Abstract
The present study was designed to test the hypothesis that sensitivity of ingestive behavior of infant rat to the pharmacological effects of ethanol changes between postnatal (P) days 9 and 12. The intake of 0.1% saccharin and water, general motor activity, and myoclonic twitching activity were assessed following administration of three doses of ethanol (0, 0.25, 0.5g/kg) while fluids were free available to the animals. The 0.5g/kg dose of ethanol attenuated saccharin intake in P9 pups and enhanced saccharin intake in P12 rats. On P12 some sex-related differences emerged at 0.5g/kg of ethanol, with saccharin intake being higher in females than in their male counterparts. Taste reactivity probe revealed that 0.5 g/kg of ethanol increased taste responsiveness to saccharin on P12 but only to infusions presented at a high rate. The results of the present study indicate that ontogenetic changes in sensitivity to the effects of ethanol on ingestive behavior occur during the second postnatal week, with P9 animals being more sensitive to the inhibitory (sedative) effects on saccharin intake and P12 rats being more sensitive to the stimulatory effects of ethanol. We suggest that acute ethanol enhanced saccharin intake via sensitization of oral response to appetitive taste stimulation.
Keywords: ethanol, intake, neonatal, taste, ingestive behavior
1. Introduction
Infancy is a unique period during which rat pups consume large quantities of ethanol without any initiating procedures. High ethanol consumption during this age has been seen either in the context of suckling behavior [1] or independent feeding [2], [3], [4], [5], [6]. When independent ingestion of ethanol was tested during infancy, intake of ethanol in low and moderate concentrations (5%-15%) gradually increased from postnatal day 4 to 12 with the peak of consumption occurring near the end of the second postnatal week [2], [3], [5].
Taste plays a substantial role in initiation of ethanol ingestion. Although several taste components determine the flavor of alcohol, the palatability of ethanol is thought to be an important predictor of high ethanol consumption in early infancy [7], [8]. Palatability of various tastants, including ethanol, has been measured in adults by analysis of short latency motor and affective reactions to intraoral infusions of small amounts of flavored fluid [9], [10]. However, assessment of taste responsiveness in the neonatal rat has usually been based on intake measured in relatively long procedures (10-20 minutes) when the animal consumed a substantial amount of ethanol (2.5% of body weight) [1], [11], [12], [13], [14], [15], [16]. With such tests it is difficult to distinguish between changes in orosensory properties of ethanol and postingestive consequences of ethanol.
When separate tests for assessment of intake and palatability were employed in our recent study, a clear dissociation between developmental changes of ethanol acceptance and responsiveness to ethanol taste was revealed [17]. It was found that ethanol intake increased while intake of saccharin decreased between postnatal (P) days 9 and 12, whereas no age-related differences in taste responsiveness to saccharin and ethanol were seen. The dissociation between ontogenetic patterns of ethanol intake and taste responsiveness suggests that increased ethanol acceptance between P9 and P12 may not be solely related to developmental changes in the pups perception of the drugs orosensory properties. It is possible that the increased intake is a consequence of changes in sensitivity of ingestive behavior to the pharmacological effects of ethanol that occur between P9 and P12.
The aim of the present study was to compare acute effects of three doses of ethanol (0, 0.25 g/kg and 0.5 g/kg) on intake and taste responsiveness to a freely available palatable fluid in P9 and P12 pups. During the active (waking) phase of the ethanol intake test conducted in our previous study [17] pups ingested about 0.2 g/kg on P9 and about 0.5 g/kg on P12. Given that intake of ethanol in a range of 0.25 – 0.5 g/kg resulted in detectable (15–25 mg/dl) blood ethanol concentrations (BEC) and reinforcement from pharmacological effects of ethanol occurred in neonatal rats at comparable BECs [12], [18], [19], the doses of 0.25 g/kg and 0.5 g/kg were used in the present study to reproduce pharmacological consequences of ethanol consumption when it is freely available to the animal.
Experiment 1 assessed ethanol effects on intake and temporal patterns of ingestion in rat pups allowed to freely extract fluid from an intraoral cannula. The fluid flow during this extraction was monitored using the flow tracking system described in our recent paper [17]. The same flow recording technique was used for assessment of taste reactivity in Experiments 2. Infant rats show prominent sensitization to and habituation of taste-elicited oral behavior [20], [21], [22], with the temporal course of habituation or sensitization determined mainly by rate of taste stimulation. To control for these processes we conducted two tests in which taste probes were presented either with low (Experiment 2A) or high frequency (Experiment 2B). To control for pharmacological effects of ethanol independently from its orosensory properties we replaced the 10% ethanol used in our previous study with 0.1% saccharin –a sweet tastant devoid of ethanol's pharmacological effects but similar to 10% ethanol in terms of palatability measured in a previous taste reactivity probe [17]. Experiment 3 examined whether BEC for a given ethanol dose differ as function of age and post-administration time. Given that ethanol effects on independent ingestive behavior may depend on baseline motor activity and behavioral state of the infant rat in terms of the time spent in sleep/wakefulness, overall motor activity and twitching activity, which represents a reliable index of active sleep, were recorded and analyzed along with changes of fluid flow.
