Abstract
An important function of receptors that signal through immunoreceptor tyrosine-based activation motifs (ITAMs) is to regulate signaling by heterologous receptors. This review describes mechanisms by which ITAM-associated receptors modulate signaling by Toll-like receptors (TLRs), tumor necrosis factor receptor family members and cytokine receptors that use the Jak–STAT signaling pathway, and the biological importance of this signal transduction cross-talk. ITAM-mediated cross-regulation can either augment or dampen signaling by other receptors. Conversely, TLRs and cytokines modulate ITAM-mediated signaling, by means including activation of β2 integrins that are coupled to the ITAM-containing adaptors DAP12 and FcRγ. Integration of ITAM signaling into signaling networks through cross-talk with other signal transduction pathways results in tight regulation and fine tuning of cellular responses to various extracellular stimuli and contributes to induction of specific activation and differentiation pathways.
The ITAM is a conserved signaling motif preferentially used by hematopoietic cells1. The ITAM motif (consensus sequence YXXLX6–8 YXXL/I) is contained in the cytoplasmic domain of transmembrane adaptor molecules that are associated with and transmit signals from various immunoreceptors. ITAM-coupled receptors include the T cell and B cell antigen receptors (TCR and BCR) and Fc receptors (FcRs), which bind immunoglobulins. In lymphocytes, ITAM-containing adaptors transmit antigen receptor signals that lead to cell activation or tolerance, depending on the intensity of receptor stimulation and the presence or absence of co-stimulatory signals2. Myeloid cells express approximately 20 ITAM-associated receptors, including FcRs, TREMs, ILTs (also called LILRs), MAIRs, PIR-A, MDL-1, DCAR, OSCAR, CD200 receptors and c-Fms (refs. 3,4). These receptors signal through two main ITAM-containing adaptors expressed by myeloid cells, termed FcRγ and DNAX activation protein-12 (DAP12). Except for FcRs, which bind immunoglobulins and immune complexes, the ligands for myeloid ITAM-coupled receptors are not well defined; they include MHC class I molecules and other, as yet unknown ligands that are expressed on myeloid cells or on tissue cells with which myeloid cells interact. Thus, in myeloid cells ITAM-coupled receptors are constitutively engaged, which leads to tonic low-level ITAM-mediated signaling (reviewed in ref. 5). More recently, it has become clear that FcRγ and DAP12 associate with β2 and β3 integrins and contribute to signaling by these receptors6–8. Thus, ITAM signaling is also induced by cell–cell and cell–extracellular matrix (ECM) interactions that are mediated by these integrins.
The ligation of ITAM-associated receptors in myeloid cells leads to a signaling cascade that begins with phosphorylation of ITAM tyrosine residues by Src-family kinases, followed by the recruitment and activation of the spleen tyrosine kinase (Syk)1,5. Syk then activates a signaling cascade leading to downstream activation of NF-κB and MAPKs, important signaling effectors and inducers of gene expression (Figs. 1 and 2). An important component of ITAM-mediated signaling is activation of phospholipase-Cγ (PLCγ), resulting in the generation of diacylglycerol (DAG) and parallel activation of calcium signaling (Fig. 2). DAG activates PKC and contributes to MAPK activation through RasGRPRas guanyl releasing protein (RasGRP). Calcium signals activate the phosphatase calcineurin, which activates nuclear factor of activated T cells (NFAT) and calcium-dependent kinases such as calmodulin-dependent kinase (CaMK) and Pyk2. CaMK and Pyk2 contribute to the activation of MAPKs and of calcium-regulated transcription factors such as CREB. Of the major signaling pathways and effectors activated by ITAM-associated receptors, NF-κB and MAPKs are involved in cell activation and are activated by many other receptors. Direct activation of calcium pathways by ITAM-associated receptors distinguishes them from macrophage-activating receptors such as TLRs and inflammatory cytokine receptors.
Figure 1.
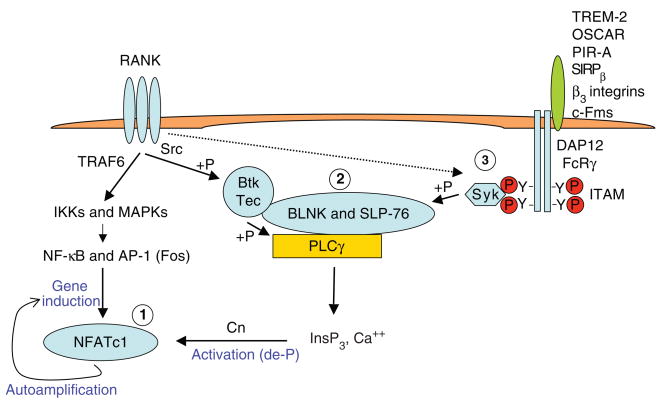
Integration of RANK and ITAM signaling to activate NFATc1. RANK signaling induces NFATc1 gene expression by way of TRAF6 and transcription factors NF-κB and AP-1 and contributes to induction of calcium signaling by activating Btk and Tec tyrosine kinases and transactivating ITAM-associated receptors. ITAM-associated receptors TREM-2 and SIRPβ are activated by ligands constitutively expressed on osteoclast precursors; OSCAR and PIR-A, by ligands expressed on stromal and osteoblast lineage cells; β3 integrins, by extracellular matrix; and c-Fms by M-CSF and IL-34, which are secreted by various cell types; some of these receptors can also be superactivated after stimulation of RANK by its ligand RANKL. ITAM-associated receptors cooperate with RANK to induce calcium signaling, which activates calcineurin (Cn), which in turn dephosphorylates (de-P) and induces nuclear translocation of NFATc1. NFATc1 is a master regulator of osteoclast differentiation that autoamplifies expression of its own promoter, activates expression of osteoclast-related genes and drives osteoclastogenesis. Specific integration points in RANK and ITAM signaling discussed in the text are labeled 1, 2 and 3. InsP3, inositol-1,4,5-trisphosphate; IKK, IκB kinase.
