells, SP600125 (20 µM) induced an increase in the annexin-V cell population (Figure 6A) and the caspase-3 activity (Figure 6B) in a time-dependent manner. Western blot analysis also demonstrated that SP600125 caused PARP cleavage and Bcl-2 downregulation (Figure 6C), suggesting that the inhibitory effects of SP600125 on leukemia cell growth are dependent on apoptosis. Because phosphorylation of Bcl-2 is induced by microtubule-targeting drugs (Ling et al., 1998), we also tested the effect of SP600125 on U937/Bcl-2 cells. Flow cytometric analysis of the cell-cycle distribution showed that SP600125 significantly induced endoreduplication in U937/Bcl-2 cells at 72 h, but induced less apoptosis than in U937 cells (Figure 6D). Therefore, SP600125 significantly induced endoreduplication until 72 h without apoptosis in ectopic Bcl-2-expressing cells. These results indicate that Bcl-2 induces endoreduplication and attenuates apoptotic death in the presence of SP600125.
Figure 6.
Ectopic Bcl-2 expression inhibits SP600125-induced delayed apoptosis at 72 h and significantly induces endoreduplication. (A) U937 cells were incubated with 20 µM SP600125 for the indicated times, and apoptosis was analyzed over time by staining for phosphatidylserine translocation using FITC-annexin V. Annexin V+ cells are represented on the x-axis and the number of cells counted is represented on the y-axis. The apoptotic annexin-V population was observed in SP600125-treated cells; n = 3 experiments using separate cultures. (B) After 48 h of treatment with SP600125, the caspase-3 activity was assayed using a caspase assay kit, following the manufacturer's protocol. (C) Equal amounts of cell lysate (50 µg) were resolved using SDS-PAGE, transferred to nitrocellulose, and probed with specific antibodies (anti-caspase-3, anti-PARP, and anti-Bcl-2). Actin was used as an internal loading control. (D) Flow-cytometric analysis of the cell-cycle distribution of ectopic Bcl-2-expressing U937 cells with SP600125 (20 µM) for 72 h. Data are expressed as mean ± SD of three independent experiments. Significance was determined using Student's t-test (*P < 0.05 vs. vehicle control).
Discussion
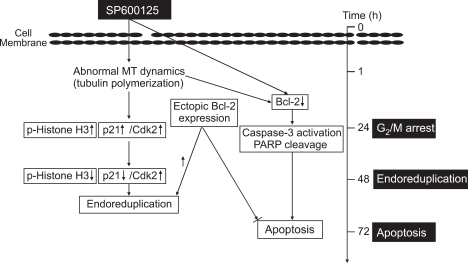
SP600125 has been implicated in G2/M arrest and apoptosis, but its precise role remains unknown (Potapova et al., 2000; Hideshima et al., 2003; Du et al., 2004; Jacobs-Helber and Sawyer, 2004; Mingo-Sion et al., 2004). The present study provides the first mechanism to explain the induction of G2/M arrest, endoreduplication, and delayed apoptosis caused by SP600125 in leukemia cells. As shown in Figure 7, we have demonstrated that SP600125 [1] arrests G2/M phases with upregulation of p21 and phosphorylation of histone H3 at 24 h; [2] promotes expression of key proteins responsible for the progression of cells into the DNA replicating phase, such as Cdk2, and gradually downregulates the expression of p21 at 48 h, suggesting that SP600125 induces endoreduplication signals; [3] promotes tubulin polymerization, a critical process in cell division; and [4] induces delayed apoptosis in leukemia cells. Therefore, SP600125 has a strong anticancer effect against leukemia cells in a dose- and time-dependent manner by promoting tubulin polymerization and disrupting the organization of the microtubule cytoskeleton.
Figure 7.
A schematic diagram of the effect of SP600125 on G2/M arrest, endoreduplication, and delayed apoptosis in human leukemia cells.
