Abstract
Perfusion-based functional brain imaging techniques such as fMRI and optical intrinsic signal (OIS) imaging are becoming increasingly important in both neuroscience research and intraoperative brain mapping. Recent studies have applied a spectroscopic approach to OIS imaging data, which we will call “two-dimensional optical spectroscopy” (2DOS), generating images of functional changes in hemoglobin oxygenation and blood volume. This improvement comes at the cost of several assumptions. Whereas the “gold standard” technique of fiber spectroscopy decomposes reflected light over a spectral axis, 2DOS retains both spatial dimensions by acquiring images at several wavelengths, sacrificing spectral resolution for the extra spatial dimension. Furthermore, 2DOS data are acquired interleaved within or between trials, but combined during the spectroscopic analysis as if acquired simultaneously. Thus far, the few studies employing this approach have assumed both that the reduced spectral resolution is tolerable, and that sufficient trial averaging can compensate for the temporally staggered data acquisition. To test these assumptions, we compared 2DOS results to those produced by traditional fiber spectroscopy by observing the hemodynamic response to hindpaw electrical stimulation over the primary somatosensory cortex in anesthetized rats. Comparisons revealed low fitting residuals and a high level of correlation between the two, but noteworthy differences in response magnitudes. Inspection of individual timecourses revealed a lower signal-to-noise ratio for 2DOS data. For visualization and interpretation of the 2DOS images, we present a parameterized visualization strategy, in which oxy-, deoxy-, and total hemoglobin are assigned to individual color channels. The resulting composite image conveniently displays the evolution of hemodynamic responses through parenchymal and vascular compartments in space and time.
Introduction
Perfusion-based functional imaging techniques such as functional magnetic resonance imaging (fMRI) and optical intrinsic signal (OIS) imaging have provided a wealth of information about brain organization. Because these modalities measure hemodynamic responses, some interpretation is required to relate them to neuronal activity. Although OIS imaging has been used extensively for high resolution functional mapping across a variety of species and cortices, one important drawback is the ambiguity of the optical signals. Changes in cortical light reflectance are a function of several biological processes (e.g., hemoglobin absorption, light scatter due to cell swelling and vascular caliber change, etc.), and most are wavelength-dependent (Frostig et al., 1990). Thus single wavelength OIS imaging adds a further degree of convolution separating the measured signals from the source of interest.
To reduce this ambiguity somewhat, optical reflectance changes can be acquired over an entire spectrum of wavelengths (spectroscopy) and fit to an appropriate model populated with the relevant physiological variables (Malonek et al., 1997). Spectroscopy can therefore distinguish between the various contributions of these processes, to the degree that they are correctly modeled, but has limited spatial information since the data is acquired through a fiber bundle or spectroscopic slit (1-dimensional).
Recently, a few studies have combined the advantages of OIS imaging and spectroscopy to produce 2-D images of the modeled physiological processes, such as changes in hemoglobin oxygenation and cerebral blood volume (Sheth et al., 2002; Devor et al., 2003; Devor et al., 2005; Dunn et al., 2005; Sheth et al., 2005; Prakash et al., 2007). In two-dimensional optical spectroscopy (2DOS), images are acquired at multiple wavelengths, and these abbreviated “spectra” are fit to an appropriate model at every pixel. This improvement provides functional images with greater biological relevance and increased comparability to fMRI, as well as the ability to investigate hemodynamic propagation within various vascular compartments.
Inherent in this form of analysis, however, are several important assumptions that require validation. In most variants of 2DOS, although the multi-wavelength data are combined during the spectroscopic analysis as if obtained simultaneously (as in fiber spectroscopy), they are actually acquired interleaved within or between trials. Sufficient trial averaging is assumed to compensate for the lack of simultaneity. In addition, there is a significant reduction in spectral resolution between 2DOS, in which only a few wavelengths are acquired, and fiber spectroscopy, in which a full spectral axis is available for curve fitting.
The primary purpose of this report, therefore, is to test the hypothesis that despite the afore-mentioned limitations of 2DOS, the results derived from this technique provide accurate physiological data on cerebral hemodynamics. We therefore studied the validity of the results derived from 2DOS by comparing them to results obtained from traditional fiber spectroscopy, which over the past decade has been shown to faithfully represent functional hemodynamic changes in a variety of models. To do so, we concurrently recorded multi-wavelength images and fiber spectroscopy data in rat somatosensory cortex during peripheral stimulation. A secondary objective was to present a visualization strategy for analyzing 2DOS results. The most relevant variables obtained from this technique are oxyhemoglobin (HbO2), deoxyhemoglobin (Hbr), and their sum total hemoglobin (Hbt). Instead of displaying a cumbersome time sequence of images for each of these three, we use a three parameter color mapping method to generate composite images that concisely and conveniently display the spatiotemporal evolution of these hemodynamic changes.