Figure 2.
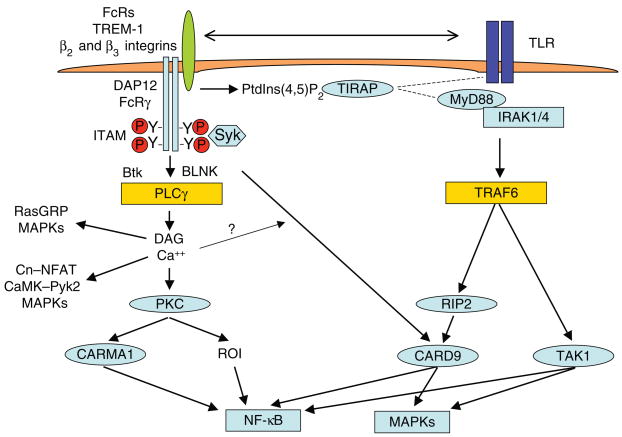
Integrated signaling by ITAM-associated receptors and TLRs leads to enhanced activation of NF-κB and MAPKs. ITAM-associated receptors and TLRs activate distinct signaling pathways that converge on common signaling effectors such as NF-κB and MAPKs. Signaling pathways can also converge on upstream signaling molecules, such as CARD9. Integrated and additive activation of signaling molecules by different signaling pathways leads to enhanced signaling and amplified cellular responses. Cn, calcineurin; IRAK1/4, interleukin 1 receptor–associated kinases 1 and 4; MyD88, myeloid differentiation factor adaptor molecule.
Extensive work has established that high-avidity cross-linking of ITAM-associated antigen receptors and FcRs strongly activates NF-κB and MAPKs and leads to cell activation (reviewed in ref. 2). This has led to the paradigm that ITAMs and associated receptors mediate activating signals that are opposed by a distinct set of receptors that signal through a cytoplasmic-domain immunoreceptor tyrosine-based inhibitory motif (ITIM)9. ITIMs recruit phosphatases including the tyrosine phosphatases SHP-1 and SHP-2 and the inositol phosphatase SHIP that attenuate ITAM-induced signaling by dephosphorylating and thereby inactivating signaling intermediates. One surprise that has emerged from recent work is that ITAMs can also generate inhibitory signals and attenuate signaling by heterologous receptors3,5,10,11. Although specific ITAM-associated receptors may be predominantly inhibitory, several experimental systems show that the same ITAM-coupled receptors can generate both positive and negative signals. For example, DAP12-associated TREMs can either synergize with TLRs in augmenting cytokine production and associated toxicity or, in a different context, can inhibit TLR-induced cytokine production12–15. FcRγ-associated FcαR (receptor for immunoglobulin A) can activate cells but also cross-inhibits several other receptors, including FcγRs, the tumor necrosis factor receptor (TNFR) and TLRs16–18. Mechanisms by which ITAM-coupled receptors generate inhibitory signals and the molecular basis of the ‘switch’ between pro- and anti-inflammatory ITAM signaling are not well understood. The avidity of receptor ligation may determine the nature of the signal that is generated, and thus ligand density governs the switch in ITAM function3,10.
ITAM-associated receptors typically do not function in isolation; instead, they cooperate with heterologous receptors to achieve cell responses most appropriate for the microenvironment1,2,19. Such cooperation can either augment or dampen signals induced by other receptors. In lymphocytes, antigen receptor–induced signals are integrated with signals from co-stimulatory receptors such as CD28 and cytokine receptors such as the interleukin (IL)-2 receptor to achieve full cell activation. Recent work has highlighted ITAM-mediated cross-regulation of TLRs, cytokine receptors that use the Jak–STAT pathway, and TNF family receptors3,5,10,11. Such cross-regulation can promote cell activation or homeostatic regulation, depending on cell context. Mechanisms of cross-regulation of heterologous receptors by ITAM-mediated signaling and the biological importance of this signaling cross-talk are discussed in this review.
Regulation of TNFR family responses
ITAM-associated receptors have been shown to regulate signaling by the TNFR family members receptor activator of NF-κB (RANK) and BLyS receptor 3 (BR3, also called BAFFR). RANK is required for differentiation of myeloid cells into osteoclasts—multinucleated cells that are specialized for bone resorption. Osteoclast generation is increased in conditions of inflammation, and osteoclast-mediated bone lysis contributes significantly to pathogenesis of autoimmune and inflammatory diseases such as rheumatoid arthritis20–22. TNFR family members signal through TRAF adaptor molecules, and RANK uses TRAF6 to activate NF-κB and MAPK pathways (Fig. 1). These TRAF6-dependent pathways, and in particular MAPK-induced Fos transcription factor, contribute to induction of expression of NFATc1, which functions as a master regulator of osteoclast differentiation and is required for osteoclastogenesis21,23,24. However, RANK-induced TRAF6-dependent activation of NF-κB and MAPK pathways is insufficient to activate NFATc1 responses, because nuclear translocation, and thus function, of NFATc1 depends on dephosphorylation by the calcium-dependent phosphatase calcineurin. Thus, activation of NFATc1 requires calcium signals that activate calcineurin. In myeloid cells, these calcium signals are provided by co-stimulatory ITAM-associated receptors that activate PLCγ, calcium oscillations and post-translational modification of NFATc1 (refs. 8,21,23,25–28) (Fig. 1). ITAM-associated receptors that have been implicated in providing co-stimulation for RANK signaling in osteoclastogenesis include DAP12-associated TREM-2, SIRPβ and αVβ3 integrins, and FcRγ-associated PIR-A and OSCAR. Deficiency in DAP12 and FcRγ results in diminished osteoclastogenesis and thus osteopetrosis (high bone mass) in mice26,28–30. DAP12 and TREM-2 are implicated in bone remodeling and osteoclastogenesis in humans31–33. When these ITAM-associated receptors provide a calcium signal that activates NFATc1 nuclear translocation, NFATc1 amplifies expression of its own promoter (Fig. 1), leading to high NFATc1 expression, induction of osteoclast-related genes, and osteoclast differentiation34.