The G2/M checkpoint is especially important in protecting normal cells from tumor formation driven by the accumulation of mutations (Hartwell and Weinert, 1989; Molinari, 2000). Therefore, elimination of the checkpoint increases the sensitivity of human tumor cell lines to anticancer agents. Some studies have reported that the G2/M arrest induced by SP600125 may be due to inhibition of cyclin B/Cdk1 kinase activity through an increase in p21 levels (Bates et al., 1998; Chang et al., 2000; Mingo-Sion et al., 2004). Increased JNK activity is important for the dissociation of p21 and JNK, following which cells enter into the S phases (Patel et al., 1998; Kim et al., 2002). Thus, inhibition of JNK activity prevents dissociation between p21 and JNK, and then prevents inhibition of cyclin B/Cdk1 activity, leading to induction of G2/M arrest. In addition, the increase in Cdk2 expression and the reduction in p21 may also support the occurrence of endoreduplication after G2/M phase arrest (Stewart et al., 1999; Gizatullin et al., 2006). This indicates that high levels of p21 and Cdk2 can be expressed at different times in G2/M arrest and endoreduplication because endoreduplication occurs after G2/M arrest. The present study has shown that an increase in p21 expression coincides with the onset of G2/M arrest at 24 h, but that endoreduplication may occur due to loss of p21 and a maximum level of Cdk2 at 48 h. Therefore, the upregulation of p21 expression may contribute to G2/M arrest in the early stages, and then Cdk2 may regulate endoreduplication by treating leukemia cells in the middle stages in the presence of SP600125. These results indicate that JNK activity may regulate cell proliferation through the regulation of cell cycles. Nevertheless, additional experiments are necessary to determine why p21 expression is significantly increased due to SP600125 treatment.
We have also provided strong evidence that the microtubule network itself is a target of SP600125 action. Using biochemical and immunofluorescence methods, we have shown that SP60015 significantly increases tubulin polymerization. microtubules are crucial cellular and structural components that induce cellular development, division, and movement (Rowinsky et al., 1993; Hari et al., 2003). Therefore, microtubule-disrupting agents provide a novel approach to cancer chemoprevention and/or cancer therapy. Recently, certain cancer chemotherapy agents have been found to exert their anticancer activities by disrupting the dynamics of microtubule assembly, thus perturbing the formation and function of the mitotic spindle apparatus and arresting cells in mitosis (Jordan et al., 1996). This action of SP600125 is similar to that of paclitaxel, which binds to tubulin and increases tubulin polymerization, causing cells to arrest in the G2/M phase thereby blocking cell cycle progression (Horwitz, 1992). Our results strongly support the idea that SP600125 inhibits cell proliferation by inhibiting mitosis through extended tubulin polymerization. In addition, topoisomerase II inhibitors, such as etoposide, amsacrine, and merbarone, can induce endoreduplication by preventing the decatenation of replicated chromosomes, which subsequently fail to complete normal segregation at mitosis (Drake et al., 1989; Ferguson et al., 1996; Sumner, 1998). An unexpected result was that SP600125 significantly increases topoisomerase II phosphorylation, but not its activity. Further studies are necessary to determine why topoisomerase II phosphorylation in the nucleus is increased in SP600125-induced endoreduplication.
Tumor cells often evade apoptosis by overexpressing anti-apoptotic proteins, such as Bcl-2, which give them a survival advantage (Mateos et al., 2005; Shore and Viallet, 2005). Recently, contrasting results have been reported. Decreased or phosphorylated Bcl-2 is implicated in the resistance of human ovarian cancer cells to tubulinpolymerizing agents, such as paclitaxel (Tang et al., 1994; Ferlini et al., 2003). Several studies have also demonstrated that phosphatase inhibitors express a phosphorylated form of Bcl-2 that induces apoptosis, suggesting that Bcl-2 phosphorylation inhibits Bcl-2 function (Haldar et al., 1995, 1996). Other reports have shown that Bcl-2 phosphorylation is a common event in mitosis (Ling et al., 1998; Scatena et al., 1998). Despite the disagreement regarding the role of Bcl-2 in microtubule-targeted drug-induced apoptosis, the role of ectopic Bcl-2 expression in the anticancer activity of microtubule-targeting agents has never been fully investigated. Our results have shown that the level of endogenous Bcl-2 expression does not affect SP600125-induced endoreduplication up to 48 h. However, ectopic Bcl-2 expression completely prevents the delayed apoptosis induced by SP600125 and significantly increases endoreduplication at 72 h. Therefore, Bcl-2, which is able to block delayed apoptosis, induces a delayed onset of SP600125-induced endoreduplication.