Methods
Animal preparation
Nine male Sprague-Dawley rats (300–450 g) were studied in accordance with the University of California, Los Angeles Chancellor’s Committee on Animal Research. Details are provided in a previous publication (Sheth et al., 2005), but the procedure is summarized here. The skull was exposed and thinned, and silicone oil applied to increase bone translucency. Anesthesia was switched to intravenous alpha-chloralose (60 mg/kg initial bolus, 30 mg/kg/hr continuous infusion) and pancuronium bromide (2 mg/kg initial bolus, 1.5 mg/kg/hr continuous infusion), and the artificial ventilation and inspired gases adjusted to maintain physiological variables within the normal range: MABP 90–110 mmHg, arterial pO2 121±6 mmHg, arterial pCO2 39±1 mmHg (mean ± SE).
Optical imaging
Imaging was performed on a Nikon SMZ1500 microscope under illumination from a DC voltage-stabilized quartz-tungsten-halogen source (PL900, Dolan-Jenner, Lawrence, MA) equipped with a heat filter. Light was collimated by two fiber optic guides with focusing lenses, and reflected light was split between the eyepiece and the 16-bit CCD imaging camera (TE/CCD-576EFT, Princeton Instruments, Trenton, NJ) with attached filter wheel (Lambda 10–2, Sutter Instruments, Novato, CA). The filter wheel housed four narrow bandpass transmission filters, centered at 569 nm (full width at half-maximum [FWHM] 3 nm; filter 569NB3), 577 nm (577NB3), 586 nm (586NB6), and 605 nm (605NB5).
The stimulation paradigm consisted of a 2 second train of 1 ms square electrical pulses (ISO-Flex, Master-8, AMPI, Israel) delivered to the left hindpaw via two steel needle electrodes inserted into the plantar surface of the foot and ~10 mm away on the medial aspect of the leg. Neuronal activity was varied by modulating the stimulation frequency (2, 5, 10, 15, 20 Hz at 1.2 mA) pseudo-randomly. Each imaging trial lasted 16 seconds (6 pre-stimulus and 10 post-stimulus), and images were acquired every 250 ms, resulting in 64 images per trial. Trials were spaced 30 seconds apart to allow the hemodynamic response to return to baseline. The four filters were alternated every trial. We acquired 12 trials per wavelength and stimulation condition, for a total of 240 trials in each subject.
Fiber spectroscopy
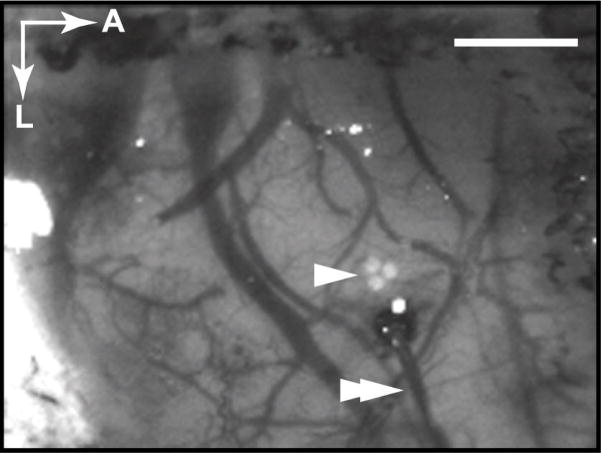
We performed concurrent fiber spectroscopy by capturing half the reflected light with a fiber optic bundle mounted on the microscope eyepiece. The fiber bundle consisted of three individual 200 μm fused silica fibers arranged in a triangular geometry on one end and a vertical column on the other. The side with the triangular arrangement was held in a micro-manipulator in front of the microscope eyepiece, and the side with the vertical arrangement aligned to the entrance slit (100 μm width) of a spectrophotometer (SpectraPro 300i, Acton Research, Acton, MA) with an attached 16-bit spectroscopic CCD camera (Spec-10:400BR, Princeton Instruments, Trenton, NJ). Spectra were acquired from 520 to 610 nm with 0.5 nm spectral resolution, and timing was identical to that of image acquisition. The area of cortex sampled by the fiber was determined by placing the slit end of the fiber in a light source and shining light in reverse, through the microscope optics, onto the cortex (Figure 1). The fiber was positioned as close to the center of activation as possible, avoiding the pial and dural vessels.
Figure 1. Spectroscopic fiber positioning.
Raw image taken at 569 nm showing the area of cortex exposed under the thinned skull. The location of the hemodynamic response to electrical hindpaw stimulation was first determined with single wavelength imaging at 569 nm. The position of the spectroscopic fiber was identified by shining light through the fiber bundle in reverse, producing an image of the triangular fiber orientation on the cortex (single arrowhead). The fiber position was adjusted to sample the capillary bed at the center of the response area. The image of the fiber position was also used as a region of interest when comparing the results from fiber spectroscopy and 2DOS. With this configuration, a microelectrode (double arrowhead) may also be used for concurrent electrophysiology (data not shown). A, anterior; L, lateral. Scale bar, 1 mm.
Reflectance spectra were analyzed using a modified form of the Beer-Lambert law as done previously (Sheth et al., 2004a):
| (Equation 1) |
where Io is the measured pre-stimulus intensity, I(t) is the intensity timecourse, the e’s are the extinction coefficients, square brackets denote absorber concentration, l is the pathlength through the tissue at a given wavelength ?, and S is scattering.