2. Methods
2.1. Subjects
A total of 472 pups, derived from 96 dams, served as subjects for all experiments. Experimental subjects were Sprague-Dawley rats born in our colony at Binghamton University. Pregnant female rats were housed individually in plastic maternity cages (47 cm long × 20 cm high × 36 cm wide) containing pine shavings as bedding material. They were checked for birth daily between 1500 and 1800 hr, and the day of birth was considered P0. Litters were culled to 10 pups, with an equal sex ratio whenever possible. All animals were housed in a temperature-controlled (22°C) vivarium maintained on a 14-/10-hr light/dark illumination cycle (lights on at 0700 hr), with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. In all respects, maintenance and treatment of the animals were in accord with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
2.2. Cannulation
Pups were separated from the dam about 1.5 hr prior to testing and placed into an incubator in group of 10 subjects at 33°C and 75% humidity., Following a 15-20 min acclimation period an intraoral cannula was inserted through the pup's cheek. For flow recording during continuous fluid availability (Experiment 1) experimental subjects were implanted with a one- channel cannula. When taste reactivity was being assessed (Experiments 2A and 2B) animals were implanted with a 2-channel intraoral cannula. The cannula preparation and implantation procedure were described extensively in our previous studies [17], [23]. In brief, cannulas were made from 6-cm sections of PE-10 polyethylene tubing (Clay Adams, Parsippany, NJ). For preparation of double-channeled cannula two pieces of tubing were fastened together and simultaneously heated at one end until a single flange linking both tubing was formed. The flange diameter was 2.0-2.5 mm. The non-flanged end of each of the two channels of the cannula was tightly fit onto one end of a separate carved stainless-steel wire (0.28 mm diameter, approximately 4 cm in length). The two wires were placed together and inserted simultaneously into the cheek 3 –4 mm caudal to the mystacial pad. Both pieces of tubing were then drawn through the cheek, leaving the flanged end in the oral cavity. A drop of a liquid bandage was used to secure the position of the cannula. The implantation of a one-channeled cannula was done in a similar way using a single wire to pull the cannula through the cheek. The cannulation procedures were accomplished within 6-12 s with no bleeding and minimal stress for the animal.
2.3. Experiment 1: Intake test
2.3.1. Procedure
Testing procedure occurred in a plastic circular arena with transparent walls (diameter 10 cm, wall height 2 cm). Absorbent paper covered the plastic floor of the arena. The floor of the test arena was heated to a temperature of 32-34°C during experiments using 9 day-old pups and at 30-32°C during experiments using P12 animals. Throughout the experiment ambient room temperature was maintained at 23 ± 0.2°C.
During the intake test (20 min) animals were allowed to voluntarily ingest 0.1% saccharin or water from an intraoral cannula. Each subject was exposed to only one fluid and this fluid was continuously available during the entire test. The intake test began 5 minutes after administration of ethanol or saline. Ethanol (190-proof Ethanol, Pharmaco, Brookfield) was administered i.p. as a 12.6% (v/v) solution in physiologic saline. The solutions were warmed to 34°C for injection. All experimental subjects were voided, weighed to the nearest 0.01 g prior to testing and weighed again immediately after the test session.
2.3.2. Recording of fluid flow
A technique of on-line monitoring of fluid flow was designed to measure the amount of fluid ingested through an intraoral cannula. We have described this technique in detail elsewhere [17], [23]. In short a piece of translucent PE-90 tubing (Clay Adams, Parsippany, NJ), 90-100 cm long, connected through a valve and adapter to intraoral cannula was used as a reservoir for the fluid to be presented to the subject. A custom built fluid tracker consisted of a photocell unit and a stepped motor rotating the wheel around which the tubing containing the fluid was twisted. The piece of PE-90 tubing was pulled through the photocell unit of the fluid tracker, with the outer end of tubing being mounted on the wheel. The photocell system was aimed through the translucent tubing to detect the border between filled and unfilled parts of the tube. An output signal from the photo sensor controlled a stepper motor that rotated the wheel and moved the tube through the photocell assembly until the light beam “hit” the filled part of the tubing. When the experimental subject consumed the test fluid, the fluid moved down the tube. The number of steps the motor performed pulling the tube through the photocell assembly in order to locate the fluid edge against the light beam was directly proportional to the amount of fluid ingested. The resolution of flow measurement was about 0.2 μl/step
The current modification of this technique includes two units set up for simultaneous flow recording in two subjects. The controller unit built on a PIC16F874 microprocessor was used to program movements of step motors and to record data from 2 flow inputs and 4 switch inputs used to mark behavioral events. The data sampled at a rate of 10 Hz were transferred to a personal computer via a RS232 input. The system was designed and programmed by W. Kashinsky at Binghamton University.
