Abstract
The incidence of juvenile obesity is increasing at an alarming rate. In adults, central insulin administration decreases hypothalamic orexigenic neuropeptides, food intake and body weight more effectively in males than females. Mechanisms regulating energy balance in juvenile animals are inherently different from those in adults due to differences in growth rates and hormonal milieu. Therefore, we sought to determine if central insulin treatment in juvenile rats (4 wk) would have similar sex-dependent effects on food intake as those reported in adult rats. Twenty four hour food intake was measured following icv saline or insulin (0.01 or 0.1 U) prior to the onset of dark phase of the light cycle. An additional set of animals was used to assess the effects of central insulin on hypothalamic orexigenic (NPY, AgRP) and anorexigenic (POMC) neuropeptide mRNA expression. In both males and females, insulin reduced meal size initially (first 4h) and later decreased meal frequency (4–24 h) to reduce cumulative food intake. Consistent with this, central insulin decreased hypothalamic NPY and AgRP and increased POMC mRNA expression. In contrast to adult studies, there were no demonstrated sex differences. These studies indicate that juvenile females and males are equally sensitive to central insulin anorexigenic effects, perhaps due to a lack of circulating gonadal hormones. The anorexigenic responsiveness of both genders suggests a potential pharmacologic approach to childhood obesity.
Keywords: food intake, meal patterns, glucose, hypothalamus, feeding, sex differences, NPY, AgRP, POMC
INTRODUCTION
Insulin is a critical endogenous regulator of energy homeostasis, and understanding the normal physiological mechanisms underlying its central anorexigenic actions is important for combating obesity. In particular, juvenile obesity is exceptionally problematic given that it is the single most important predictor of adult obesity. In addition, maintaining energy homeostasis is necessarily different in juveniles compared with adults since their energy demands for growth and metabolism are markedly different. Outside the study of diet-induced obesity, limited research has been done to determine the normal neuroendocrine mechanisms that regulate energy balance in juvenile animals.
One key advancement in understanding the neuroendocrine mechanisms that regulate energy balance at any age occurred with the discovery that endocrine factors important for the regulation of food intake, including insulin and leptin, act on specific hypothalamic receptors (Banks et al., 1996; Baura, et al., 1993). More specifically, both chronic and acute central insulin treatment decrease food intake and body weight in a variety of species. (Air, et al., 2002; Baura, et al., 1993; Bruning, et al., 2000; Florant, et al., 1991; Porte, et al., 2005). Similarly, blocking insulin action in the brain with neutralizing antibodies, antisense or receptor knockout increases food intake and body weight. (Bruning, et al., 2000; Obici, et al., 2002; Strubbe and Mein, 1977). Insulin receptors are distributed in several brain regions including the hypothalamus, and the catabolic action of insulin appears to be mediated via the hypothalamic melanocortin and NPY pathways. (Air, et al., 2002; Benoit, et al., 2002; Niswender and Schwartz, 2003; Schwartz, et al., 1992). Therefore, insulin is a critical endogenous regulator of energy balance that elicits a net catabolic response. (Niswender, et al., 2003; Obici, et al., 2002; Porte, et al., 2005; Schwartz, et al., 1991; Schwartz et al., 1993; Woods, et al., 1996).
There are well documented sex differences in the effects of central insulin on energy balance in adults, presumably due to differences in gonadal hormone milieu (Clegg, et al., 2003, 2006). For example, in rats and humans, males are more sensitive to the central catabolic actions of insulin (Benoit, et al., 2000; Hallschmid, et al., 2004). More specifically, 1 mU insulin given intracerebroventricularly (icv) decreases 24 h food intake and body weight in male rats but not in female rats, male rats given estradiol or ovariectomized female rats (Clegg et al., 2006). Although, a sufficiently high dose (8 mU) of icv insulin will decrease food intake in ovariectomized female rats (Clegg et al., 2006). Therefore, it appears that low serum estradiol levels enhance insulin sensitivity by are not necessarily sufficient for the anorexigenic effects of insulin.
Male and female juvenile rats have low steroid hormone levels, exhibiting similar serum estradiol and testosterone levels, 35–40 pg/ml and 0.25–0.5 ng/ml, respectively (Dohler and Wuttke, 1975; Parlow et al., 1973). Unlike juveniles, adult animals have considerable sex differences in gonadal hormone levels. Female rats have 70–80 pg/ml serum estradiol while male rats have only 30–35 pg/ml, and male rats have 5–6 ng/ml serum testosterone while female rats only have 0.2–0.4 ng/ml (Dohler and Wuttke, 1975; Parlow et al., 1973). These studies show that serum estradiol levels of male and female juvenile rats most closely resemble those of adult males. Therefore, consistent with the research done on the axnorexigenic effects of central insulin in adults, we hypothesized that central insulin treatment would cause anorexigenic responses in both male and female juvenile rats as a result of their low serum estradiol (Dohler and Wuttke, 1975). Specifically, these studies aim to determine if central insulin treatment reduces food intake in juvenile rats and if there are any sex differences in the effects of insulin on food intake and hypothalamic neuropeptide expression.