In summary, our findings indicate a role for both targeting (tubulin polymerization) and signaling (Bcl-2) in human leukemia cells for SP600125. Increased p21 and p-histone H3 protein expressions were found to be responsible for SP600125-induced G2/M arrest at 24 h and high levels of Cdk2 expressed in SP600125-induced endoreduplication at 48 h. SP600125-induced delayed apoptosis was related to Bcl-2 expression, which was closely related to endoreduplication. Further studies are necessary to clarify the exact mechanisms that are induced by SP600125 involved in specific stages of cell distribution.
Methods
Reagents
The specific JNK inhibitor SP600125 was purchased from Calbiochem (La Jolla, CA). The inhibitor was reconstituted in DMSO to make a 10 mM stock solution, and DMSO (0.1%) was used as a control vehicle. MTT, 4,6-diamidino-2-phenylindole (DAPI), and propidium iodide (PI) were purchased from Sigma (St. Louis, MO). Trizol reagent and FBS were purchased from GIBCO (Gaithersburg, MD). An enhanced chemiluminescence (ECL) kit was purchased from Amersham (Arlington Heights, IL). Antibodies against p21, Cdk2, cyclin E, cyclin A, histone H3, phospho (p)-histone H3 (Ser10), p-topoisomerase II (Ser1106), topoisomerase II, α-tubulin, Bcl-2, and PARP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against c-Jun and p-c-Jun were purchased from Cell Signaling (Beverly, MA), and anti-β-actin was purchased from Sigma. Peroxidase-labeled anti-rabbit and sheep anti-mouse immunoglobulin were purchased from KOMA Biotechnology (Seoul, Korea).
Cell lines and cell culture
The human leukemia cell lines U937, THP-1, K562, and HL60 were obtained from the American Type Culture Collection (Manassas, VA). Ectopic Bcl-2-expressing U937 (U937/Bcl-2) cells were provided by Prof. T.K. Kwon (Department of Immunology, School of Medicine, Keimyung University, Korea). The cells were maintained in RPMI-1640 medium (Invitrogen; Carlsbad, CA) supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin (Sigma) in 5% CO2 at 37℃. Cells were seeded at 5 × 104 cells/ml and treated with SP600125 at the indicated times. Cell growth was determined using MTT assays.
Flow cytometric analysis
The cell cycle was analyzed using flow cytometry of PI-stained cells. Cells (1 × 106) were fixed in 70% ethanol overnight at 4℃, and then washed in PBS with 0.1% BSA. Cells were incubated with 1 U/ml of RNase A (DNase free) and 10 µg/ml of PI overnight at room temperature in the dark. For annexin-V staining, cells were washed with PBS, and then incubated with annexin V-fluorescein isothiocyanate (R&D Systems; Minneapolis, MN). Cells were analyzed using a FACSCalibur flow cytometer (Becton Dickenson; San Jose, CA). Data were analyzed using Cell Quest software (Becton Dickenson) and 10,000 events were analyzed for each sample.
Western blot analysis
Cellular lysates were prepared by suspending 1 × 106 cells in a lysis buffer (137 mM NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM MgCl2, 0.1% Triton X-100, 25 mM MOPS, 100 µM PMSF, and 20 µM leupeptin adjusted to pH 7.2) at 4℃ for 30 min. The protein concentration was quantified using a Bio-Rad detergent-compatible protein assay reagent (Bio-Rad Laboratories; Hercules, CA). We separated 50 µg of total cell extract on 10% polyacrylamide gel, followed by transfer to nitrocellulose membrane using standard procedures. Membranes were blocked in 5% powdered milk in TBST and incubated overnight with primary antibodies at 4℃. Blots were washed three times for 10 min in TBST and probed with either mouse or rabbit secondary antibodies for 1 h. The membranes were washed three times for 10 min in TBST, and then developed using ECL reagent.