Cooperation of RANK and ITAM-associated receptor signals to activate NFATc1 provides a good example of integration of signals from different receptors to achieve specific cell functions. Recent work has revealed that dual signals from RANK and ITAM-associated receptors are required to activate PLCγ and generate an effective calcium signal27,35,36. Thus, RANK and ITAM signaling are integrated not only distally, at the level of NFATc1 activation (Fig. 1, integration point 1), but also proximally, at the level of PLCγ activation (Fig. 1, integration point 2). Activation of PLCγ requires formation of a signaling complex that includes the BLNK and SLP-76 adaptors, Btk and Tec tyrosine kinases, and PLCγ (refs. 35–38). RANK contributes to PLCγ activation by activating Btk and Tec35,36. RANK-mediated activation of Btk and Tec is independent of ITAM-associated receptors and also independent of TRAF-mediated signaling and instead probably proceeds by a Src kinase-dependent pathway (Fig. 1). ITAM-associated receptors contribute to PLCγ activation by Syk-mediated phosphorylation of BLNK and SLP-76, which promotes assembly of a Btk-, Tec- and PLCγ-containing ‘signalosome’ and Btk-and Tec-mediated phosphorylation and activation of PLCγ. Syk can also contribute to Btk and Tec activation, further underscoring the tight coupling of RANK and ITAM signaling pathways.
There is also evidence suggesting a third level of cross-talk, namely RANK-induced and Src-mediated transactivation of ITAM-associated receptors that leads to increased phosphorylation of DAP12 and FcRγ ITAM motifs and Syk activation26,27 (Fig. 1, dotted line, integration point 3). In this model, RANK provides the most upstream signal, which results in TRAF6-dependent activation of NF-κB and MAPKs, Src-dependent activation of Btk and Tec, and transactivation of ITAMs that results in calcium signaling. The mechanisms that mediate trans-activation of ITAM-associated receptors are not known. This model does not fully accommodate a role for ITAM-associated receptors and their ligands and does not explain the presence of basal DAP12 and PLCγ phosphorylation in osteoclast precursors; indeed, RANK-induced DAP12 and PLCγ phosphorylation seems modest as these signals are readily detected only when basal phosphorylation is diminished by serum and macrophage colony stimulating factor (M-CSF) starvation, and acute RANK-induced calcium fluxes are difficult to measure23,26,27.
An alternative model, not mutually exclusive, is that ITAM signaling is regulated by ligation of ITAM-associated receptors and, in myeloid cells, is tonically active secondary to constitutive engagement of ITAM-associated receptors. These receptors are engaged by mostly unknown ligands expressed on myeloid cells themselves or on interacting stromal and osteoblast-lineage cells. The presence of constitutive ITAM-associated receptor ligation and tonic ITAM and calcium signaling are supported by observations that DAP12 and FcRγ are basally tyrosine phosphorylated, that basal calcium concentrations are elevated, and that Syk and calcium-dependent Pyk2 are basally active in myeloid cells (refs. 28,39–41 and K.-H. Parkmin and L.B.I., unpublished data). In this model, low-avidity constitutive receptor ligation and tonic ITAM signaling ‘license’ differentiation of myeloid cells into noninflammatory osteoclasts in response to RANK by providing a signal that activates NFATc1 function. The amplitude of this signal can be further ‘fine-tuned’ by regulation of expression of ITAM-associated receptors and their ligands and by transactivation of ITAMs by RANK. At the same time that this tonic ITAM signaling promotes osteoclastogenesis, it suppresses inflammatory TLR signaling (see below) and thereby suppresses differentiation of inflammatory macrophages. Thus, ITAM-associated receptor signaling is implicated in cell fate decisions in the late stages of differentiation in the myeloid lineage.
With the exception of ECM components that engage DAP12-associated αVβ3 integrins and M-CSF and IL-34, which engage c-Fms, ligands for ITAM-associated receptors important in osteoclastogenesis have not been definitively identified. Emerging evidence suggests that TREM-2 can be activated indirectly by ligands for receptors that interact with TREM-2. For example, TREM-2 associates with plexin A receptors and TREM-2–DAP12 signaling is activated by the plexin A1 ligand Semaphorin 6D (ref. 42). Indirect activation of ITAM signaling by Semaphorin 6D is important not only in osteoclast differentiation but also in regulating cytokine production and antigen-presenting function of dendritic cells (which express TREM-2 and plexin A1). In addition, TREM-2–DAP12 signaling is activated in myeloid dendritic cells by PD-L2 (also called B7-DC), a molecule important in mediating effects of dendritic cells on T cell phenotype43. Thus, ITAM signaling can be triggered by various receptors and ligands important in immune responses, and in turn, ITAM signaling modulates the function of the TNFR family member RANK and myeloid cell activation and differentiation. It is clear that ITAM-associated receptors are enmeshed in complex signaling networks, and a systems approach may be helpful in dissecting their regulation and function.
A fruitful area for future research is investigation of ITAM-mediated regulation of other members of the TNFR family. A recent report showed that tonic ITAM signaling by the BCR modulates signaling by the TNFR family member BLyS receptor-3 (ref. 44). Tonic BCR-mediated ITAM signaling induces expression of the noncanonical NF-κB pathway factor p100, which serves as a substrate for activation by BR3 and promotes cell survival. Noncanonical NF-κB signaling is important in mediating cellular responses to various TNFR family members, including RANK45,46. Thus, it is likely that ITAM-associated receptors regulate various TNFR family members by several mechanisms.
