Abstract
Background and Purpose
Though clinical cardiovascular and cerebrovascular diseases are established risk factors for cognitive decline and dementia, less is known about the relations between vascular health and cognition among individuals without these diseases. Carotid intimal medial thickness (IMT), a measure of subclinical vascular disease, is associated with concurrent decrements in cognitive function, but relatively little research has examined longitudinal relations between carotid IMT and prospective cognitive decline.
Methods
We examined relations of carotid IMT to prospective trajectories of cognitive function among 538 (aged 20-93, 39% male, 66% white) participants in the Baltimore Longitudinal Study of Aging (BLSA) free of known cardiovascular, cerebrovascular, and neurological disease. Participants underwent initial carotid ultrasonography and repeat neuropsychological testing on up to eight occasions over up to 11 years of follow-up. Mixed-effects regression analyses were adjusted for age, gender, race, education, mean arterial pressure, body mass index, total cholesterol, smoking, depressive symptoms, and cardiovascular medication use.
Results
Individuals with greater carotid IMT displayed accelerated decline in performance over time on multiple tests of verbal and nonverbal memory, as well as a test of semantic association fluency and executive function.
Conclusions
Carotid IMT predicts accelerated cognitive decline, particularly in the domain of memory, among community-dwelling individuals free of vascular and neurological disease.
Keywords: carotid intimal medial thickness, subclinical vascular disease, atherosclerosis, cognitive function, neuropsychology
Clinical cardiovascular and cerebrovascular diseases are established risk factors for cognitive decline and dementia.1, 2 Reduced cerebral perfusion, metabolic dysfunction, a proinflammatory state, and/or shared genetic, behavioral, or cardiovascular factors may account for these associations.2, 3 Relations between vascular health and cognition among individuals without clinical cardiovascular diseases remain less explored. Measures of subclinical disease allow assessment of vascular risk in asymptomatic individuals, providing opportunity for early intervention.4 Carotid artery intimal medial thickness (IMT), a measure of subclinical vascular disease, predicts future clinical coronary events, coronary heart disease deaths, stroke, frailty, physical decline, and all-cause mortality.5, 6 Carotid IMT has also been associated with concurrent decrements in cognitive function among community and clinical samples,7, 8 although findings are equivocal.7
Longitudinal research linking carotid IMT with prospective cognitive function is more limited. A small number of studies have identified longitudinal relations in population-based samples,9, 10 but these associations have been restricted largely to performance on brief cognitive screening measures such as the Mini Mental State Examination (MMSE). Knopman and colleagues found no evidence for an association between carotid IMT and cognitive decline over two time points among middle-aged adults.11
We investigated relations of carotid IMT to trajectories of cognitive function among participants in the Baltimore Longitudinal Study of Aging (BLSA) free of known cardiovascular, cerebrovascular, and neurological disease. Participants completed neuropsychological testing on up to eight occasions over a span of up to 11 years of follow-up. We examined the association between carotid IMT and prospective performance on two cognitive screening measures and an extensive battery of neuropsychological tests assessing verbal and nonverbal memory, attention, concentration, perceptuo-motor speed, executive functions, language, and visuospatial function. To account for potential confounding by variables highly related to carotid IMT and/or neuropsychological outcomes,12, 13 we included age, education, gender, race, smoking, mean arterial pressure (MAP), body mass index (BMI), total cholesterol, and depressive symptoms as covariates in all analyses.