Topoisomerase II activity in nuclear extracts
Topoisomerase II activity in nuclear extracts, either vehicle controlled or incubated for different times with SP600125, was assayed using an assay kit (TopoGen; Columbus, OH) based on the decatenation of kinetoplast DNA (kDNA). Reaction products were resolved using DNA agarose gel electrophoresis. After incubation for 40 min at 37℃, samples were loaded onto 1% agarose gel and subjected to electrophoresis for 1 h at 100 V.
Immunofluorescence analysis
Cells were fixed in PIPES buffer [0.1 M PIPES (pH 6.8), 5 mM MgCl2, and 50 mM EGTA pH 7.4] containing 2% paraformaldehyde and 0.1% glutaraldehyde for 10 min at room temperature. Fixed cells were permeabilized in 0.5% Triton X-100, washed, and quenched for 30 min with 66 mM sodium borohydride in 50% ethanol. Cells were incubated with α-tubulin and the antibody was detected using anti-mouse IgG conjugated with Texas Red (Molecular Probes; Eugene, OR). The nucleus was stained using DAPI, and the nuclear and α-tubulin morphologies were evaluated using fluorescence microscopy.
Extraction of monomeric and polymeric tubulin
After treatment with the indicated compounds, cells were washed twice with PBS, then extracted into 0.4 ml of a monomeric extraction buffer [20 mM piperazine-N,N'-bis (2-ethanesulfonic acid) (pH 6.8), 0.14 M NaCl, 1 mM MgCl2, 1 mM EGTA, 0.5% Nonidet P-40 (NP-40), 0.5 mM PMSF, and 4 mM taxol], transferred to a new tube, and centrifuged at 13,000 × g for 10 min at room temperature. The NP-40-soluble extract containing monomeric tubulin was transferred to a new tube. Polymeric tubulin was extracted from the remaining insoluble material using 0.4 ml of RIPA buffer [10 mM Tris (pH 7.4), 0.15 M NaCl, 1% deoxycholate, 1% NP-40, and 0.1% SDS]. Equivalent aliquots consisting of 100 ng of total protein from polymeric fractions were fractionated using 10% SDS-PAGE, and Western-blotted for α-tubulin.
In vitro tubulin polymerization assay
We prepared 50 µl of 5 mg/ml tubulin (>90%) (Cytoskeleton; Denver, CO) at a steady state by incubation at 37℃ for 30 min in G-PEM buffer [100 mM PIPES (pH 6.9), 1 mM EGTA, 1 mM MgCl2, and 1 mM GTP] containing 10% glycerol in a 96-well plate (0.33 cm2/well). The effects of the tested compounds on polymerization/depolymerization were quantified over time by measuring the increase/decrease in their absorbance at 340 nm (A340).
In vitro caspase activity assay
The enzymatic activity of induced caspase-3 was assayed using a colorimetric assay kit (R&D Systems; Minneapolis, MN) according to the manufacturer's protocol. The cells were briefly lysed in a lysis buffer for 30 min in an ice bath. The lysed cells were centrifuged at 12,000 g for 10 min, and 100 µg of the protein was incubated with 50 µl of a reaction buffer and 5 µl of colorimetric tetrapeptides at 37℃ for 2 h. Tetrapeptides included Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA) for caspase-3. The optical density of the reaction mixture was quantified spectrophotometrically at a wavelength of 405 nm.
Statistical analysis
All data from cell counts, MTT assays, FACS analyses, topoisomerase II activity, and caspase-3 activity experiments were derived from at least three independent experiments. Images were visualized using Chemi-Smart 2000 (Vilber Lourmat; Cedex, France). Images were captured using Chemi-Capt (Vilber Lourmat) and loaded into Photoshop. Scion Imaging software (http://www.scioncorp.com) was used to quantify the Western blots. Statistical analyses were conducted using SigmaPlot software, and values are presented as mean ± SD. Significant differences between groups were determined using an unpaired Student's t-test. A value of *P<0.05 was accepted as an indication of statistical significance.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0076654).