METHODS
Animals
All procedures were approved by the Animal Research Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA (LABioMed) and were in accordance with Public Health Service and United States Department of Agriculture and with the American Association for Accreditation of Laboratory Care and National Institutes of Health guidelines. Juvenile male and female Sprague Dawley rats 18 days of age were obtained from Charles River Laboratories (Hollister, CA). The rats were housed in a facility with constant temperature (21±2.0 °C) and humidity and a controlled 12:12 h light:dark cycle. Rats were fed standard laboratory chow (Lab Diet 5002, Brentwood, MO) ad libitum.
Cannula Implantation
Cannulae were stereotaxically implanted into the third ventricle as described previously (Clark et al., 1985). Briefly, the animals were anesthetized with isoflurane and the fur at the top of the head was removed to expose the area to be incised. A hole was drilled at the intersection of bregma and the midsaggital sinus and the guide cannula (26 gauge stainless steel; Plastics One, Roanoke, VA) was lowered using the following stereotaxic coordinates (level skull, anterior-posterior from bregma 0, medial-lateral from midsaggital sinus 0, and dorsal-ventral from the top of the skull −8.0 mm) targeted for placement just above the third ventricle. The guide cannula was secured to the skull using cyanoacrylate ester gel, 3/16 mm jeweler’s screws and dental acrylic. A removable obturator sealed the opening in the guide cannula throughout the experiment except when it was removed for the injections. Rats were allowed one week to recover fully before starting any additional experimental procedures.
Intracerebroventricular Injection Protocol
The inner cannula (33 gauge stainless steel, Plastics One, Roanoke, VA) extended 8.5 mm below the top of the skull and all rats were injected with a 2.0 μl volume. All injections were given at the beginning of the dark phase of the photoperiod. On the day of testing, animals had their food removed 6 h prior to treatment. Animals were lightly restrained by hand during the 30 s injection and the injection needle remained in place ~30 s before withdrawal.
Experimental Design
Experiment 1: Effect of central insulin on food intake in male and female juvenile rats
Sixteen male (n=8) and female (n=8) juvenile rats from four different litters were used in this experiment. At 21 days of age rats had cannulae stereotaxically implanted into the third ventricle as described above, and after one week recovery time, animals were administered either saline or insulin (0.01 U or 0.1 U (Eli Lilly)) icv. Every animal received each treatment in a counterbalanced fashion to control for order effects with two days between treatments. As noted above food was removed 6 h prior to the injection and returned directly after the injection. Food intake was monitored for 24 h post-injection using the CLAMS apparatus as described below.
Experiment 2: Effect of icv insulin treatment on hypothalamic feeding neuropeptide mRNA expression in male and female juvenile rats
Twenty-four male (n=12) and female (n=12) juvenile (21 days old) rats were implanted with 3rd ventricular cannulae as described above. Following 1 week of recovery, animals were injected icv with either saline or 0.1 U insulin. Ninety minutes following icv injections, animals were rapidly decapitated, and their hypothalami were removed and placed on dry ice until RNA extraction at a later date.
Experiment 3: Effect of central or peripheral insulin on serum glucose in male and female juvenile rats
Rats from Experiment 1 were used in this experiment as well. Forty-eight h after the feeding experiments, rats were injected icv with either saline or insulin (0.1 U). After another 48 h, rats were then injected intraperitoneally (ip) with either saline or insulin (1 U/kg), to confirm biologic activity. Following both the icv and ip injections, blood was taken from the tail vein at 0, 15 and 30 minutes post-injection for the measurement of blood glucose levels.
Measurement of Food Intake
Two days before testing, rats were placed into the Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH) for acclimation. Animals received access to their normal diet in ground-up form and water ad libitum in the CLAMS chambers. The food intake data were generated by automated monitoring of the food balance every 2 minutes, and documenting the accumulated food intake, meal size and meal number. In this experiment, the end of a feeding bout (meal) was identified as the time when the balance had been stable for >10 s and a minimum of 0.1 g of food had been eaten.
Measurement of Blood Glucose
Blood for glucose measurement was taken by repeated needle sticks from the tail vein at 0, 15 and 30 min after injection. During the bleed times, the rats were loosely restrained in a cloth towel. Blood glucose measurements were performed on whole blood using the Truetrack Glucometer smart system (Home Diagnostics, Inc., Fort Lauderdale, FL; range 20–600 mg/dL).