Materials and Methods
Participants
Participants were enrolled in the BLSA, a prospective study of community-dwelling volunteers initiated in 1958. Approximately every 2 years, participants visit the National Institute on Aging in Baltimore for medical, psychological, and cognitive testing. Data collection for the present study began in 1994 when carotid ultrasonography was introduced to the BLSA protocol. A total of 804 participants were available for the present study. We excluded participants with coronary artery disease (n=150), congestive heart failure (n=6), peripheral arterial disease (n=30), diabetes (n=42), carotid endarterectomy (n=0), cerebrovascular diseases including stroke (n=23), dementia (n=14; following protocol published previously14), and other neurological diseases (n=1) across all assessment visits. For additional information regarding exclusion procedures, please refer to supplemental Table I, available online at http://stroke.ahajournals.org. Following exclusions, 538 participants were available for analyses. Because the BLSA uses continuous enrollment procedures, participants have differential start times, numbers of visits, and follow-up times in the project (see Table 1). Participants had an average of 2.3 visits (SD=1.3 visits; range=1-8 visits), and the average time between visits was 2.1 years (SD=0.9 years; range=0.5-5.5 years). Participants were followed for up to 11 years (mean=4.0 years; median=4.0 years; SD=3.6 years). Institutional Review Board approval was obtained from the Johns Hopkins Bayview Medical Center before 2002 and the MedStar Research Institute afterwards. All participants provided written informed consent, and all procedures followed were in accordance with institutional guidelines. The Institutional Review Board of the University of Maryland, Baltimore County approved these data analyses.
Table 1.
Sample Size by Number of Visits*
| Number of Visits | n (% of Sample) |
|---|---|
| 1 | 538 (100.0) |
| 2 | 350 (65.1) |
| 3 | 208 (38.7) |
| 4 | 88 (16.4) |
| 5 | 30 (5.6) |
| 6 | 6 (1.1) |
| 7 | 3 (0.6) |
| 8 | 2 (0.4) |
The BLSA is not a fixed cohort; the study uses rolling recruitment. Thus, what appears as attrition are participants who were not in the study long enough for higher numbers of visits.
Carotid Intimal Medial Thickness
High resolution B-mode ultrasonography of the right common carotid artery was performed with a linear-array, 5- to 10-MHz transducer (Ultramark 9 HDI, Advanced Technology Laboratories, Inc., Seattle, Washington). A region 1.5cm proximal to the carotid bifurcation was identified, and the IMT of the far arterial wall was evaluated as the distance between the lumen/intimal interface and the medial/adventitial interface. Specific care was taken to measure IMT in areas devoid of plaque. IMT was measured on a frozen-frame image, magnified to achieve higher resolution of detail. The IMT measurement was obtained from five contiguous sites at approximate 1 mm intervals; the mean of these values was used in statistical analyses. Measurements were performed by a single sonographer. Intra-observer correlation between repeated carotid IMT measurements on 10 BLSA participants was 0.96 (p<.001).15
Neuropsychological Assessment
At each BLSA visit, standard neuropsychological tests12 were administered by highly trained psychometricians. The numbers that follow each test indicate respective sample sizes because of test-specific missing data. The Blessed Information-Memory-Concentration (I-M-C) Test (n=538) and the MMSE (n=257) are cognitive screening measures. The Digits Forward (n=438) and Digits Backward (n=440) portions of the Wechsler Adult Intelligence Scale-Revised assessed attention and concentration. The California Verbal Learning Test (CVLT; n=437) measured verbal learning and memory, including immediate free recall, short- and long-delayed free recall, and learning slope. The Benton Visual Retention Test (BVRT; n=439) and the immediate and delayed recall trials of the Rey Complex Figure Test (RCFT; n=160) evaluated nonverbal memory. The Trail Making Test-Part A (n=257) and Part B (n=251) assessed attention, perceptuo-motor speed, visuomotor scanning, and mental flexibility, an executive function. Letter Fluency (n=256) and Category Fluency (n=255) examined phonemic and semantic association fluency, respectively, and executive function. The Boston Naming Test (n=256) assessed confrontation naming, a language ability. The copy trial of the RCFT measured visuospatial constructional ability, and the Card Rotations Test (n=435) also measured visuospatial function.
Covariates
Covariate data were collected at each BLSA visit. Age (based on self-reported date of birth) and self-reported education were assessed in years. Binary covariates included gender, race (self-reported white, non-white), smoking (ever/never), and use of antihypertensive or lipid-lowering medications (yes/no). The Center for Epidemiological Studies-Depression Scale16 assessed depressive symptomatology. Body mass index (BMI) was calculated as the ratio of weight (in kilograms) to height (in meters) squared. After an overnight fast, blood for lipid assay was drawn from an antecubital vein between 7:00 and 8:00 a.m. while the participant was supine. Concentrations of total cholesterol were determined enzymatically (ABA-200 ATC Biochromatic Analyzer; Abbott Laboratories, Irving, TX). Resting brachial systolic and diastolic blood pressure (SBP, DBP) values were obtained three times bilaterally with participants in the seated position following a 5-minute resting period. SBP and DBP were defined by Korotkoff phases I and V, respectively. Mean arterial pressure (MAP) was calculated as MAP=(2*DBP+SBP)/3, using the mean of the second and third blood pressure measurements.