Wavelength dependence (superscript ?) was determined using an in vitro phantom model to simulate the absorption and scattering properties of rat cortex (Sheth et al., 2004b). The phantom consisted of rat blood as the absorber and Intralipid (Fresenius Kabi, Clayton, NC), an aqueous suspension of lipid droplets, as the scatterer. A 1% Intralipid solution provides a reduced scattering coefficient (μs′) of 1 mm−1, similar to that of brain tissue (Nakai et al., 1997), and the blood was diluted to 1% (~50 μM), the approximate mean value in rat brain (Cooper et al., 1998).
Defining α as the product of the extinction coefficient and pathlength and removing time dependency, equation (1) becomes:
| (Equation 2) |
where Io is the reference spectrum consisting of 0% hematocrit (scattering only), , and . By holding the hematocrit fixed and measuring the reflected intensity, we calculated and , which represent the absorption spectra of HbO2 Hbr and HbO2 adjusted for the wavelength dependent pathlength. To isolate and measure the α values, we fully oxygenated the phantom suspension ([Hbr]=0) by bubbling 100% O2 for several minutes, and fully deoxygenated it by allowing yeast (0.5% by weight) to consume oxygen and then adding a small amount of the reducing agent sodium hydrosulfite (Na2S2O4). Both oxygenation states were confirmed by measuring oxygen tension with a blood gas monitor. The timecourse of [Hbr] and [HbO2] changes due to neuronal activation was calculated from (1) using a least-squares analysis and the values of and obtained from the phantom.
Two-Dimensional Optical Spectroscopy (2DOS)
Multi-wavelength optical imaging data were analyzed using the identical spectroscopic approach. Instead of measuring a continuous spectrum at a single point as described above, however, we treated the four wavelengths of data at each pixel as a discrete abbreviated spectrum, thereby sacrificing spectral resolution for the retention of two spatial dimensions of information. These pixel-by-pixel “spectra” were subjected to a conventional spectroscopic analysis using equation (1). We used the same in vitro phantom model to measure the pathlength-adjusted absorption coefficients e for the four wavelengths.
Because the spectral resolution of 2DOS is only 4 points (in our case) compared to fiber spectroscopy (180 points), we verified the former by comparing full response timecourses generated by both methods. Changes in Hbr, HbO2, and Hbt (sum of Hbr and HbO2) were calculated in a region of interest (ROI) based on the position of the spectroscopic fiber (Figure 1) for each subject.
2DOS provides a timecourse of two-dimensional images for three distinct but related hemodynamic parameters. To better appreciate their interaction and compare their spatiotemporal evolution, we applied a visualization strategy based on a parameterized composite image of the functional representations. Pixel values in each image were uniformly scaled and clipped to range between 0 and 1 in Matlab (MathWorks, Natick, MA). At every time point, the HbO2 functional image was assigned to the red channel, the Hbr image to the blue channel, and the Hbt image to the green channel (Figure 2). As the BOLD fMRI signal increases with decreasing Hbr, Hbr images in Figure 2 were inverted such that a decrease in Hbr over time corresponded to an increase in pixel intensity. The information provided by this composite image can be described using the cylindrical coordinate model shown in Figure 2. Pixel hue (f, angular position) varies through the color spectrum, and saturation (r, radial position) varies from a gray shade to color. These two coordinates map the position in a color wheel plane and represent the relative contribution of Hbr, HbO2, and Hbt at that pixel. Brightness (h, height) varies from black to white and represents the magnitude of the response. Composite images thus simultaneously display the spatial distribution and relative contribution of functional hemoglobin concentration and oxygenation changes. Because Hbt is calculated as the sum of Hbr and HbO2, only two of the three components of this composite map are independently measured. Nevertheless, assigning a separate color channel to Hbt adds useful visual information.
Figure 2. Composite visualization strategy.
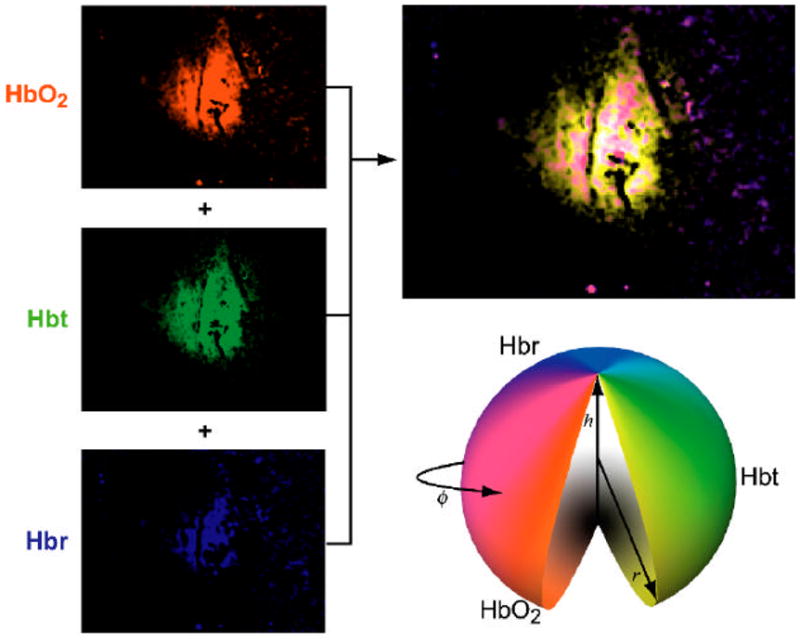
The two dimensional spatial profiles of HbO2, Hbt, and Hbr generated by 2DOS are depicted as a single composite image by assigning responses to the red, blue, and green channels, respectively. The composite image simultaneously displays the spatial distribution of the three functional hemodynamic responses. Their varying contributions cover a volume that can be described with a cylindrical coordinate model. Hue (f) and saturation (r) map the position in a color wheel plane and specify the relative contribution of HbO2, Hbt, and Hbr at that pixel, and brightness (h) denotes response magnitude (also see Methods). Overlapping responses are therefore represented with mixed colors (e.g., magenta = HbO2+Hbr; yellow = HbO2+Hbt; white = all three).