Measurement of Hypothalamic mRNA
Real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was used to measure the expression of the hypothalamic NPY, AgRP and POMC. The primer sequences for NPY, POMC and AgRP have been described previously (Beloosesky et al., 2006). Briefly, samples were homogenized in RA-1 Buffer (USB Corporation, Cleveland, OH) using an ultrasonic cell disruptor (Misonix, Inc, Farmingdale, NY), and total RNA was isolated using the PrepEase RNA Spin kit according to the manufacturer’s protocol (USB Corporation, Cleveland, OH). The RNA concentration was determined by spectrophotometry at an absorbance of 260 nm, and integrity was assessed by agarose gel electrophoresis and ethidium bromide staining. Reverse transcription was performed as described previously (Belkacemi et al., 2008). Briefly, in a 20 μl reaction 2μg total RNA was reverse transcribed using the Qiagen Omniscript kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. All RNA samples were treated with the Turbo DNA free kit (Applied Biosystems, Austin, TX) as per manufacturer’s instructions. To assess genomic DNA contamination, controls were set up where no reverse transcriptase was included in reverse transcription reaction for all RNA samples.
Real time polymerase chain reaction (PCR) was carried out as described previously (Belkacemi et al., 2008) using the Sequence Detection System 7000 (Applied Biosystems, Foster City, CA). Briefly, real time PCR was performed using the qPCR Master Mix Plus for SYBR green I (Eurogentec, San Diego, CA). in a total volume of 25 μL consisting of 1X SYBR Master Mix, 200 nM of each primer, and 2 μL of cDNA sample. All reactions were performed with an initial denaturation of 2 minutes at 50°C, followed by 95°C for 10 minutes, followed by 40 PCR cycles of denaturing at 95°C for 15 seconds, and annealing/extension at 60°C for 1 minute. Levels of β-actin were used to control for sample amount, and all assays were performed in duplicate. Detection of PCR product was accomplished by real-time detection of the increase in fluorescence of SYBR green caused by the primer extension. After each RT-PCR experiment, data were analyzed to select a threshold level of fluorescence that was in the linear phase of the PCR product accumulation. The cycle at which each reaction reached threshold fluorescence was defined as the threshold cycle (CT) for that reaction. The analysis was performed using the comparative threshold (CT) cycle method (Livak and Schmittgen, 2000). CT values were tabulated after each run, and the presence of NPY, AgRP or POMC expression was subsequently determined. In order to achieve quantitative values, β-actin CT values were first subtracted from a test CT value for each well to give a ΔCT value. The expression of mRNA of our gene of interest relative to that of controls was evaluated using the expression 2−ΔΔCT, and values were expressed as percent difference from the control condition, with the control values being set to 100%.
Statistical Analyses
Cumulative food intake, meal size, meal frequency and blood glucose responses to icv and ip insulin treatment were analyzed by two-way, repeated measures ANOVA and Tukey’s Multiple Comparison tests were used for individual pair-wise comparison. For meal latency a one-way ANOVA and Tukey’s posthoc tests were used for pair-wise comparison, and for hypothalamic mRNA changes in response to insulin treatment, student’s t-test was used. All statistical analyses were done using GraphPad Prism Software (San Diego, CA). Differences between means were considered statistically significant if P<0.05.
RESULTS
Experiment 1
Body weights of female and male animals were not significantly different at the time of saline (124.4 ± 6.2, 119.1 ± 5 g), 0.01 U insulin (124.7 ± 3.5, 120.9 ± 3.7 g), and 0.1 U insulin injection (122.2 ± 6.6, 119.4 ± 4.1 g, respectively). Intracerebroventricular injection of insulin in male and female 4 week old rats decreased cumulative food intake up to 24 h post-injection (Fig. 1). Both females (Fig. 1A) and males (Fig. 1B) exhibited similar decreases in food intake, and there was no dose response observed. Insulin treatment at both dosages initially decreased food intake 29–40% during the 0–1, 1–2 and 2–4 h post-injection periods in both males and females. Decreases in cumulative food intake were much smaller by 24 h post-injection, ranging from 10 to 13% (Fig. 1).
Fig. 1.