Data Analyses
Statistical analyses were performed using SAS version 9.1 (Cary, NC). Mixed-effects regression analyses examined longitudinal relations of carotid IMT to cognitive function. This statistical approach handles inconsistent measurement intervals within and across BLSA participants and accounts for the correlation among repeated measurements on the same participants.17 Carotid IMT measures were obtained only at one visit, whereas cognitive function and covariates were assessed at that visit and on up to 8 subsequent occasions. Mixed-effects regression adequately handles variable numbers of data points per participant, as one of its strengths is its use of all available data, regardless of length of individual trajectories. Results demonstrate whether carotid IMT predicts trajectories of age-related change in cognitive performance thereafter.
Each cognitive measure was entered as a single outcome variable in separate sequential mixed-effects regression models. For consistency with prior literature, we first examined the two cognitive screening measures (MMSE, Blessed I-M-C) as outcome variables, although significant results were unexpected due to their limited sensitivity in non-demented samples.12 Primary analyses then examined each neuropsychological test individually. Age, years of education, MAP, BMI, total cholesterol, and depressive symptoms were treated as continuous covariates, and gender, race, smoking, and cardiovascular medications were treated as categorical covariates. Except for gender and race, all covariates were treated as time-varying in all analyses. Age was modeled as a random effect to index time, as is recommended for studies with continuous enrollment procedures.18 Carotid IMT (representing overall differences in carotid IMT regardless of longitudinal changes) and a 2-way interaction of carotid IMT with age (representing longitudinal change in cognitive outcomes associated with carotid IMT over time) served as fixed terms of interest.
Results
Table 2 shows sample characteristics at first assessment. As expected, preliminary mixed-effects regression analyses revealed non-significant findings for Blessed I-M-C and MMSE. In subsequent mixed-effects regression analyses (Table 3), significant interactions of carotid IMT and age (indicating change over time) were found for BVRT (b=0.169, p=.049), CVLT immediate free recall (b=-0.653, p=.026), CVLT long-delay free recall (b=-0.185, p=.024), RCFT immediate recall (b=87.0, p=.035), RCFT delayed recall (b=87.9, p=.026), and Category Fluency (b=-0.373, p=.023). A significant main effect of carotid IMT (that was not qualified by an age interaction) was noted for CVLT short-delay free recall (b=10.4, p=.031). For complete results, please refer to supplemental Table II, available online at http://stroke.ahajournals.org. Of note, because raw tests scores were used in analyses, regression coefficients are not directly comparable across test measures. Several outcome measures, including MMSE, Blessed I-M-C, Boston Naming Test, and Trail Making Test Part B, did not meet regression distributional requirements. Following logarithmic transformation (base 10) of these outcomes and associated resolution of distributional violations, we observed no meaningful changes in results.
Table 2.
Characteristics of Study Sample at First Assessment
| Variable | Mean (SD) | Range |
|---|---|---|
| Age (years) | 54.9 (14.0) | 20-93 |
| Gender (% male) | 39.8 | |
| Race (% white)* | 66.2 | |
| Education (years) | 16.7 (2.5) | 8-23 |
| Mean arterial pressure (mm Hg) | 96.4 (13.3) | 67-137 |
| Total cholesterol (mg/dl) | 198.3 (35.8) | 112-320 |
| Body mass index (kg/m2) | 26.3 (4.5) | 18.1-45.5 |
| Smoking (% ever) | 49.8 | |
| Cardiovascular medication use, % | 20.0 | |
| CES-D (total score) | 6.6 (9.6) | 0-47 |
| Mean carotid IMT (mm) | .49 (.12) | .20-1.0 |
27.3% of participants were African-American, and 6.6% were Asian/Pacific Islander, American Indian, or Other non-white. SD=standard deviation; CES-D=Center for Epidemiological Studies-Depression scale; IMT=intimal medial thickness
Table 3.