Abbreviation
- SP
SP600125
References
- 1.Andreassen PR, Martineau SN, Margolis RL. Chemical induction of mitotic checkpoint override in mammalian cells results in aneuploidy following a transient tetraploid state. Mutat Res. 1996;372:181–194. doi: 10.1016/s0027-5107(96)00138-8. [DOI] [PubMed] [Google Scholar]
- 2.Bates S, Ryan KM, Phillips AC, Vousden KH. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–1703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 3.Cantero G, Pastor N, Mateos S, Campanella C, Cortés F. Cisplatin-induced endoreduplication in CHO cells: DNA damage and inhibition of topoisomerase II. Mutat Res. 2006;599:160–166. doi: 10.1016/j.mrfmmm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Chang BD, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC, Roninson IB. p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene. 2000;19:2165–2170. doi: 10.1038/sj.onc.1203573. [DOI] [PubMed] [Google Scholar]
- 5.Cortes F, Mateos S, Pastor N, Dominguez I. Toward a comprehensive model for induced endoreduplication. Life Sci. 2004;76:121–135. doi: 10.1016/j.lfs.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Drake FH, Hofmann GA, Mong SM, Bartus JO, Hertzberg RP, Johnson RK, Mattern MR, Mirabelli CK. In vitro and intracellular inhibition of topoisomerase II by the antitumor agent merbarone. Cancer Res. 1989;49:2578–2583. [PubMed] [Google Scholar]
- 7.Du L, Lyle CS, Obey TB, Gaarde WA, Muir JA, Bennett BL, Chambers TC. Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK activity: evidence that mitotic Bcl-2 phosphorylation is JNK-independent. J Biol Chem. 2004;279:11957–11966. doi: 10.1074/jbc.M304935200. [DOI] [PubMed] [Google Scholar]
- 8.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson LR, Whiteside G, Holdaway KM, Baguley BC. Application of fluorescence in situ hybridization to study the relationship between cytotoxicity, chromosome aberrations, and changes in chromosome number after treatment with the topoisomerase II inhibitor amsacrine. Environ Mol Mutagen. 1996;27:255–262. doi: 10.1002/(SICI)1098-2280(1996)27:4<255::AID-EM2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Ferlini C, Raspaglio G, Mozzetti S, Distefano M, Filippetti F, Martinelli E, Ferrandina G, Gallo D, Ranelletti FO, Scambia G. Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Mol Pharmacol. 2003;64:51–58. doi: 10.1124/mol.64.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI. The aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res. 2006;66:7668–7677. doi: 10.1158/0008-5472.CAN-05-3353. [DOI] [PubMed] [Google Scholar]
- 12.Gui Y, Yin H, He JY, Yang SH, Walsh MP, Zheng XL. Endoreduplication of human smooth muscle cells induced by-methoxyestradiol: a role for cyclin-dependent kinase 2. Am J Physiol Heart Circ Physiol. 2007;292:H1313–H1320. doi: 10.1152/ajpheart.00867.2006. [DOI] [PubMed] [Google Scholar]
- 13.Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 14.Haldar S, Jena N, Croce CM. Inactivation of bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hari M, Wang Y, Veeraraghavan S, Cabral F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol Cancer Ther. 2003;2:597–605. [PubMed] [Google Scholar]
- 16.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 17.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 18.Hideshima T, Hayashi T, Chauhan D, Akiyama M, Richardson P, Anderson K. Biologic sequelae of c-Jun NH(2)-terminal kinase (JNK) activation in multiple myeloma cell lines. Oncogene. 2003;22:8797–8801. doi: 10.1038/sj.onc.1206919. [DOI] [PubMed] [Google Scholar]
- 19.Hochedlinger K, Wagner EF, Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–2445. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz SB. Mechanism of action of taxol. Trends Pharmacol Sci. 1992;13:134–136. doi: 10.1016/0165-6147(92)90048-b. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs RJ, Davies SL, Sandri MI, Redwood C, Wells NJ, Hickson ID. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs-Helber SM, Sawyer ST. Jun N-terminal kinase promotes proliferation of immature erythroid cells and erythropoietin-dependent cell lines. Blood. 2004;104:696–703. doi: 10.1182/blood-2003-05-1754. [DOI] [PubMed] [Google Scholar]