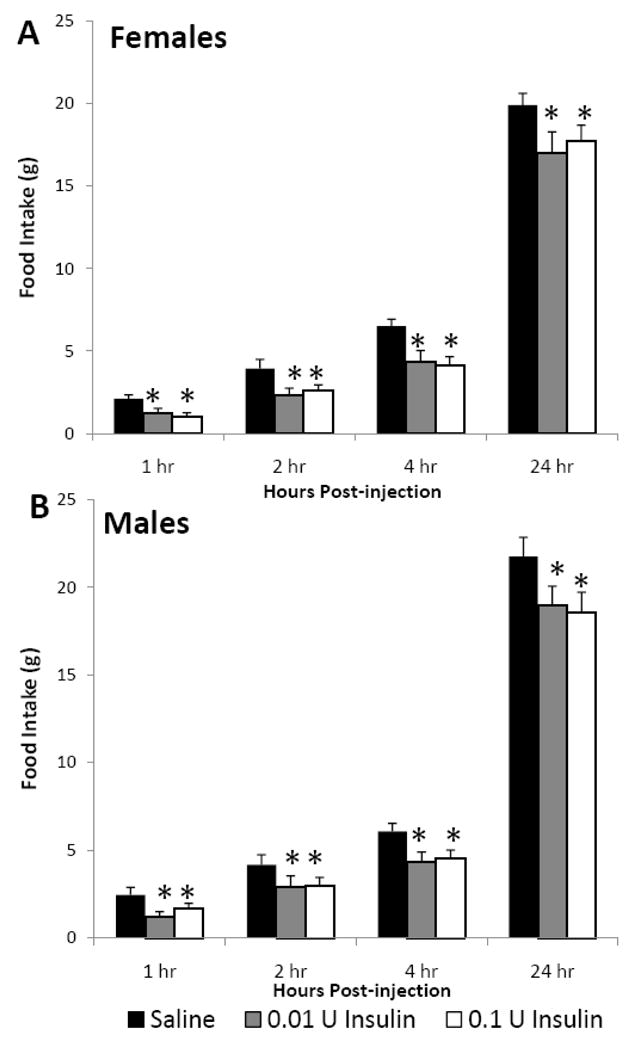
The effect of icv insulin injection on cumulative food intake in juvenile (A) female (n=8) and (B) male (n=8) rats at 1, 2, 4 and 24 h post-injection. Rats were injected icv with saline, 0.01 or 0.1 U insulin in a repeated-measures design. Data are expressed as the mean ± SEM. *=P<0.05 compared with saline treatment for that time interval.
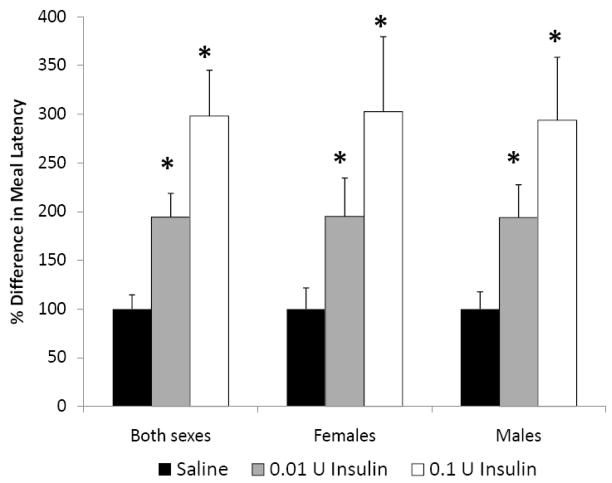
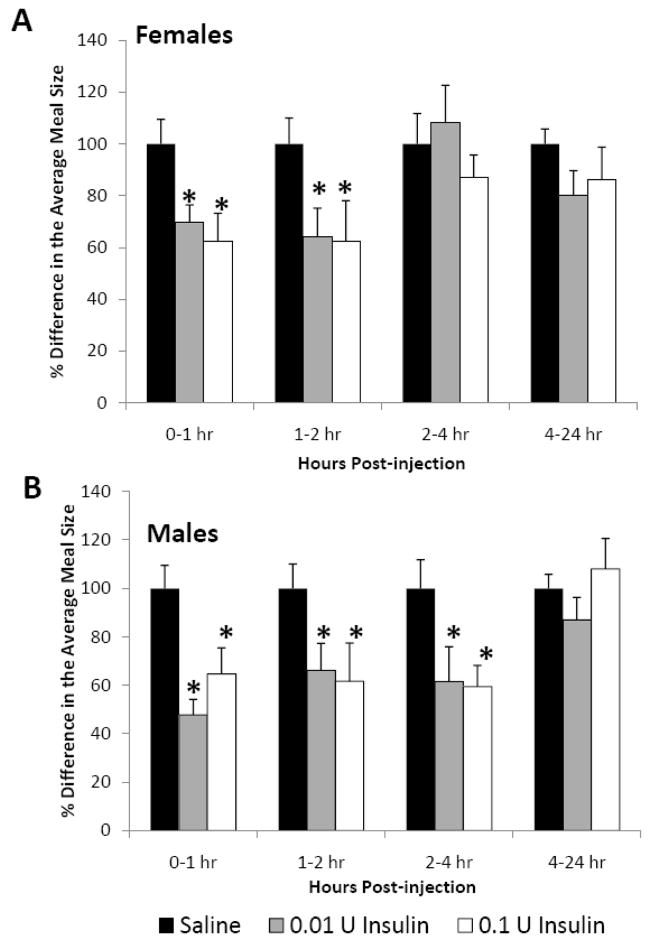
In both female and male rats, insulin increased the length of time it took animals to start eating, as indicated by a significant increase in meal latency (Fig. 2, P<0.05). The low dose of insulin (0.01 U) caused a 195% increase, while the higher dose (0.1 U) caused a 298% increase in meal latency. Insulin also had a significant impact on meal size in both females (Fig. 3A, P<0.05) and males (Fig. 3B, p<0.05). In females, icv insulin treatment at both 0.01 and 0.1 U significantly decreased meal size during the first 2 h and had no effect from 2–24 h post-injection (Fig. 3A). In males, both doses of insulin significantly decreased meal size during the first 4 h post-injection and had no effect during the 4–24 h time interval (Fig. 3B). The only sex difference observed in these studies was that central insulin treatment decreased meal size during the 2–4 h post-injection period only in males. This sole sex difference in meal size during this one 2 h period was apparently not significant enough to cause a difference in cumulative food intake. Similar to the results on cumulative food intake, we did not observe any dosage effect on meal size in either sex.