Results
We measured the hemodynamic response to peripheral stimulation in rat somatosensory cortex using simultaneous 2DOS and fiber spectroscopy. Imaging data were acquired at four wavelengths and fit to a spectroscopic model incorporating absorption due to changes in hemoglobin concentration and oxygenation. The resulting functional maps displayed the spatiotemporal evolution of functional hemodynamic signals over cortex. Concurrently obtained spectra were fit to the same model, allowing comparison between 2DOS and fiber spectroscopy results.
Validation of two-dimensional optical spectroscopy
Absorbance data from phantom measurements are shown in Figure 3. The full spectrum was measured at a single point with fiber spectroscopy, and the four-point abbreviated imaging “spectrum” was measured as an average over the entire field of view. 2DOS data corresponded well with the full spectrum.
Figure 3. Phantom absorbance spectra.
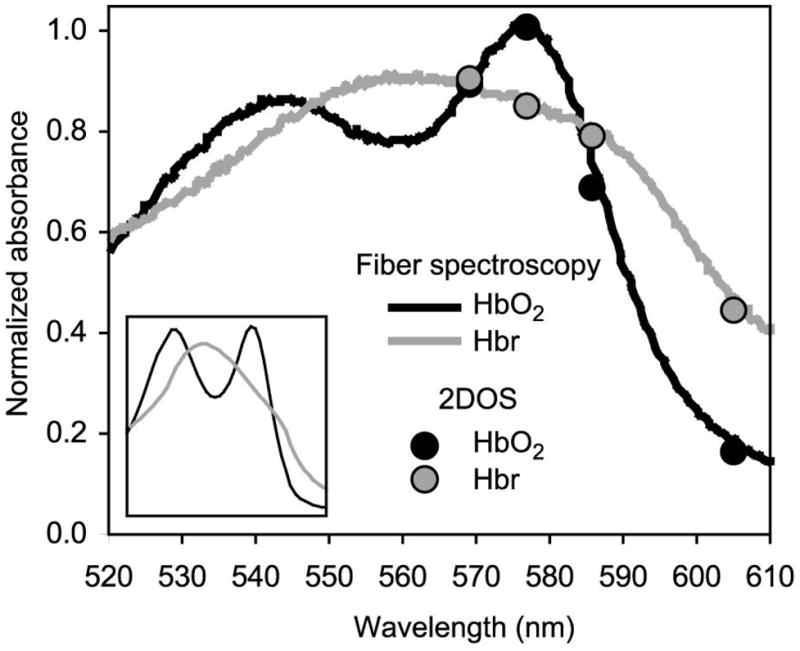
Absorbance spectra were measured in an in vitro phantom simulating the absorbance and scattering properties of brain tissue. Solid lines represent measurements made with fiber spectroscopy, and circles represent measurements averaged over an image using narrow bandpass filters at 569, 577, 586, and 605 nm. 2DOS absorbance values correspond well with those measured by fiber spectroscopy. The high degree of scattering in biological tissue introduces wavelength dependency in the path length. Measured absorbance values include this differential path length, thus distorting the shape of the spectrum compared to textbook Hb absorbance spectra (inset).
Response timecourses from fiber spectroscopy are shown in Figure 4, averaged over all subjects. Both peak response magnitudes and latency (time to peak) increased with decreasing stimulation frequency, reaching a plateau around 2 to 5 Hz. We observed a small and transient increase in Hbr (“initial dip”) immediately following stimulation onset, peaking at 0.75 to 1 s. Hbt peaked between 2 and 3 s, and Hbr and HbO2 reached minimum and maximum values, respectively, between 3 and 4 s.
Figure 4. Fiber spectroscopy hemodynamic responses.