2.3.3. Behavioral observations
The selected categories (see below) of behavioral activity were scored online by an experimenter blind to treatment condition. The behavioral scores were recorded using a flow tracking device and stored in one file with flow data. The behavior of all subjects was also continuously monitored by a video camera (Panasonic Model AF-X8) and recorded on DVD (LiteOn 5002 DVD recorder). The data acquisition module included a video overlay unit that provided simultaneous display of video images, flow counts and markers of behavioral events.
The behavioral analysis included three categories of behavioral activity: appetitive/ingestive reactions, overall motor activity and myoclonic twitching. The appetitive/ingestive reactions included probing (activity mimicking licking or picking food from floor) and paw licking. The behavior was scored as overall motor activity if it was not ingestion-related behavior (i.e. mouthing, probing, paw licking) or aversive behavior (such as gapes, chin scraping, wall climbing). Twitching activity, which represents a highly reliable measure of active sleep in the infant rat [24], [25], was defined as phasic, rapid, and independent movements of any part of the hind limbs and the tail. Twitches were recorded as event data; for further analysis we computed the number of 5-s intervals during which at least 2 twitches were observed without any coordinated movements of the body.
2.3.4. Data analysis
To avoid possible confounding of litter with treatment, no more than two subjects per litter (one male and one female) were assigned to the same treatment group. Order of testing for the different treatment groups was counterbalanced across the experiment. Numbers of males and females in each group were equated.
Body weight gain was measured as the difference (in mg) between body weights assessed immediately before and immediately after the intake test. Fluid flow through the cannula was measured as the volume (μL) of fluid extracted from cannula. This measure was used to evaluate total fluid intake and per minute intake (μL/min). The level of motor activity was indexed as time spent in this behavior. Twitching activity as a measure of active sleep yielded two indices: (a) duration of initial waking period (time from start of the test to the first occurrence of twitching activity); and (b) time spent with myoclonic twitching, which indexed duration of active sleep.
Total flow intake, body weight gain and behavioral indexes (total time spent in motor and twitching activities as well as latency of active sleep onset) were analyzed via separate 2(fluid) × 2(age) × 3(dose) × 2(sex) between-groups ANOVA procedures (Statistica 6.0, StatSoft, Inc., Tulsa, OK 74104 USA). All behavioral scores were converted to percent of time spent in particular activity and were arc-sin transformed before being submitted to ANOVA. Post-hoc comparisons were conducted using Fisher's PLSD test. The type 1 error level (alpha) was set at 0.05, and for multiple comparisons the critical alpha level was corrected using the Bonferroni adjustment. Unless otherwise specified population estimates are presented in the form of means ±SEM.
The temporal changes of per-minute flow rate and per-minute scores of behavioral activities were analyzed using a MANOVA with successive one-minute intervals of the intake test treated as repeated measure. When a temporal trend was observed, linear and non-linear regression analysis (Graph Pad Prism 5.0, Graph Pad Software, Inc., La Jolla, CA 92037 USA) was used to find the best-fit model and to compare fitting parameters between treatments.