Fig. 2.
The effect of icv insulin injection on meal latency in juvenile female (n=8) and male (n=8) rats. Rats were injected icv with saline, 0.01 or 0.1 U insulin in a repeated-measures design. Data are expressed as the mean ± SEM. *=P<0.05 compared with saline treatment.
Fig. 3.
The effect of icv insulin injection on meal size in juvenile (A) female (n=8) and (B) male (n=8) rats at 1, 2, 4 and 24 h post-injection. Rats were injected icv with saline, 0.01 or 0.1 U insulin in a repeated-measures design. Data are expressed as the mean ± SEM. *=P<0.05 compared with saline treatment for that time interval.
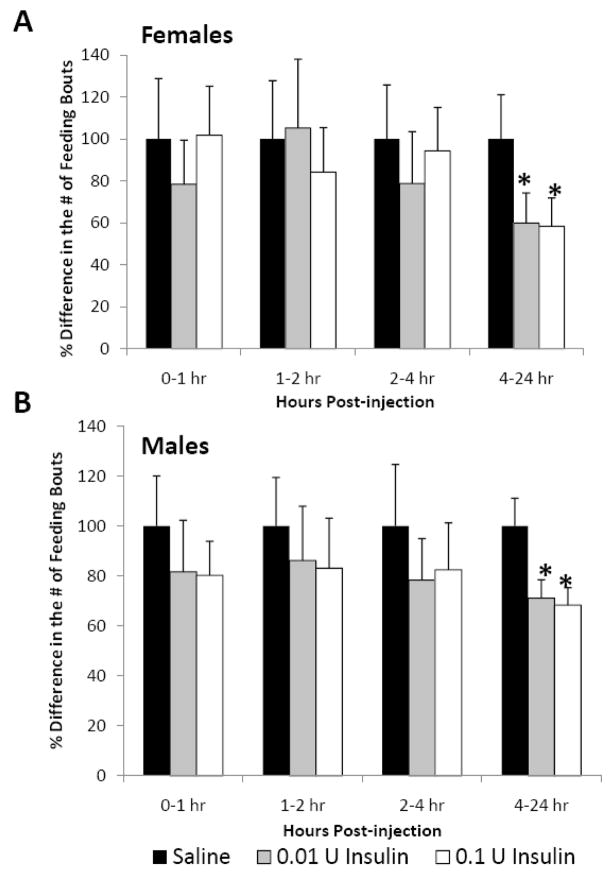
In addition to cumulative food intake and meal size, we also examined the effects of icv insulin treatment on the number of feeding bouts or “meals” during the 24 h post-injection. During the first 4 h post-injection neither dose of insulin affected meal number in either sex. However, during the 4–24 h post-injection time interval, both doses of insulin significantly decreased meal number in both female (Fig. 4A, 41%) and male (Fig. 4B, 30%) juvenile rats.
Fig. 4.
The effect of icv insulin injection on meal number in juvenile (A) female (n=8) and (B) male (n=8) rats at 1, 2, 4 and 24 h post-injection. Rats were injected icv with saline, 0.01 or 0.1 U insulin in a repeated-measures design. Data are expressed as the mean ± SEM. *=P<0.05 compared with saline treatment for that time interval.
Experiment 2
There was a significant decrease in NPY and AgRP (Fig. 5 A and B, P<0.05) and a significant increase in hypothalamic POMC (Fig. 5C, P<0.05) mRNA expression in both female and male rats 90 minutes after injection with 0.1 U insulin icv. Both sexes appeared to have similar relative changes in hypothalamic neuropeptide expression, indicating similar neuropeptide sensitivities to icv insulin treatment.
Fig. 5.