- 23.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 25.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38α and JNK1 stabilizep21Cip1 by phosphorylation. J Biol Chem. 2002;277:29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakis JM, Avruch J. pp54 microtubule-associated protein 2 kinase. A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-L-lysine. J Biol Chem. 1990;265:17355–17363. [PubMed] [Google Scholar]
- 27.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 28.Ling YH, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- 29.Mateos S, Domínguez I, Pastor N, Cantero G, Cortés F. The DNA demethylating 5-azaC induces endoreduplication in cultured Chinese hamster cells. Mutat Res. 2005;578:33–42. doi: 10.1016/j.mrfmmm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Min BW, Kim CG, Ko J, Lim Y, Lee YH, Shin SY. Transcription of the protein kinase C-δ gene is activated by JNK through c-Jun and ATF2 in response to the anticancer agent doxorubicin. Exp Mol Med. 2008;40:699–708. doi: 10.3858/emm.2008.40.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596–604. doi: 10.1038/sj.onc.1207147. [DOI] [PubMed] [Google Scholar]
- 32.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niculescu AB, 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel R, Bartosch B, Blank JL. p21WAF1 is dynamically associated with JNK in human T-lymphocytes during cell cycle progression. J Cell Sci. 1998;111:2247–2255. doi: 10.1242/jcs.111.15.2247. [DOI] [PubMed] [Google Scholar]
- 35.Potapova O, Gorospe M, Dougherty RH, Dean NM, Gaarde WA, Holbrook NJ. Inhibition of c-Jun N-terminal kinase 2 expression suppresses growth and induces apoptosis of human tumor cells in a p53-dependent manner. Mol Cell Biol. 2000;20:1713–1722. doi: 10.1128/mcb.20.5.1713-1722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowinsky EK, Eisenhauer EA, Chaudhry VC, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 37.Rowinsky EK. Paclitaxel pharmacology and other tumor types. Semin Oncol. 1997;24:1–12. [PubMed] [Google Scholar]
- 38.Scatena CD, Stewart ZA, Mays D, Tang LJ, Keefer CJ, Leach SD, Pietenpol JA. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and taxol-induced growth arrest. J Biol Chem. 1998;273:30777–30784. doi: 10.1074/jbc.273.46.30777. [DOI] [PubMed] [Google Scholar]
- 39.Shah JV, Cleveland DW. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell. 2000;103:997–1000. doi: 10.1016/s0092-8674(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 40.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 41.Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematology. 2005;1:226–230. doi: 10.1182/asheducation-2005.1.226. [DOI] [PubMed] [Google Scholar]
- 42.Stewart ZA, Leach SD, Pietenpol JA. p21Waf1/Cip1 inhibition of cyclinE/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–215. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sumner AT. Induction of diplochromosomes in mammalian cells by inhibitors of topoisomerase II. Chromosoma. 1998;107:486–490. doi: 10.1007/s004120050333. [DOI] [PubMed] [Google Scholar]
- 44.Tang C, Willingham MC, Reed JC, Miyashita T, Ray S, Ponnathpur V, Huang Y, Mahoney ME, Bullock G, Bhalla K. High levels of p26BCL-2 oncoprotein retard taxol-induced apoptosis in human pre-B leukemia cells. Leukemia. 1994;8:1960–1969. [PubMed] [Google Scholar]
- 45.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 46.Vogt PK. Fortuitous convergences: the beginnings of JUN. Nat Rev Cancer. 2002;2:465–469. doi: 10.1038/nrc818. [DOI] [PubMed] [Google Scholar]
- 47.Wang JC. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 48.Weiss A, Herzig A, Jacobs H, Lehner CF. Continuous cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Curr Biol. 1998;8:239–242. doi: 10.1016/s0960-9822(98)70090-9. [DOI] [PubMed] [Google Scholar]