Integration with TLR responses
Many microbial products and complex inflammatory stimuli contain ligands for both ITAM-associated receptors and TLRs and activate the two receptor systems in tandem. For example, the yeast cell wall preparation zymosan activates cells simultaneously through TLR2 and through the Dectin-1 receptor for β-glucans, which signals through a variant ITAM motif47–49; nucleic acid-containing immune complexes simultaneously stimulate macrophages and dendritic cells through FcRs and TLR9 (ref. 50). Linked activation of these receptors results in synergistic activation of inflammatory cytokine production and enhances antigen-presenting functions. This synergy may be particularly important under conditions of low ligand concentration or may function to achieve specific outcomes—for example, zymosan-induced production of IL-23 and the capacity to promote TH-17 differentiation51. Ligation of ITAM-associated receptors and TLRs by ligands that are not physically linked also increases cell activation. For example, DAP12-coupled TREM-1 synergizes with TLR4 in vitro and in vivo to increase cytokine production and potentiate endotoxemia15. In addition, β2 and β3 integrins, which signal in part through DAP12 and FcRγ, augment TLR-induced cell activation and inflammatory cytokine production52–54.
TLRs activate myeloid cells and induce inflammatory cytokine production through downstream signaling molecules NF-κB, MAPKs and IRFs55. The molecular basis of synergistic induction of cytokine production by ITAM-associated receptors and TLRs seems to be enhanced activation of NF-κB and MAPKs (Fig. 2); as yet, there is no evidence supporting a distinct ITAM-activated pathway that directly activates cytokine genes. Increased activation of NF-κB and MAPKs may occur by several mechanisms—dual activation of signaling molecules upstream of NF-κB and/or MAPKs by distinct signals emanating from ITAMs and TLRs. For example, ITAM-associated receptors and TLRs both activate the signaling adaptor CARD9 (refs. 51,56–60) (Fig. 2). ITAM-associated receptors activate CARD9 by a Syk-dependent pathway that leads to assembly of a CARD9-Bcl-10-MALT1 signaling complex and to downstream NF-κB activation. In contrast, TLRs activate CARD9 through RIP2, leading to down-stream MAPK activation. It is not yet clear why activation of CARD9 by ITAM-associated receptors and TLRs leads to, respectively, NF-κB and MAPK activation and why the role of CARD9 in TLR signaling seems cell-type and context dependent. Synergy may occur secondary to additive activation of NF-κB and MAPKs by distinct signaling pathways emanating from ITAM-associated receptors and TLRs (Fig. 2). One example is activation of a reactive oxygen burst (ROI production) by Dectin-1 and of a TGF-β–activated kinase (TAK1)-mediated signal by TLR2 that work together to superactivate NF-κB48 (Fig. 2). ROI generation is dependent on PKC, which can also activate NF-κB through the adaptor CARMA1 (Fig. 2); this pathway is used in a cell type–specific manner. Recent evidence suggests that Dectin-1 also modulates TLR-mediated NF-κB activation by inducing acetylation of the NF-κB p65 subunit by a Raf-1 dependent pathway61. TLRs activate MAPKs through TAK1- and CARD9-mediated pathways, whereas ITAM-associated receptors activate MAPKs through DAG- and calcium-mediated pathways (Fig. 2). Thus, additive and integrated activation of MAPKs by these distinct pathways results in enhanced activation of downstream signaling molecules and transcription factors. Direct interactions between ITAM-associated receptors and TLRs can augment signaling (Fig. 2, top). β3 integrins interact with TLR2 and augment TLR2-induced signaling and gene activation, possibly by facilitating presentation of microbial ligands to TLR2 (ref. 53). In addition to direct receptor interactions, ITAM-associated receptors can control the function of TLRs. β2 integrins augment TLR4 signaling by generating phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5) P2), which serves to recruit the signaling adaptor TIRAP (also called Mal)54. Integrins can also modulate signaling by controlling the formation, trafficking and disassembly of microclusters of signaling molecules62. It is not known which, if any, of the mechanisms by which integrins augment TLR signaling are dependent on DAP12 or FcRγ—integrins activate distinct signaling pathways through signaling motifs present in their cytoplasmic domains, and these ITAM-independent signals may function to modulate TLR responses.
The overall picture emerging is that ITAM-associated receptors augment TLR signaling through several mechanisms, thereby allowing regulation over a broad amplitude of response. Recent research indicates that subcellular localization of receptors can be important in cross-talk between ITAM-associated receptors and TLRs. Activated BCRs traffic to an autophagosome-like compartment and recruit TLR9-containing endosomes to autophagosomes by a phospholipase D–dependent mechanism, and subsequent BCR-TLR interactions result in hyperactivation of MAPKs63. ITAM signaling may also modulate the function of other pattern-recognition receptors, as Syk-mediated signaling is required for dendritic-cell activation by, and high-affinity interaction with, monosodium urate crystals that precipitate gout attacks64. As monosodium urate crystals subsequently activate Nod-like receptors, leading to inflammasome activation and caspase-1–mediated processing of IL-1, these findings suggest a role for ITAM signaling in modulating Nod-like receptor and inflammasome signaling and function. Thus, it seems that ITAM-associated receptors cooperate with many receptors that sense and respond to infectious pathogens and inflammatory stimuli.