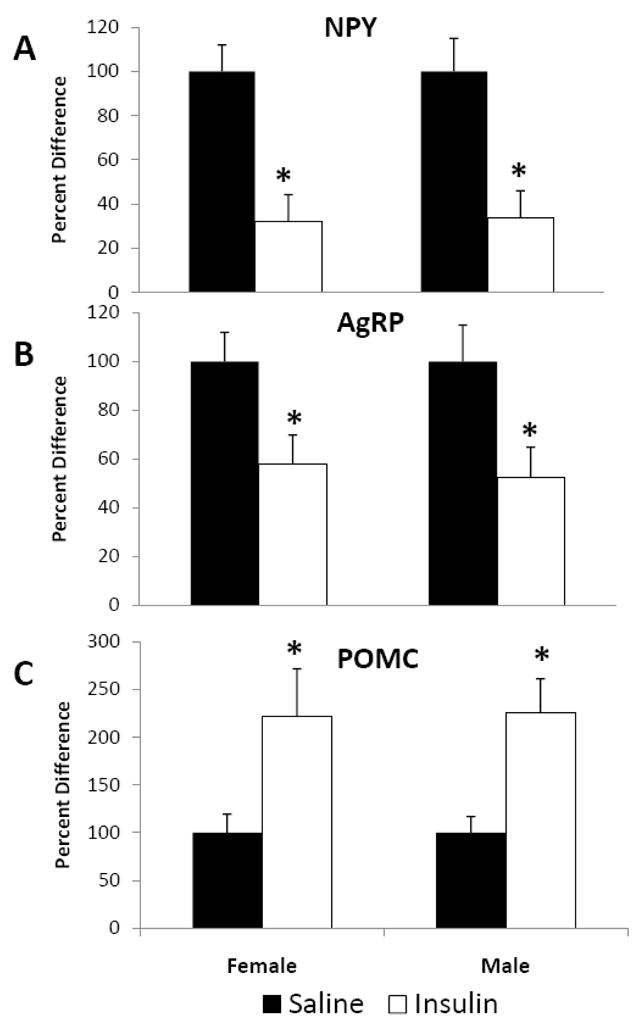
The effect of icv insulin injection on hypothalamic mRNA expression in juvenile female (n=12) and male (n=12) rats injected icv with either saline or 0.1 U insulin. Hypothalamic (A) NPY, (B) AgRP and (C) POMC were measured 90 minutes post-injection by quantitative real-time PCR. Data are expressed as the mean ± SEM. *=P<0.05 compared with saline treatment.
Experiment 3
Peripheral treatment with 1 U/kg insulin significantly decreased blood glucose at 15 and 30 minutes post-injection, regardless of gender (Fig. 6A, P<0.05). Unlike peripheral insulin, central 0.1U insulin did not alter blood glucose levels during the 30 minutes after injection (Fig. 6B).
Fig. 6.
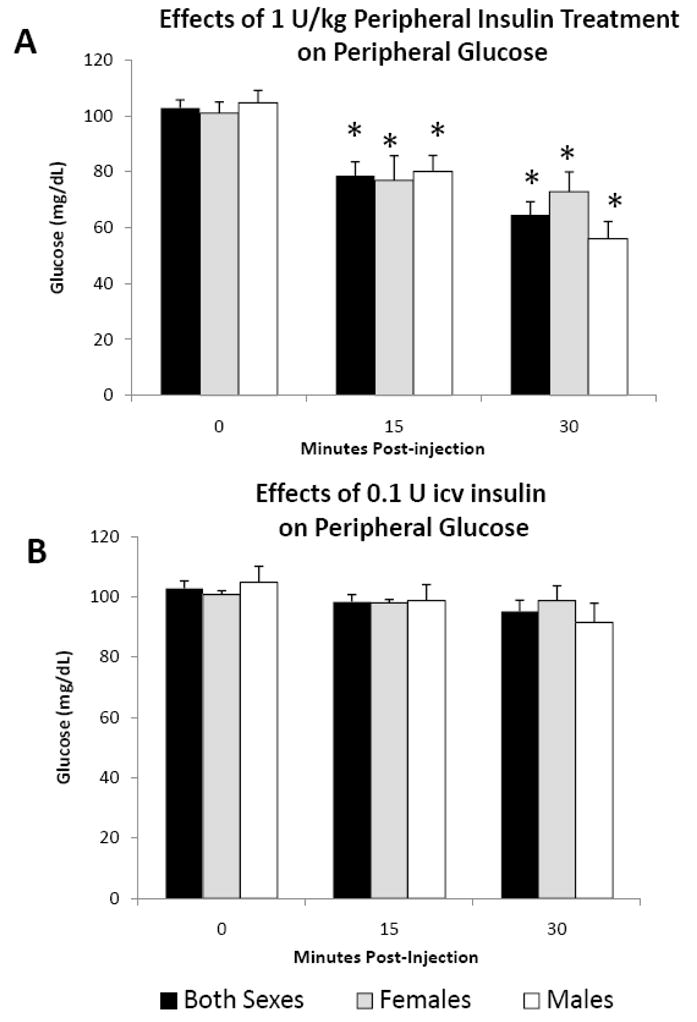
The effect of (A) ip and (B) icv insulin injection on blood glucose levels in juvenile female (n=8) and male (n=8) rats at 0, 15 and 30 minutes post-injection. Data are expressed as the mean ± SEM. *=P<0.05 compared with time 0.