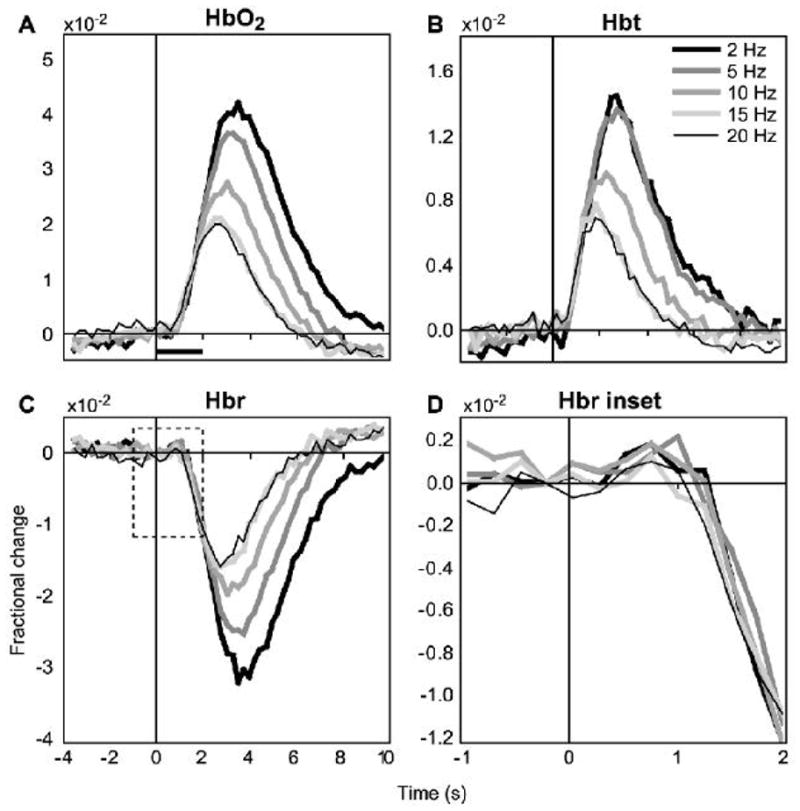
The timecourse of functional changes in HbO2, Hbt, and Hbr determined from fiber spectroscopy are shown averaged over all subjects. Response size increased with decreasing stimulation frequency. We observed a small, transient increase in Hbr (“initial dip”; panel D, dashed region in C). Horizontal bar, stimulus duration (2 seconds).
2DOS provided functional images of changes in cerebral blood oxygenation and volume. Figure 5 depicts an example of the evolution of the three hemodynamic variables over space and time in a representative subject. Acquisition of full-field images permits the calculation of functional changes in different regions, or across parenchymal and vascular compartments. In Figure 6, average response timecourses are shown in three compartments: central parenchymal, arterial, and venous. Further analysis of hemodynamic propagation and its relevance for functional imaging are the subject of a separate publication (Sheth et al., 2005).
Figure 5. 2DOS time series.
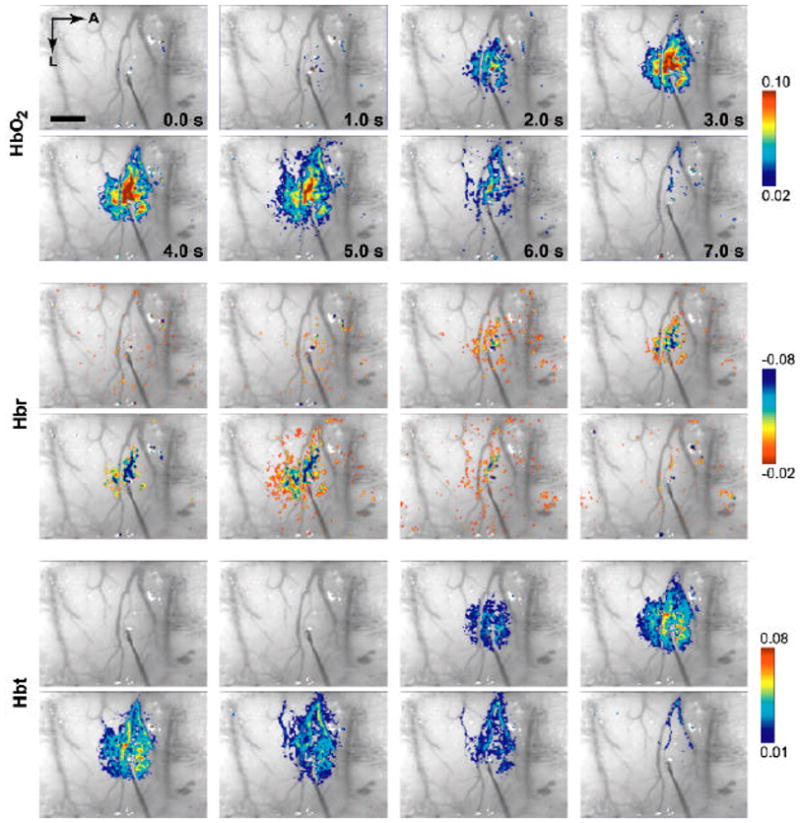
Applying a spectral analysis to multi-wavelength optical imaging data produced 2D images of functional changes in HbO2, Hbt, and Hbr (2DOS). A representative example is shown here at several time points, overlaid on raw structural images taken at 605 nm. Hemodynamic responses originated from a common focus and subsequently evolved through vascular compartments. A, anterior; L, lateral. Scale bar, 1 mm.
Figure 6. Compartment-specific hemodynamic timecourses.