Inhibition of TLR responses
ITAM-associated receptors can also dampen TLR signaling. Constitutive ligation of DAP12-associated TREM-2 by as yet unknown macrophage– and dendritic cell–expressed ligands leads to tonic low-level ITAM signaling that attenuates cytokine production induced by TLRs12–14,65. This negative regulation of TLR responses is physiologically important, as DAP12-deficient mice show increased cytokine production and increased toxicity in the D-galactosamine-potentiated endotoxemia model13. However, the role of DAP12 in mediating cytokine production and inflammation in vivo is complex, as DAP12 deficiency is protective in LPS-induced endotoxemia and in the cecal puncture and ligation model of bacterial peritonitis15. The mechanisms by which TREM-2 and DAP12 inhibit TLR signaling have not been identified, although DAP12 seems to regulate TLR-induced activation of the kinase ERK. Insight into negative regulatory mechanisms has been provided by findings that low-avidity ligation of FcRγ-coupled FcαR (receptor for immunoglobulin A) by monovalent ligands cross-inhibits signaling by FcγRs, CCR2, TNFRs and TLRs16–18. In contrast, high-avidity multivalent ligation of FcαR using antibodies or immune complexes activates inflammatory signaling. A molecular explanation for these observations is suggested by the findings that low-avidity FcαR engagement recruits SHP-1, which dephosphorylates and inactivates heterologous receptors and signaling intermediates. In contrast, high-avidity ligation recruits Syk and activates positive signaling17. These findings have led to a model whereby the activating versus inhibitory function of an ITAM-coupled receptor is determined by avidity of ligation and the resulting pattern of partial or complete ITAM phosphorylation and differential recruitment of signaling molecules. However, this model cannot explain all of the reported observations—for example, the inhibition of TLR9 responses in plasmacytoid dendritic cells by high-avidity antibody-mediated cross-linking of FcRγ-associated ILT7 (ref. 66). These findings point to alternative and complementary mechanisms by which ITAM-associated receptors inhibit TLRs, such as inhibition of TLR signaling by the ITAM-activated phosphatase calcineurin39 or by PLCγ-mediated dephosphorylation of PtdIns(4,5)P2 (ref. 54), leading to diminished TIRAP recruitment to the plasma membrane. These various inhibitory mechanisms have been discussed in recent reviews3,5,10,11.
A hallmark of signal transduction is the induction of feedback inhibitory pathways and molecules that limit cell activation67,68; such feedback inhibitors have the potential to cross-inhibit heterologous receptors. For example, ITAM-associated receptors induce suppressors of cytokine signaling (SOCS proteins)69,70 in a manner dependent on NF-κB and MAPKs70,71 and high-avidity ITAM-associated receptor ligation, in distinction to the low-avidity ligation–dependent inhibitory mechanisms discussed above. In addition, FcγRs synergize with TLRs to induce production of the anti-inflammatory cytokine IL-10, which can cross-inhibit TLR responses72,73. FcγR-induced and ERK-mediated phosphorylation of histone-3 on Ser10 promotes increased chromatin accessibility at the Il10 locus, which facilitates recruitment of TLR-induced transcription factors74,75. A similar ERK-mediated mechanism is likely to explain the large amounts of IL-10 that are produced by dendritic cells in response to zymosan76. The induction of signaling inhibitors by ITAM-mediated pathways is an important mechanism for suppressing inflammation, and it will be important to fully characterize the inhibitors that are induced and proximal signaling events required for their induction.
Regulation of cytokine Jak–STAT responses
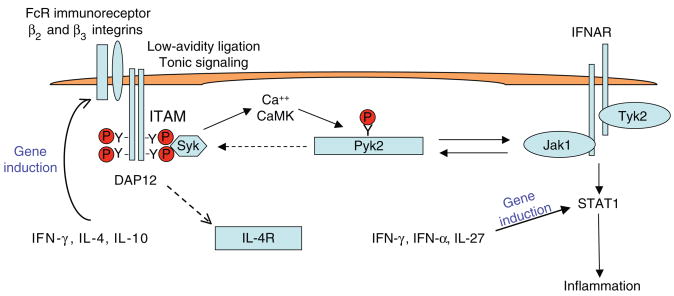
Integration of signaling by ITAM-associated receptors and interferon (IFN) receptors that augments activation of transcription factor STAT1 and expression of downstream inflammatory target genes was recently reported40,41. Enhanced IFN receptor signaling was observed under conditions of tonic ITAM signaling generated in part by integrin-mediated cell adhesion. Tonic ITAM signaling maintains constitutive activity of a signaling pathway dependent on DAP12 and Syk that leads to basal calcium-dependent activity of calmodulin-dependent kinase (CaMK) and Pyk2 (Fig. 3). Pyk2 relays the ITAM signal by interacting with and amplifying the activation of Jak kinases associated with IFN receptors. In addition to integrins, FcγRs play a positive role in enhancing IFN-α signaling77, and thus various ITAM-associated receptors have the potential to augment IFN signaling. Furthermore, integration of DAP12 and IL-4 signaling, and activation of FcRγ by the IL-3 receptor, have recently been demonstrated78,79, suggesting more extensive integration of signaling by ITAM-associated receptors and receptors for various cytokines that use the Jak–STAT pathway.
Figure 3.
Integration of ITAM and cytokine Jak–STAT signaling. Low-avidity ligation of ITAM-associated receptors leads to tonic calcium signaling that maintains basal activity of a CaMK–Pyk2 pathway that amplifies Jak activity and enhances downstream activation of STAT1. Cytokine receptors and Jaks also activate Pyk2, and Pyk2 can thus relay signals between ITAM-associated receptors and cytokine receptors bidirectionally. ITAM-containing DAP12 also modulates cell responses to IL-4. Cytokines such as IFN-γ, IFN-α, IL-4, IL-10 and IL-27 regulate expression of ITAM-associated receptors and STAT1, and thus cytokine and ITAM-associated receptor signaling pathways are tightly integrated. Adapted from Nat. Immunol. 9, 186–193 (2008).
Early in vitro experiments demonstrated that, in contrast to tonic ITAM signaling, high-avidity ligation of TCRs inhibits signaling by the IL-2 and IL-4 receptors, which share a common γ signaling subunit (γc), and by IFN-α and IL-6 receptors80,81. The physiological importance of such inhibition is supported by the recent in vivo demonstration of a phenomenon termed ‘coreceptor tuning’82. In ‘coreceptor tuning’, TCR ligation inhibits signaling by the IL-7 receptor, which uses γc, and thereby suppresses IL-7–induced expression of the co-receptor CD8. This mechanism ensures that CD8+ T cells do not express amounts of CD8 high enough to lead to autoreactivity. Cell surface expression of cytokine receptors is not affected by TCR cross-linking. Instead, activation of receptor-associated Jak kinases, the most proximal step in the Jak–STAT signaling pathway, is inhibited by PKC- and ERK-dependent pathways80,81. Inhibition of Jaks is induced rapidly in the absence of de novo protein synthesis and thus occurs by a direct pathway.