DISCUSSION
In these studies, central insulin treatment in juvenile animals decreased food intake by altering ingestive behavior patterns: increasing meal latency and decreasing both meal size and meal number in a time dependent manner. Concomitant with these effects on food intake, icv insulin also altered hypothalamic appetite regulatory neuropeptide mRNA expression, decreasing the orexigenic neuropeptides, NPY and AgRP, and increasing the anorexigenic neuropeptide, POMC. The lack of an observed decrease in blood glucose after central insulin injection is consistent with a central site of insulin action. Unlike adults (Air et al., 2002; Clegg et al., 2003, 2006), juvenile female and male rats were equally responsive to exogenous icv insulin treatment, exhibiting similar decreases in food intake and changes in hypothalamic gene expression.
The insulin doses selected for these studies were based on adult studies of central insulin effects on food intake (Clegg et al., 2003; 2006). In these previous studies, injection of up to 8 mU insulin was not able to reduce food intake in adult intact females or castrated males, but decreased food intake 18% in ovariectomized female rats. Therefore, our minimum dose in this study was 10 mU (0.01 U) to ensure detectable effects on food intake. It is possible that lower doses of insulin would have effectively reduced food intake, and further studies are needed to determine the lowest effective dose of central insulin for juvenile animals. The anorexigenic response to exogenous insulin treatment was not dose or sex dependent in this study, and a dose response in the anorexigenic effects of central insulin treatment also has not been reported in adult male or ovariectomized female rats (Air et al., 2002; Clegg et al., 2003, 2006). Previous studies reporting sex differences in the anorexigenic effects of insulin in adults had treatment group sizes (n=8–10) similar to those used in this study (n=8) in juvenile rats (Brown et al., 2006; Clegg et al., 2003, 2006). Therefore, if a sex difference in insulin sensitivity existed in juvenile rats, there should have been sufficient statistical power in this study to detect it. In studies, where 8 mU of insulin was administered icv to adult males or ovariectomized females there was an 18% decrease in both 4 h and 24 h cumulative food intake. In contrast, the effects of exogenous central insulin treatment in our juvenile rat study was time dependent with greater decreases (29–40%) in food intake during the initial 4 h post-injection as compared to the decrease (10–13%) for 24 h cumulative food intake, regardless of dose.
Because insulin is an important endogenous anorexigenic factor, it is critical to understand how it acts on the brain to regulate appetite in younger animals. The observed changes in hypothalamic peptide expression indicate that central insulin decreases juvenile food intake via a mechanism similar to that observed in adult male or ovariectomized female rats. Previous studies in adults indicate that intact females are not responsive to the anorexigenic effects of central insulin, but females become more insulin sensitive after ovariectomy (Clegg, et al., 2006). These studies suggest that estradiol reduces central insulin sensitivity. Both male and female juvenile rats have low estradiol levels (30–35 pg/ml), similar to what has been observed previously in adult male and ovariectomized female rats (Clegg et al., 2006; Dohler and Wuttke, 1975). Central insulin treatment decreased food intake in both male and female juvenile rats in this study, and there was no observed sex difference in the capacity for central insulin treatment to decrease food intake. Therefore, the similar sensitivity to central insulin observed in male and female juvenile rats is likely due, at least in part, to low serum estradiol. These results are congruent with previous findings showing that low estradiol is necessary, although not sufficient, for the central anorexigenic effects of insulin (Clegg et al., 2006).
Studies to date have not examined the effects of central insulin on ingestive behavior patterns. We observed that insulin decreased meal size and meal number in a time-dependent fashion. Initial insulin delayed eating and decreased meal size, which may indicate an initial increase in satiety. After the first 4 h, meal size was no longer different in the saline and insulin treated groups. Instead central insulin treatment reduced the meal number during the 4–24 h post-injection period. Therefore, it appears that central insulin treatment has effects that persist even after it has been metabolized (4 h), suggesting the importance of downstream mechanisms which could involve persistent changes in hypothalamic neuropeptide expression or insulin-mediated neuronal firing (Davidowa and Plagemann, 2001).
In addition to examining ingestive behavior, we examined the effects of central insulin treatment on hypothalamic neuropeptide mRNA expression 90 min. post-injection. Prior studies indicate that treatment with either NPY or AgRP increases meal size in adult rats (Tiesjema et al., 2007; Santollo and Eckel, 2008), and that NPY may specifically be acting in the lateral hypothalamus to increase meal size (Tiesjema et al., 2007). In addition, treatment with the α-MSH analog, MTII decreases meal size in adult rats (Bellinger et al., 2003). Consistent with prior reports, icv insulin treatment reduced orexigenic neuropeptide mRNA expression (NPY, AgRP) and enhanced anorexigenic neuropeptide precursor mRNA expression (POMC), which likely contributed to the reductions in cumulative food intake observed during the first 2 hours post-injection. The alterations in neuropeptide mRNA expression were correlated with increased meal latency and decreased meal size, and without changes in meal number. Therefore, the observed increases in NPY and AgRP and decreases in POMC are consistent with the reported effects of these neuropeptides in reducing meal size in adults. These same neuropeptides are reportedly able to alter meal number in adults as well, but we did not observe any changes in meal number until after 4 h post-injection in the juvenile rats. Because we did not measure changes in hypothalamic gene expression during the time period when meal number was reduced, we are unable to determine if these neuropeptide changes persisted through the period when alterations in meal patterns were observed. Future studies would be necessary to determine potential mechanisms underlying the effects of central insulin on meal frequency.