Mixed-Effects Regression Models Predicting Neuropsychological Test Performance from Carotid IMT and Covariates
| Carotid IMT | Carotid IMT × Age | |||
|---|---|---|---|---|
| Neuropsychological Test | b | SE | b | SE |
| Benton Visual Retention Test | -9.09 | 5.41 | 0.17* | 0.09 |
| Boston Naming Test | -12.2 | 17.3 | 0.13 | 0.25 |
| Card Rotations Test | 35.7 | 61.2 | -1.19 | 0.92 |
| CVLT, Learning Slope | 1.17 | 0.95 | -0.02 | 0.01 |
| CVLT, Immediate Free Recall | 49.2* | 18.2 | -0.65* | 0.29 |
| CVLT, Short-Delay Free Recall | 10.4* | 4.78 | -0.14 | 0.08 |
| CVLT, Long-Delay Free Recall | 13.0* | 5.15 | -0.19* | 0.08 |
| Digits Forward | 1.35 | 4.19 | -0.04 | 0.06 |
| Digits Backward | 0.73 | 4.59 | -0.02 | 0.07 |
| Category Fluency | 23.8* | 11.4 | -0.37* | 0.16 |
| Letter Fluency | 4.29 | 12.3 | -0.10 | 0.17 |
| RCFT, Copy | 17.0 | 22.2 | -0.23 | 0.31 |
| RCFT, Immediate Recall | 87.0* | 41.7 | -1.30* | 0.58 |
| RCFT, Long-Delay Recall | 87.9* | 39.7 | -1.32* | 0.55 |
| Trail Making Test–Part A | -46.6 | 49.3 | 0.76 | 0.72 |
| Trail Making Test–Part B† | -1.15 | 0.60 | 0.02 | 0.01 |
p<.05
Transformed logarithmically prior to analysis due to non-normal distribution
CVLT=California Verbal Learning Test, RCFT=Rey Complex Figure Test
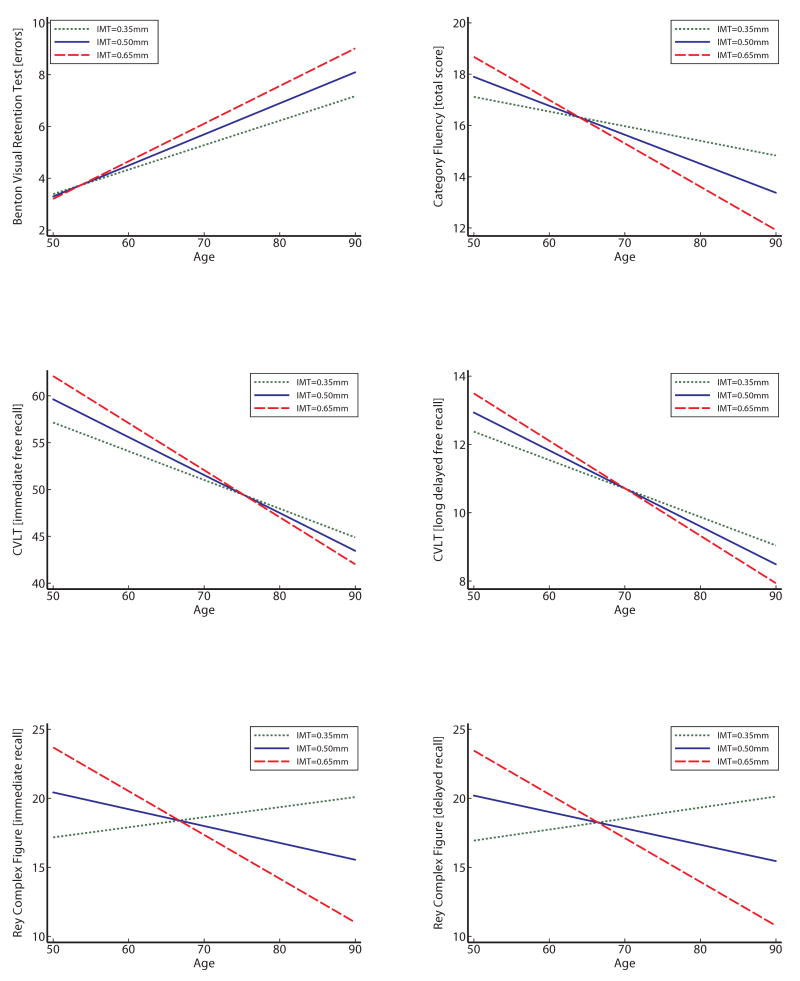
Figure 1 depicts significant carotid IMT × age interactions for BVRT, CVLT immediate free recall, RCFT, and Category Fluency, respectively. Each graph depicts age-related change in cognitive performance as a function of carotid IMT using all information in the analyses, regardless of the number of repeated assessment. Graphs were generated using predicted scores associated with mean carotid IMT +/- 1 SD. Except for BVRT, better performance is indicated by increasing scores over time.