Additional studies in myeloid cells have shown rapid and direct inhibition of IL-10 receptor (IL-10R) and IFN-α receptor (IFNAR) signaling after high-avidity ligation of FcγRs or receptors for zymosan69,83,84. Like inhibition of cytokine signaling in T cells, inhibition of IFNAR signaling in macrophages is PKC dependent. Activation of PKC inhibits IFN-α–induced Jak phosphorylation by inducing recruitment of the phosphatase SHP-2 to the IFNAR receptor complex. IL-10R signaling is also inhibited by a PKC-dependent mechanism, but in this case cell surface expression IL-10R is down-regulated, probably by internalization. Thus, rapidly induced, direct inhibition of cytokine signaling by ITAM-coupled receptors is mediated by PKC and targets cytokine receptors and proximal steps in Jak–STAT signal transduction. The negative role of PKC in inhibiting cytokine signaling contrasts with its positive role in augmenting TLR signaling. Sustained activation of FcγRs also leads to delayed inhibition of IFNγ signaling that seems to be independent of ITAM signaling and is mediated by internalization of the IFNγ receptor85. Another main mechanism of inhibition of cytokine signaling occurs through induction of expression of SOCS proteins, which interact with phosphotyrosine residues in receptor cytoplasmic domains, compete with STATs for binding to receptor docking sites, inactivate Jak catalytic activity and target receptors for proteasomal degradation70. High-avidity ligation of ITAM-coupled receptors induces SOCS3 expression69,70,80, and it is likely that SOCS3 contributes to inhibition of cytokine signaling at later time points, after engagement of ITAM-associated receptors.
Overall, ITAM-associated receptors seem to augment cytokine signaling under conditions of tonic ITAM signaling that occur under physiological conditions of homeostasis. Such amplification of cytokine signaling confers on macrophages the capacity to respond to low concentrations of cytokines produced under physiological conditions and to respond rapidly and strongly to cytokines produced by changes in the environment, such as challenges produced by stress or infection. In contrast, strong ligation of ITAM-associated receptors that by itself activates macrophages attenuates cell responses to cytokines and thereby prevents excessive activation and associated toxicity.
Regulation of ITAM signaling
Cross-talk among signaling pathways allows for bidirectional regulation. For example, IFN-γ induces expression of FcγRI and TNF induces expression of PIR-A, thereby amplifying signaling by ITAM-mediated pathways86,87. In addition, TLRs induce expression of ZAP70, a tyrosine kinase closely related to Syk, and thus can augment ITAM-dependent signaling88. Notably, TNF modulates signaling in response to immune complexes by increasing expression of ‘activating’, ITAM-containing FcγRIIA while decreasing expression of ‘inhibitory’, ITIM-containing FcγRIIB, thereby altering the balance of activatory and inhibitory signals89,90. Several other cytokines regulate expression of ITAM-coupled receptors, including IL-4 and IL-10, and we have found extensive remodeling of the pattern of ITAM-associated receptor expression during maturation of human monocytes to macrophages (L.B.I., unpublished data). These cytokine- and differentiation-induced changes in expression of ITAM-associated receptors will affect the avidity of receptor ligation and also will alter the pattern of ligands to which cells are responsive, and thus will qualitatively change cell responses to the environment.
Less is known about direct mechanisms that regulate ITAM signaling, such as the RANK-induced transactivation of ITAM phosphorylation discussed above. Recently, a direct association of the IL-3 receptor with FcRγ has been demonstrated; this association couples IL-3 to the activation of Syk and channels IL-3 signals into ITAM-mediated pathways79. Activation of Pyk2 by cytokines and Jaks91,92 (Fig. 3) suggests that cytokine signaling also modulates farther downstream components of ITAM signaling pathways. Association of several more cytokine receptors with heterologous receptors or signaling adaptors, such as IL-15R with DAP10 (ref. 93), G-CSF receptor with integrin α9β1 (ref. 94), and IFNAR with TAM receptors95, suggests that direct association of receptors is a common mechanism that underlies cytokine receptor and ITAM-associated receptor cross-talk.
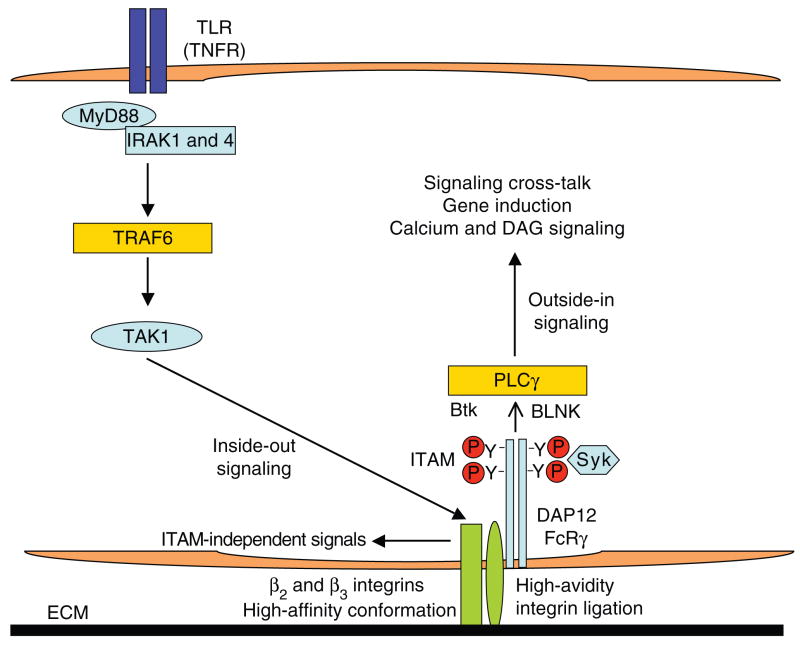
In addition, TLRs and cytokines potentially can regulate integrin-induced ITAM-dependent and ITAM-independent signals. TLRs and cytokines activate integrins to assume a high-affinity ligand-binding conformation by a process termed inside-out signaling37,96 (Fig. 4). Inside-out signaling is now appreciated to result in increased adhesion to ECM, cell spreading and enhanced cell–cell interactions. This increased affinity of integrin–ligand interactions will lead to increased avidity of integrin ligation, which will in turn generate an integrin-mediated ‘outside-in’ signal. In the case of β2 and β3 integrins, such outside-in signals will include signaling through associated DAP12 and FcRγ, with downstream activation of Syk and calcium signaling (Fig. 4). In support of this model, TNF-induced activation of Syk that is indirect and mediated by β2 integrins has been reported97. Additional direct interactions between integrins and TLRs, as exemplified by β3 integrin–TLR2 interactions53 and β2 integrin–TLR4 interactions54, are also likely to modulate ITAM-mediated signaling.