In summary, the present studies demonstrated that icv insulin reduces food intake and alters hypothalamic gene expression in juveniles, similar to that observed in adult male rats and mice as well as ovariectomized female rats. Although systemic levels of insulin cross the blood brain barrier and contribute importantly to central satiety regulation following meals, central insulin does not appear to cross significantly to the periphery. The demonstrated anorexigenic effect of central insulin in both juvenile females and males offers a potential pharmacologic opportunity for the prevention and treatment of juvenile obesity.
Acknowledgments
This work was supported by the NIH 1R01HD054751-01A2 and K01 DK 063994.
This study was supported by National Institutes of Health grants: K01 DK 063994, and 1R01HD054751-01A2. The authors thank Patricia Liljelund for her technical support and Dr. Tom Magee for his technical assistance and advice with the quantitative RT-PCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer ZA, Rayner DV, Mercer JG. Hypothalamic gene expression is altered in underweight but obese juvenile male Sprague Dawley rats fed a high energy diet. J Nutr. 2004;134:1369–1374. doi: 10.1093/jn/134.6.1369. [DOI] [PubMed] [Google Scholar]
- Archer ZA, Corneloup J, Rayner DV, Barrett P, Moar KM, Mercer JG. Solid and liquid obesogenic diets induce obesity and counter-regulatory changes in hypothalamic gene expression in juvenile Sprague Dawley rats. J Nutr. 2007;137:1483–1490. doi: 10.1093/jn/137.6.1483. [DOI] [PubMed] [Google Scholar]
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143(6):2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi L, Beall MH, Magee TR, Pourtemour M, Ross MG. AQP1 gene expression is upregulated by arginine vasopressin and cyclic AMP agonists in trophoblast cell. Life Sci. 2008;82(25–26):1272–1280. doi: 10.1016/j.lfs.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Bellinger L, Cepeda-Benito A, Bullard RL, Wellman PJ. Effect of icv infusion of the alpha-MSH agonist MTII on meal patterns in male rats following nicotine withdraw. Life Sci. 2003;73(14):1861–1872. doi: 10.1016/s0024-3205(03)00485-5. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22(20):9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Schwartz MW, Baskin D, Woods SC, Seeley RJ. CNS melanocortin system involvement in the regulation of food intake. Horm Behav. 2000;37:299–305. doi: 10.1006/hbeh.2000.1588. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Rold of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117(6):2435–2442. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Reidy CA, Blake Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Davdiowa H, Plagemann A. Inhibition by insulin of hypothalamic VMN neurons in rats overweight dur to postnatal over feeding. NeuroReport. 2001;12:3201–3204. doi: 10.1097/00001756-200110290-00012. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin, and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Florant GL, Singer L, Scheurink AJW, Park CR, Richardson RD, Woods SC. Intraventricular insulin reduces food intake and body weight of marmosets during the summer feeding period. Physiol Behav. 1991;49:335–338. doi: 10.1016/0031-9384(91)90053-q. [DOI] [PubMed] [Google Scholar]
- Harder T, Plagemann A, Rohde W, Dorner G. Syndrome X-like alterations in adult female rats due to neonatal insulin treatment. Metabolism. 1998;47(7):855–862. doi: 10.1016/s0026-0495(98)90126-3. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-delta delta CT method. Methods. 2000;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendo. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8(12):1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Parlow AF, Coyotupa J, Kovacic N. The sequence of changes in prepubertal male rat serum testosterone following intravenous injection of rat LH. J Reprod Fert. 1973;32:163–165. doi: 10.1530/jrf.0.0320163. [DOI] [PubMed] [Google Scholar]
- Porte D, Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: A critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 200;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191(2):173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser SI, Smid SA, Mashford ML, Desmond PV. Sex hormones differentially regulate isoforms of UDP-Glucuronosyltransferase. Pharm Res. 1997;14(9):1115–1121. doi: 10.1023/a:1012130118186. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav. 1977;19:309–313. doi: 10.1016/0031-9384(77)90343-2. [DOI] [PubMed] [Google Scholar]
- Tiesjema B, Adan RA, Luijendijk MC, Kalsbeek A, la Fleur SE. Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and the lateral hypothalamus on feeding behavior. J Neurosci. 2007;27(51):14139–14146. doi: 10.1523/JNEUROSCI.3280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Chavez M, Park CR, Riedy C, Kaiyala K, Richardson RD, Figlewicz DP, Schwartz MW, Porte D, Jr, Seeley RJ. The evaluation of insulin as a metabolic signal influencing behavior via the brain. Neurosci Biobehav Rev. 1996;20(1):139–144. doi: 10.1016/0149-7634(95)00044-f. [DOI] [PubMed] [Google Scholar]