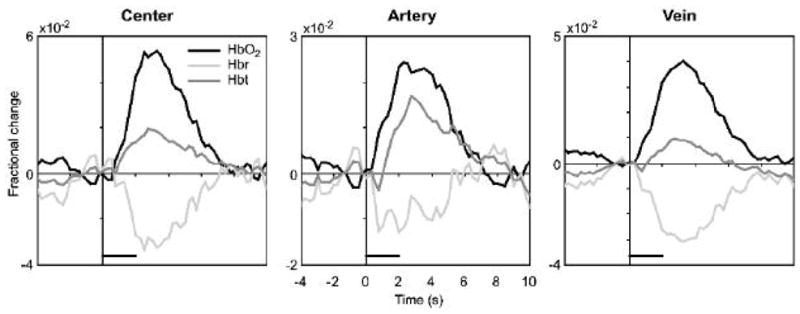
Calculation of hemodynamic parameters over an entire image allows the investigation of their temporal evolution through parenchymal and vascular compartments. “Center” refers to the response within the region of interest (ROI) corresponding to the spectroscopy fiber location (Figure 1), which was positioned over the capillary bed at the response center. We also created arterial and venous ROIs for each subject, corresponding to the timecourses labeled “Artery” and “Vein”, respectively.
Functional HbO2, Hbr, and Hbt responses calculated by 2DOS were validated by comparing 2DOS timecourses with those calculated using traditional fiber spectroscopy. Responses to 2 Hz stimulation calculated using both methods are shown in Figure 7A. The overall profiles of 2DOS responses were very similar to fiber spectroscopy responses, in terms of latency, time to peak, and general shape. Signal-to-noise ratios (SNR) for fiber spectroscopy and 2DOS data, respectively, were as follows: HbO2 32.1 and 16.5; Hbr 27.4 and 6.6; Hbt 31.2 and 11.2. For 2DOS, 48 trials were acquired per stimulation condition (4 wavelengths × 12 repetitions), requiring 24 minutes. Fiber spectroscopy required 12 trials per stimulation condition, resulting in a 6 minute acquisition (see Methods). Because both were performed concurrently, four times as many fiber spectroscopy trials compared to 2DOS trials were collected, increasing the expected SNR for fiber spectroscopy by approximately two-fold. This difference may account for the improved SNR observed for fiber spectroscopy compared to 2DOS. The residuals from both techniques were small, indicating that despite decreased spectral resolution, 2DOS provides an accurate measure of hemoglobin concentration and oxygenation changes.
Figure 7. Validation of 2DOS measurements.
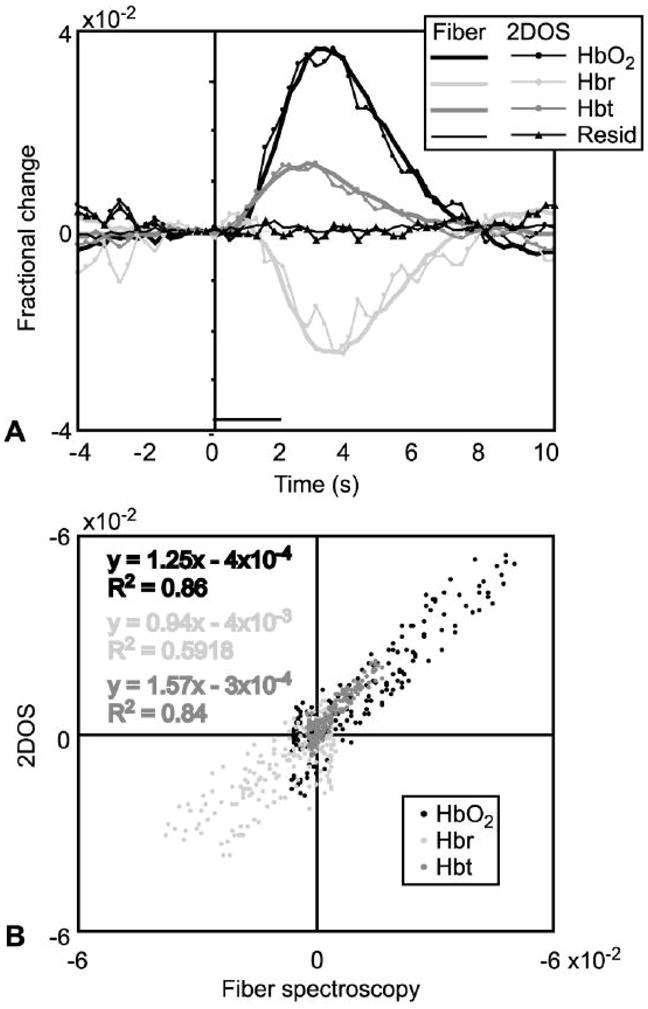
Functional changes in HbO2, Hbr, and Hbt measured by fiber spectroscopy (“Fiber”) and 2DOS (“2DOS”) were compared to validate the latter. Imaging responses were calculated in the area sampled by the spectroscopy fiber (Figure 1). A, Response timecourses to 2 Hz stimulation using both methods. To facilitate comparison of temporal properties, 2DOS response timecourses were normalized by scaling the peak magnitude to match that of fiber spectroscopy responses. Both were similar in terms of latency, time to peak, general shape, and small residuals from curve fitting. Horizontal bar, stimulus duration (2 seconds). B, Raw (not normalized) response size at every post-stimulus time point and stimulation condition (40 time points × 5 conditions) measured by 2DOS versus fiber spectroscopy.