Figure 4.
Indirect activation of calcium signaling by TLRs and TNFR through integrins. Engagement of TLRs and TNFRs activates signaling pathways that induce a conformational change in integrins (in a process termed inside-out signaling), leading to high-affinity ligand binding and associated high-avidity ligation of integrins. High-avidity integrin ligation results in outside-in signaling that activates integrin-mediated signaling pathways (which in the case of β2 and β3 integrins includes activation of DAP12- and FcRγ-mediated signaling).
Overview and biology
Many canonical signaling pathways are conserved across several cell types and are capable of acting in isolation to elicit cellular responses. For example, the conserved core Jak–STAT signaling pathway, which operates in many cell types, proceeds to the nucleus in a linear, unbranched manner and elicits a relatively focused cellular response by selectively activating downstream effector molecules and target genes. In contrast, ITAM-mediated signals, which operate only in hematopoietic cells, proceed in a branching manner and activate several signaling cascades and second messengers (Fig. 2); in addition, ITAM signals are typically not sufficient in isolation to induce full cellular activation or differentiation. What are the benefits of a system in which a receptor can activate several branching pathways but by itself is not capable of eliciting cell activation or differentiation?
One advantage of a requirement for integration among heterologous receptor systems is that cells are not likely to be inappropriately activated by one receptor system, as activation requires two or more signals from the environment. Such regulation is particularly important for immune cells that need to interpret signals in the context of ‘danger’ and adopt an appropriate response. The TCR, BCR and FcRs are involved in recognition of foreign antigens and pathogens, and it is important that cells do not become activated in the absence of further evidence of infection, such as that provided by pathogen-induced cytokines or by sensing of pathogens by TLRs. It is also important to avoid inappropriate cell fate decisions—for example, differentiation of osteoclasts in soft tissues where they are not necessary and could be harmful20. Another advantage of integrating ITAM pathways into signaling networks is that this confers upon ITAM-associated receptors the capacity to ‘fine tune’ the amplitude and qualitative nature of cellular responses to heterologous receptors. In this formulation, proposed by M. Colonna19, ITAM-associated receptors sense the environment and modulate cellular responses to ensure they are appropriate for that environment.
ITAM signaling activates several DAG- and calcium-dependent signaling molecules, such as PKC, CaMKs, Pyk2 and calcineurin-NFAT, that are not strongly activated by other immune cell signaling pathways. It is precisely these PLCγ- and calcium-dependent molecules that have been implicated in cross-talk between ITAM-mediated and other signaling pathways. Thus, calcium signaling plays a prominent role in connecting signaling networks in immune cells.
Acknowledgments
I thank M. Nakamura for discussions and X. Hu and K.-H. Park-Min for critical review of the manuscript. Supported by the US National Institutes of Health.
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;2007:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 2.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 3.Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- 4.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8:816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abtahian F, et al. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–6949. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocsai A, et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W, et al. Syk, c-Src, the αvβ3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 11.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Hamerman JA, et al. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 13.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull IR, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull IR, et al. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–369. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanamaru Y, et al. Inhibitory ITAM signaling by FcαRI-FcRγ chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008;180:2669–2678. doi: 10.4049/jimmunol.180.4.2669. [DOI] [PubMed] [Google Scholar]
- 17.Pasquier B, et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRγ ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro da Silva F, et al. CD16 promotes Escherichia Coli sepsis through an FcRγ inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- 19.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 22.Teitelbaum SL. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res Ther. 2006;8:201. doi: 10.1186/ar1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 24.Aliprantis AO, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphrey MB, et al. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 26.Koga T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 27.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCγ2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocsai A, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaifu T, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nataf S, et al. Brain and bone damage in KARAP/DAP12 loss-of-function mice correlate with alterations in microglia and osteoclast lineages. Am J Pathol. 2005;166:275–286. doi: 10.1016/S0002-9440(10)62251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella M, et al. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paloneva J, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 33.Paloneva J, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asagiri M, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008;283:11526–11534. doi: 10.1074/jbc.M708935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara M, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 37.Bezman N, Koretzky GA. Compartmentalization of ITAM and integrin signaling by adapter molecules. Immunol Rev. 2007;218:9–28. doi: 10.1111/j.1600-065X.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 38.Epple H, et al. Phospholipase Cγ2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol Cell Biol. 2008;28:3610–3622. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YJ, et al. Calcineurin negatively regulates TLR-mediated activation pathways. J Immunol. 2007;179:4598–4607. doi: 10.4049/jimmunol.179.7.4598. [DOI] [PubMed] [Google Scholar]
- 40.Tassiulas I, et al. Amplification of IFN-α-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–1189. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, et al. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008;9:186–193. doi: 10.1038/ni1548. [DOI] [PubMed] [Google Scholar]