Figure 1.
Longitudinal change in performance on the Benton Visual Retention Test, California Verbal Learning Test (immediate and long-delay free recall), Rey Complex Figure Test (immediate and long-delay recall), and Category Fluency as a function of carotid IMT.
Discussion
This study examined associations between carotid IMT and longitudinal trajectories of cognitive function among community-dwelling individuals without clinical vascular or neurological disease. Over time, individuals with greater carotid IMT showed significantly greater prospective decline in performance on multiple measures of verbal and nonverbal memory, including CVLT immediate free recall, CVLT delayed free recall, RCFT immediate recall, RCFT delayed recall, and total errors on the BVRT. In addition, individuals with greater carotid IMT showed a performance decline in Category Fluency, a measure of semantic association fluency and executive function. These findings are important in the context of previous work linking risk of stroke with diminished cognitive performance among individuals without clinical vascular disease or dementia.19 Subclinical vascular disease is considered a potential mechanism by which stroke risk may translate into reduced cognitive function,20 and the present study corroborates such a pathway.
Our findings extend previous longitudinal work relating greater carotid IMT with poorer performance on measures of cognitive function among community-dwelling populations.9-11 Our results linking carotid IMT with memory function are consistent with the findings of at least one previous longitudinal study,10 although there are also discrepant memory findings.11 Additionally, our findings are an extension of the literature linking various forms of clinical atherosclerosis with diminished cognitive function and decline.21, 22 Associations between subclinical atherosclerosis and cognition provide further support for a continuous, rather than threshold, effect of suboptimal vascular health on cognition.23 That is, vascular disease is not required to exceed a certain threshold of severity (clinical or otherwise) to impact cognitive function; instead decrements in cognitive function are likely to be proportional to the degree of underlying vascular disease.
Our finding that carotid IMT was most pervasively associated with measures of memory, both verbal and nonverbal, is interesting in light of recent evidence linking vascular risk factors with mild cognitive impairment and Alzheimer's disease.2, 24 Evidence indicates that individuals with vascular dementia and Alzheimer's disease demonstrate more considerable overlap in risk factors, brain pathology, and cognitive profiles than previously thought,2, 25, 26 and individuals with memory difficulties are at particularly increased risk for all-cause dementia.27
At first glance, the Semantic Fluency finding in the present study is consistent with the decrements in executive function associated with clinical vascular disease.1, 28 However, it remains difficult to draw a strong conclusion linking carotid IMT with lower levels of executive function in the absence of significant Letter Fluency and Trail Making Test-Part B results, which also measure primarily executive functions. Semantic Fluency may be a more sensitive diagnostic test than Letter Fluency for individuals with Alzheimer's disease and other types of neurological disease.12 This discrepancy may indicate enhanced assessment of memory deterioration by Semantic Fluency than Letter Fluency29– a pattern that is consistent with our otherwise pervasive memory findings.