To compare timecourses over all stimulation conditions, we plotted 2DOS versus fiber spectroscopy raw response size for every time point after stimulation onset, and for every stimulation condition (40×5=200 points) (Figure 7B). This point-by-point comparison showed that all three responses correlated significantly (HbO2 R2=0.86, p<10−90; Hbr R2=0.61, p<10−43; Hbt R2=0.86, p<10−89) as they evolved over time.
To compare the magnitudes of responses, the slope of the regression lines was calculated. A slope of unity for the regression lines would indicate that the magnitude of 2DOS responses equaled that of fiber spectroscopy responses. The HbO2 regression line slope was significantly different from unity (p<0.005, two-tailed Student’s t test), as was that of Hbt (p<10−4), but not Hbr (p>0.05).
Visualization of spatiotemporal evolution
To better visualize the spatiotemporal evolution of functional hemoglobin concentration and oxygenation changes, we created composite maps showing the relative contribution of HbO2, Hbr, and Hbt at every pixel. Figure 8 depicts a representative composite image time series. In these images red denotes HbO2, blue Hbr, and green Hbt (which is proportional to blood volume). White pixels therefore represent spatial overlap of the three signals, and magenta/purple pixels denote concomitant changes in HbO2 and Hbr consistent with pure oxygenation shifts with little change in blood volume.
Figure 8. Spatiotemporal evolution of functional hemodynamic changes.
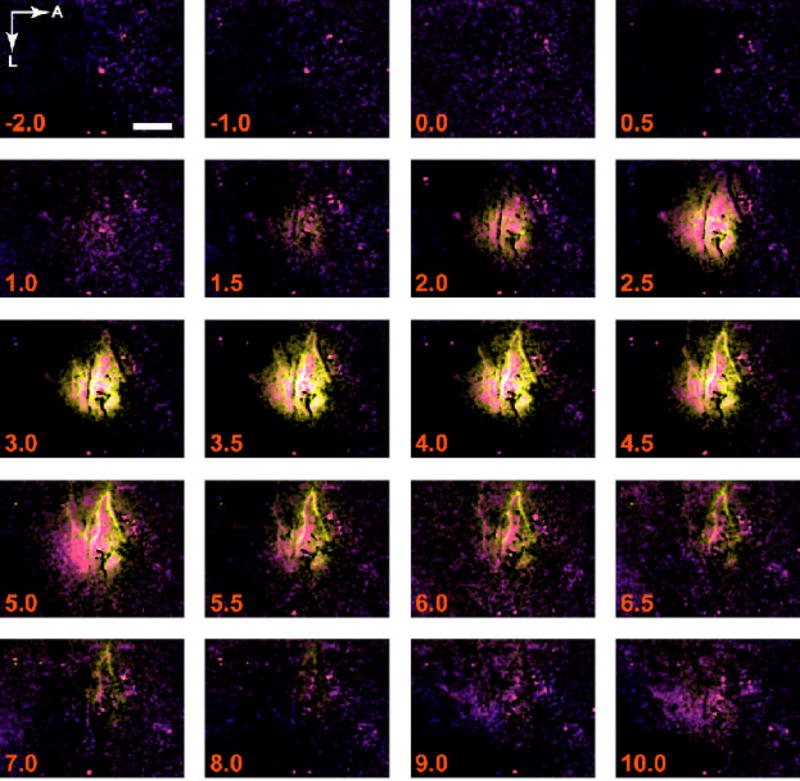
Composite HbO2-Hbr-Hbt images are shown in a representative subject. The propagation of hemodynamic responses from a common central focus within the first few seconds to segregated vascular compartments over time is readily apparent with this visualization strategy. The parameterized composite images succinctly provide a means to temporally and spatially compare relative response magnitude and location for the three relevant hemodynamic variables without shifting attention between three different time series, as in Figure 5. A, anterior; L, lateral. Scale bar, 1 mm.
At early time points (2–3 sec), the three signals overlapped near the center of activation, resulting in a central white region. Peripheral regions a few hundred microns from the center showed segregation between Hbt and oxygenation changes, evidenced by distinct regions of green and magenta/purple. Hbt changes tended to localize in activated arterioles, identified by referring to structural images taken at 569 nm, which show both arteries and veins, and 605 nm, which emphasize venous structures.
At later times (>5 sec), overlap between the three signals was decreased. Response size in the central region was diminished, and vascular structures were more prominent. Overlap between blood volume and oxygenation was limited to venous structures. Variations in the degree to which blood volume increases in veins produced pixel colors ranging from magenta (minimal increase) to white/yellow (significant increase). This variation may depend upon the size of the vein or its proximity to the focus of neural activity.
Discussion
We have shown that despite the limited spectral resolution of 2DOS, this technique accurately represents functional changes in physiologically relevant hemodynamic parameters. The timecourse of these changes through vascular compartments seen in two-dimensional images is a significant advantage over fiber spectroscopy. Moreover, the visualization strategy presented here succinctly encapsulates the evolution of several parameters through space and time in a single image sequence.