2.4. Experiment 2: Taste reactivity assessment
2.4.1. Procedure
The housing of the animals, acclimation procedure and cannulation were exactly the same as those described in Experiment 1. Assessment of taste reactivity occurred in the same plastic arenas used for the intake test. Subjects were allowed a 5-6 minutes as a habituation period prior to the start of the taste reactivity test. During habituation water was available through an intraoral cannula and pups also received four water infusions. Ethanol or saline was injected i.p. 5 minutes before assessment of taste reactivity
For assessment of taste reactivity, one channel (piece of PE-10 tubing) of the double-channel cannula was connected via PE-50 tubing to a Gilmont syringe mounted on a rotary pump (AutoPump, Kashinsky 5/2000, Binghamton, NY). This channel of the cannula was used for delivery of test fluid (0.1% saccharin), whereas the second channel provided recording of water flow before, during, and after infusion of saccharin. Water was continuously available through this channel of the cannula, and animals could voluntarily extract it during the entire test [23]. Due to the closely spaced openings of the fluid delivery channel and flow recording channel on the cannula flange pups inevitably extracted water from the recording tubing, while responding to appetitive taste probe by licking. Consequently, measurement of the volume of water extracted from the cannula shortly after infusion gives an estimate of the amplitude of oromotor response to taste stimulation. Saccharin infusions (4 μL/2 s) were delivered either every 75 s (Experiment 2A) or every 15 s (Experiment 2B). The first taste probe was presented after one minute of recorded water flow. The test lasted 375 s in Experiment 2A and 300 s in Experiment 2B. To normalize flow reactions against individual variability of baseline water flow we measured reactions as deviations from baseline flow rate, which was computed for 15 seconds immediately preceding every infusion (Experiment 2A) or immediately preceding the first infusion (Experiment 2B). Consequently, the mean amplitude of flow deviation during the 15 s interval immediately following saccharin infusion was the main index of taste responsiveness. We also scored paw licking and probing as constituents of animal reaction to the taste probe and recorded non-ingestive motor activity as an index of nonspecific behavioral activation. The behavioral scores were measured as percent time spent in behavioral activities and were averaged across the whole test.
2.4.2. Data analysis
Treatment assignment within each litter was the same as in Experiment 1 and included three ethanol doses (0, 0.25 g/kg and 0.5 g/kg) and sex (males and females). Flow and behavioral reactions averaged across all infusions in a series were analyzed using 2 (age) × 3(dose) × 2(sex) between-subjects analyses of variance (ANOVA). A multivariate approach (MANOVA) was applied to testing repeated measure effects (infusion number or block number).
2.5. Experiment 3: Blood ethanol analysis
One hundred sixty pups derived from 20 litters were used in Experiment 3. Pups were either i.p. injected with 0.25 g/kg or 0.5 g/kg of 12.6 % ethanol (v/v) and were placed in the same plastic arenas used for the intake test (floor temperature- 30-32 C°). For each group blood samples were collected 5 or 15 minutes after ethanol administration. The times of blood sampling approximately corresponded to minutes 1-2 and minutes 10-11 of the intake test (Experiment 1).
Trunk blood was collected in heparinized tubes, centrifuged at 6000 rpm, plasma was frozen, and blood alcohol concentration (BEC) was assessed later. BECs were assessed via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II gas chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μL aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 minutes and then extracted and injected a 1.0-mL sample of the gas headspace into the chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions. BECs were analyzed using a 2(age)×2 (acute ethanol dose)×2 (time) × 2 (sex) ANOVA.
3. Results
3.1. Experiment 1: Effects of ethanol on voluntary consumption of 0.1% saccharin and water
3.1.1. Total flow and Body weight gain
A 2(fluid) × 2(age) × 3(dose) × 2(sex) ANOVA revealed a significant main effect of fluid on flow, F(1,144)=174.63, p<0.001, and on BWG, F(1,144)=129.35, p<0.001, with P9 and P12 pups taking in more saccharin than water. The ANOVA also revealed significant dose × age interactions for total fluid flow F(2,144)=7.40, p<0.01 and BWG F(2,144)=8.93, p<0.01. Furthermore significant dose × fluid × age interactions for total flow F(2,144)=14.42, p<0.001 and BWG F(2,144)=11.96, p<0.001 were found.