Subclinical vascular disease may directly or indirectly affect cognitive function through a variety of mechanisms. Measures of carotid atherosclerosis have been associated with various cardiovascular risk factors, including demographic, metabolic, immunologic, and lifestyle factors, which have been associated with lower levels of cognitive function.3, 4, 30 Other hypothesized mechanisms include a common genetic vulnerability (e.g., presence of apolipoprotein E ε4 allele), chronic cerebral hypoperfusion, silent micro- and macro-cerebrovascular disease, and other associated structural brain changes, such as cortical atrophy.8, 31-33 For example, among asymptomatic individuals, greater carotid IMT is associated with increased frequency of silent cerebral lesions,34 which in turn are associated with reduced cognitive function.35
Strengths of this investigation include its longitudinal design, that minority participants comprised about one third of the sample, frequency of testing, length of follow-up, and extensive neuropsychological battery. Our inclusion of time-dependent covariates also represents a strength. Limitations include a single assessment of carotid IMT and use of an ever/never definition of cigarette smoking. Also, the study was based on a convenience sample of typically highly educated participants. The homogeneity and non-representative nature of the sample may limit the study's generalizability, although the sample's homogeneity may also restrict the influences of confounding demographic variables. Future research should continue to examine which cognitive domains are particularly related to carotid IMT and other subclinical vascular disease measures. Current inconsistencies across findings in this area are likely a byproduct of variability in study populations, follow-up times, number of follow-up visits, domains tested, and neuropsychological tests utilized. Future longitudinal investigations would benefit from careful examination of these factors and their associated effects on study results and interpretations. Variables mediating or moderating the relation between carotid IMT and accelerated cognitive decline also necessitate further examination.
Summary
Our findings suggest that relatively healthy individuals with greater carotid IMT demonstrate more pronounced memory decline over time than those with lesser carotid IMT. Evidence that carotid IMT predicts accelerated cognitive decline among such persons underscores the importance of early intervention to delay or reduce atherosclerosis and improve vascular health prior to symptom manifestation. Such intervention (e.g., pharmacologic, behavioral) may delay onset of cognitive decline, slow cognitive aging, and perhaps delay and/or protect against clinical cognitive diagnoses.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
None of the authors report any conflicts of interest relevant to this manuscript.
References
- 1.O'Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ. Cardiovascular disease and alzheimer's disease: Common links. J Intern Med. 2006;260:211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 3.Elias MF, Elias PK, Robbins MA, Wolf PA, D'Agostino RB. Cardiovascular risk factors and cognitive functioning: An epidemiological perspective. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Erlbaum; 2001. pp. 83–104. [Google Scholar]
- 4.Devereux RB, Alderman MH. Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation. 1993;88:1444–1455. doi: 10.1161/01.cir.88.4.1444. [DOI] [PubMed] [Google Scholar]
- 5.Chaves PH, Kuller LH, O'Leary DH, Manolio TA, Newman AB. Subclinical cardiovascular disease in older adults: Insights from the cardiovascular health study. Am J Geriatr Cardiol. 2004;13:137–151. doi: 10.1111/j.1076-7460.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Shemanski L, Psaty BM, Borhani NO, Gardin J, Haan MN, O'Leary DH, Savage PJ, Tell GS, Tracy R. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 7.Auperin A, Berr C, Bonithon-Kopp C, Touboul PJ, Ruelland I, Ducimetiere P, Alperovitch A. Ultrasonographic assessment of carotid wall characteristics and cognitive functions in a community sample of 59- to 71-year-olds. Stroke. 1996;27:1290–1295. doi: 10.1161/01.str.27.8.1290. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RA, Poppas A, Forman DE, Hoth KF, Haley AP, Gunstad J, Jefferson AL, Tate DF, Paul RH, Sweet LH, Ono M, Jerskey BA, Gerhard-Herman M. Vascular and cognitive functions associated with cardiovascular disease in the elderly. J Clin Exp Neuropsychol. 2008:1–15. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SC, O'Meara ES, Manolio TA, Lefkowitz D, O'Leary DH, Goldstein S, Carlson MC, Fried LP, Longstreth WT., Jr Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- 10.Komulainen P, Kivipelto M, Lakka TA, Hassinen M, Helkala EL, Patja K, Nissinen A, Rauramaa R. Carotid intima-media thickness and cognitive function in elderly women: A population-based study. Neuroepidemiology. 2007;28:207–213. doi: 10.1159/000108112. [DOI] [PubMed] [Google Scholar]