The acquisition of high resolution images of physiological variables has already enabled detailed studies of cerebral microcirculatory hemodynamics, including bi-directional spread through vascular compartments (Sheth et al., 2005) and center-surround blood redistribution patterns (Devor et al., 2005) evoked by stimulation. The relationship between neuronal activity and hemodynamic responses has been investigated with concurrent field potential (Sheth et al., 2005) and single- and multi-unit (Devor et al., 2003) recordings, and the addition of 2D cerebral blood flow imaging may be used to produce maps of cerebral oxygen metabolism (Dunn et al., 2003; Dunn et al., 2005), and to compare scaling parameters of functional maps across species (Prakash et al., 2007). Additionally, the technique inherently and easily accommodates simultaneous acquisition of other useful measures, such as electrophysiology and laser Doppler flowmetry.
2DOS may be especially useful for intraoperative functional imaging. Several studies using single wavelength optical imaging have investigated the organization of language cortex (Pouratian et al., 2000; Pouratian et al., 2002), the perfusion response to direct cortical stimulation (Haglund and Hochman, 2004; Suh et al., 2006), and the hemodynamic correlates of epileptiform discharge (Zhao et al., 2007). Given the limitations of single wavelength imaging mentioned above, 2DOS would provide more accurate physiological information than what has been obtained thus far. Image acquisition time could be significantly reduced compared to that in this study by using a beam splitter with multiple filters, such that four or more wavelength data could be obtained simultaneously (Prakash et al., 2007). The time savings would be extremely valuable for intraoperative imaging, as the clinical confines of patient safety demand expediency in data collection. In addition, the convenient visualization method described here efficiently displays several hemodynamic parameters in a single image, allowing the neurosurgeon to make clinical decisions quickly.
Acknowledgments
Work was supported by grants from the NIH (GM08042, MH67432, MH52083) and the Adelson Program in Neural Repair and Rehabilitation
We thank Masahito Nemoto for his comments during data analysis and preparation of the manuscript.
Role of the funding source: Work was supported by grants from the NIH (GM08042, MH67432, MH52083). The role of the sponsor (AWT) was to guide the other authors in experimental design, data analysis, and editing the manuscript.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cooper CE, Delpy DT, Nemoto EM. The relationship of oxygen delivery to absolute haemoglobin oxygenation and mitochondrial cytochrome oxidase redox state in the adult brain: a near-infrared spectroscopy study. Biochem J. 1998;332:627–632. doi: 10.1042/bj3320627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci U S A. 2005;102:3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27 doi: 10.1016/j.neuroimage.2005.04.024. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Bolay H, Andermann ML, Moskowitz MA, Dale AM, Boas DA. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt Lett. 2003;28:28–30. doi: 10.1364/ol.28.000028. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund MM, Hochman DW. Optical imaging of epileptiform activity in human neocortex. Epilepsia. 2004;45:43–47. doi: 10.1111/j.0013-9580.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T, Nishimura G, Yamamoto K, Tamura M. Expression of optical diffusion coefficient in high-absorption turbid media. Phys Med Biol. 1997;42:2541–2549. doi: 10.1088/0031-9155/42/12/017. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg. 2002;97:21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, O’Farrell AM, Sicotte NL, Cannestra AF, Becker D, Toga AW. Optical imaging of bilingual cortical representations: Case report. Journal of Neurosurgery. 2000;93:686–691. doi: 10.3171/jns.2000.93.4.0676. [DOI] [PubMed] [Google Scholar]
- Prakash N, Biag JD, Sheth SA, Mitsuyama S, Theriot J, Ramachandra C, Toga AW. Temporal profiles and 2-dimensional oxy-, deoxy-, and total-hemoglobin somatosensory maps in rat versus mouse cortex. Neuroimage. 2007;37:S27–36. doi: 10.1016/j.neuroimage.2007.04.063. Epub 2007 May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004a;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Walker M, Guiou M, Toga AW. Hemoglobin concentration mapping using two-dimensional spectrophotometry. 8th Annual Meeting of the Organization for Human Brain Mapping Abstracts.2002. [Google Scholar]
- Sheth SA, Nemoto M, Guiou MW, Walker MA, Toga AW. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. J Cereb Blood Flow Metab. 2005;2:2. doi: 10.1038/sj.jcbfm.9600091. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Hageman N, Toga AW. Columnar specificity of microvascular oxygenation and volume responses: Implications for functional brain mapping. J Neurosci. 2004b;24:634–641. doi: 10.1523/JNEUROSCI.4526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh M, Bahar S, Mehta AD, Schwartz TH. Blood volume and hemoglobin oxygenation response following electrical stimulation of human cortex. Neuroimage. 2006;31:66–75. doi: 10.1016/j.neuroimage.2005.11.030. Epub 2006 Feb 2015. [DOI] [PubMed] [Google Scholar]
- Zhao M, Suh M, Ma H, Perry C, Geneslaw A, Schwartz TH. Focal increases in perfusion and decreases in hemoglobin oxygenation precede seizure onset in spontaneous human epilepsy. Epilepsia. 2007;48:2059–2067. doi: 10.1111/j.1528-1167.2007.01229.x. Epub 2007 Jul 2030. [DOI] [PubMed] [Google Scholar]