- 42.Takegahara N, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 43.Radhakrishnan S, et al. TREM-2 mediated signaling induces antigen uptake and retention in mature myeloid dendritic cells. J Immunol. 2008;181:7863–7872. doi: 10.4049/jimmunol.181.11.7863. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Stadanlick JE, et al. Tonic B cell antigen receptor signals supply an NF-κB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita T, et al. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 46.Vaira S, et al. RelB is the NF-κB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci USA. 2008;105:3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown GD, et al. Dectin-1 mediates the biological effects of β-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers NC, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Boule MW, et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 52.Cuzzola M, et al. β2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J Immunol. 2000;164:5871–5876. doi: 10.4049/jimmunol.164.11.5871. [DOI] [PubMed] [Google Scholar]
- 53.Gerold G, et al. A Toll-like receptor 2-integrin β3 complex senses bacterial lipopeptides via vitronectin. Nat Immunol. 2008;9:761–768. doi: 10.1038/ni.1618. [DOI] [PubMed] [Google Scholar]
- 54.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 55.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 56.Gross O, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 57.Gross O, et al. Multiple ITAM-coupled NK-cell receptors engage the Bcl10/Malt1 complex via Carma1 for NF-κB and MAPK activation to selectively control cytokine production. Blood. 2008;112:2421–2428. doi: 10.1182/blood-2007-11-123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara H, et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8:619–629. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 59.Hara H, et al. Cell type-specific regulation of ITAM-mediated NF-κB activation by the adaptors, CARMA1 and CARD9. J Immunol. 2008;181:918–930. doi: 10.4049/jimmunol.181.2.918. [DOI] [PubMed] [Google Scholar]
- 60.Hsu YM, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 61.Gringhuis SI, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin vLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng G, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu CL, et al. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRγ. Eur J Immunol. 2008;38:166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao W, et al. Plasmacytoid dendritic cell–specific receptor ILT7-FcεRI γ inhibits Toll-like receptor–induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 68.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of IFN and TLR signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji JD, et al. Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med. 2003;197:1573–1583. doi: 10.1084/jem.20021820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 71.Bode JG, et al. The MKK6/p38 mitogen-activated protein kinase pathway is capable of inducing SOCS3 gene expression and inhibits IL-6-induced transcription. Biol Chem. 2001;382:1447–1453. doi: 10.1515/BC.2001.178. [DOI] [PubMed] [Google Scholar]
- 72.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 73.Polumuri SK, Toshchakov VY, Vogel SN. Role of phosphatidylinositol-3 kinase in transcriptional regulation of TLR-induced IL-12 and IL-10 by Fcγ receptor ligation in murine macrophages. J Immunol. 2007;179:236–246. doi: 10.4049/jimmunol.179.1.236. [DOI] [PubMed] [Google Scholar]
- 74.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcγR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Edwards JP, Mosser DM. Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription. J Immunol. 2006;177:1282–1288. doi: 10.4049/jimmunol.177.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dillon S, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhodapkar KM, et al. Selective blockade of the inhibitory Fcγ receptor (FcγRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Helming L, et al. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci Signal. 2008;1:ra11. doi: 10.1126/scisignal.1159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hida S, et al. Fc receptor γ-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009;10:214–222. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]
- 80.Lee IH, Li WP, Hisert KB, Ivashkiv LB. Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J Exp Med. 1999;190:1263–1274. doi: 10.1084/jem.190.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu J, et al. Transient inhibition of interleukin 4 signaling by T cell receptor ligation. J Exp Med. 2000;192:1125–1134. doi: 10.1084/jem.192.8.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park JH, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 83.Du Z, et al. Selective regulation of IL-10 signaling and function by zymosan. J Immunol. 2006;176:4785–4792. doi: 10.4049/jimmunol.176.8.4785. [DOI] [PubMed] [Google Scholar]
- 84.Du Z, et al. Inhibition of IFN-α signaling by a PKC- and protein tyrosine phosphatase SHP-2-dependent pathway. Proc Natl Acad Sci USA. 2005;102:10267–10272. doi: 10.1073/pnas.0408854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park-Min KH, et al. FcγRIII-dependent inhibition of interferon-γ responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 86.Ochi S, et al. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proc Natl Acad Sci USA. 2007;104:11394–11399. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pearse RN, Feinman R, Ravetch JV. Characterization of the promoter of the human gene encoding the high-affinity IgG receptor: transcriptional induction by γ-interferon is mediated through common DNA response elements. Proc Natl Acad Sci USA. 1991;88:11305–11309. doi: 10.1073/pnas.88.24.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bekeredjian-Ding I, et al. TLR9-activating DNA up-regulates ZAP70 via sustained PKB induction in IgM+ B cells. J Immunol. 2008;181:8267–8277. doi: 10.4049/jimmunol.181.12.8267. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, et al. Cytokine-mediated regulation of activating and inhibitory Fcγ receptors in human monocytes. J Leukoc Biol. 2005;77:767–776. doi: 10.1189/jlb.0904532. [DOI] [PubMed] [Google Scholar]
- 90.Belostocki K, et al. Infliximab treatment shifts the balance between stimulatory and inhibitory Fcγ receptor type II isoforms on neutrophils in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:384–388. doi: 10.1002/art.23200. [DOI] [PubMed] [Google Scholar]
- 91.Miyazaki T, et al. Pyk2 is a downstream mediator of the IL-2 receptor-coupled Jak signaling pathway. Genes Dev. 1998;12:770–775. doi: 10.1101/gad.12.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takaoka A, et al. Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-γ but not IFN-α, signaling. EMBO J. 1999;18:2480–2488. doi: 10.1093/emboj/18.9.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 2007;8:1345–1352. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- 94.Chen C, et al. The integrin α9β1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity. 2006;25:895–906. doi: 10.1016/j.immuni.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 95.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 96.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Avdi NJ, et al. Tumor necrosis factor-α activation of the c-Jun N-terminal kinase pathway in human neutrophils. Integrin involvement in a pathway leading from cytoplasmic tyrosine kinases apoptosis. J Biol Chem. 2001;276:2189–2199. doi: 10.1074/jbc.M007527200. [DOI] [PubMed] [Google Scholar]