- 11.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 12.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 2004 [Google Scholar]
- 13.Poredos P. Intima-media thickness: Indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vascular Medicine. 2004;9:46–54. doi: 10.1191/1358863x04vm514ra. [DOI] [PubMed] [Google Scholar]
- 14.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of alzheimer's disease: The baltimore longitudinal study of aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 15.Nagai Y, Metter EJ, Earley CJ, Kemper MK, Becker LC, Lakatta EG, Fleg JL. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–1509. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The ces-d scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 17.Singer JD. Using sas proc mixed to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355. [Google Scholar]
- 18.McArdle JJ, Bell RQ. An introduction to latent growth models for developmental data analysis. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multilevel data: Practical issues, applied approaches, and specific examples. Mahwah, NJ: Erlbaum; 2000. pp. 69–107. [Google Scholar]
- 19.Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Beiser A, Au R, Seshadri S, DeCarli C, Wolf PA. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 20.Desmond DW, Tatemichi TK, Paik M, Stern Y. Risk factors for cerebrovascular disease as correlates of cognitive function in a stroke-free cohort. Arch Neurol. 1993;50:162–166. doi: 10.1001/archneur.1993.00540020040015. [DOI] [PubMed] [Google Scholar]
- 21.Vinkers DJ, Stek ML, van der Mast RC, de Craen AJ, Le Cessie S, Jolles J, Westendorp RG, Gussekloo J. Generalized atherosclerosis, cognitive decline, and depressive symptoms in old age. Neurology. 2005;65:107–112. doi: 10.1212/01.wnl.0000167544.54228.95. [DOI] [PubMed] [Google Scholar]
- 22.Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: Evidence from the whitehall ii study. J Am Geriatr Soc. 2003;51:1445–1450. doi: 10.1046/j.1532-5415.2003.51464.x. [DOI] [PubMed] [Google Scholar]
- 23.Waldstein SR, Tankard CF, Maier KJ, Pelletier JR, Snow J, Gardner AW, Macko R, Katzel LI. Peripheral arterial disease and cognitive function. Psychosom Med. 2003;65:757–763. doi: 10.1097/01.psy.0000088581.09495.5e. [DOI] [PubMed] [Google Scholar]
- 24.Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: Part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 25.de la Torre JC. Is alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 26.White L, Launer L. Relevance of cardiovascular risk factors and ischemic cerebrovascular disease to the pathogenesis of alzheimer disease: A review of accrued findings from the honolulu-asia aging study. Alzheimer Dis Assoc Disord. 2006;20:S79–83. doi: 10.1097/00002093-200607001-00012. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- 28.Desmond DW. The neuropsychology of vascular cognitive impairment: Is there a specific cognitive deficit? J Neurol Sci. 2004;226:3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the alzheimer's type: A meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Everson SA, Helkala E, Kaplan GA, Salonen JT. Atherosclerosis and cognitive functioning. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Erlbaum; 2001. pp. 105–120. [Google Scholar]
- 31.Bots ML, van Swieten JC, Breteler MM, de Jong PT, van Gijn J, Hofman A, Grobbee DE. Cerebral white matter lesions and atherosclerosis in the rotterdam study. Lancet. 1993;341:1232–1237. doi: 10.1016/0140-6736(93)91144-b. [DOI] [PubMed] [Google Scholar]
- 32.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of apoe epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 33.Manolio TA, Burke GL, O'Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of cerebral mri findings to ultrasonographic carotid atherosclerosis in older adults: The cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto M, Inoue K, Moriki A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertens Res. 2007;30:767–773. doi: 10.1291/hypres.30.767. [DOI] [PubMed] [Google Scholar]
- 35.Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, DeCarli C, Sacco R, Stern Y. White matter hyperintensities and subclinical infarction: Associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.