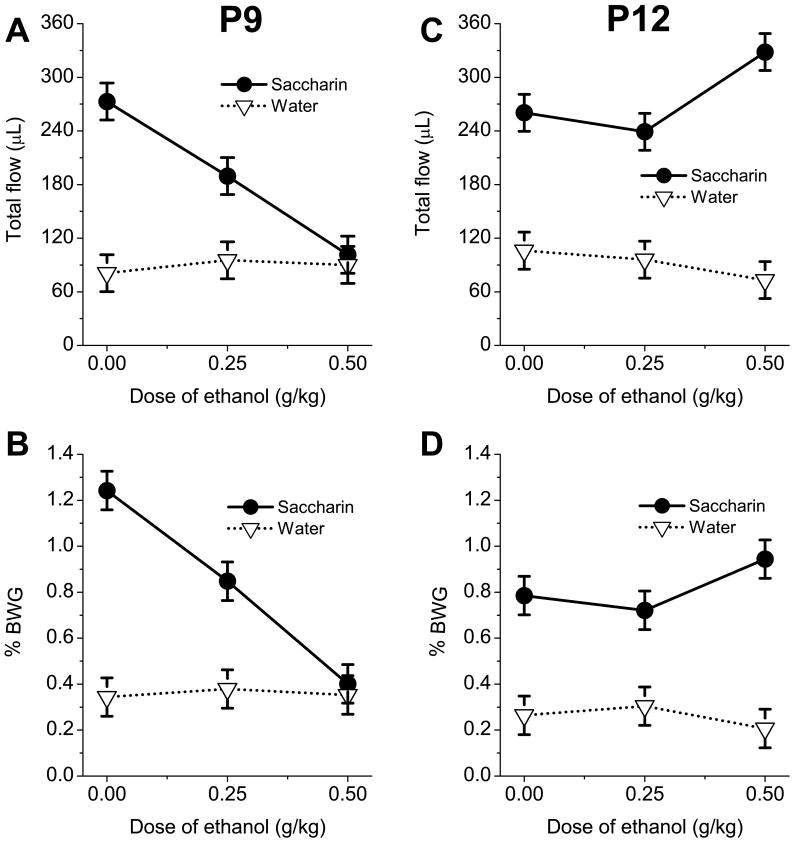
Acute exposure to ethanol affected intake of saccharin but had no effect on ingestion of water (fig.1). Total saccharin flow was not significantly different between P9 and P12 subjects in the saline-treated groups. However, intake of saccharin measured as BWG was higher for P9 animals than for P12 subjects given saline injections. In other words, in the absence of ethanol effects intake of saccharin for these two age groups was similar when measured by total fluid flow but differed when assessed via BWG. The effects of ethanol exposure on fluid flow were dose- and age-dependent. Compared to saline-treated animals, 0.5g/kg ethanol significantly decreased saccharin intake in P9 pups but enhanced saccharin ingestion in P12 rats (fig.1). The increase in saccharin intake on P12 after 0.5g/kg dose of ethanol was significant only for total flow. Total flow of P12 pups injected with 0.5g/kg of ethanol was significantly higher than total flow intake of their P9 counterparts. No changes of intake were observed in P9 and P12 pups after injection of 0.25 g/kg ethanol compared to animals treated with saline. However, a clear trend of decreased saccharin intake following the low dose of ethanol was evident in P9 subjects (see fig. 1).
Fig.1.
Total intake of 0.1% saccharin and water measured as fluid volume extracted through a cannula (A,C) and as body weight gain (B,D) in P9 (left) and P12 (right) pups injected with saline or two doses of ethanol.
Significant main effects of sex on total flow, F(1,144)=21.57, p<0.001, and on BWG, F(1,144)=15.71, p<0.001, were also revealed as well as an interaction of sex × fluid × age for total flow, F(1,144)=5.58, p<0.05, and for BWG, F(1,144)=5.71, p<0.05. Furthermore, a sex × dose × fluid × age interaction was found, F(2,144)=4.40, p<0.05 for total flow.
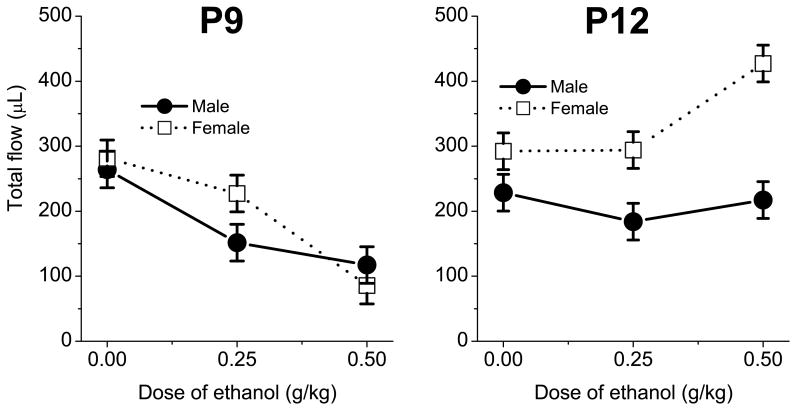
Females ingested more saccharin than males, but this sex-related difference emerged only on P12 after treatment with 0.5 g/kg ethanol (fig. 2). Total saccharin flow and BWG in P12 females treated with 0.5g/kg of ethanol were significantly higher than in male P12 rats. In other words, while 0.5 g/kg of ethanol significantly decreased saccharin intake in females as well as in males on P9, the same dose significantly increased total saccharin flow in P12 females but not in males.
Fig.2.
Total saccharin intake in P9 and P12 pups as function of ethanol dose, age and sex of the animals.
3.1.2. Temporal patterns of flow
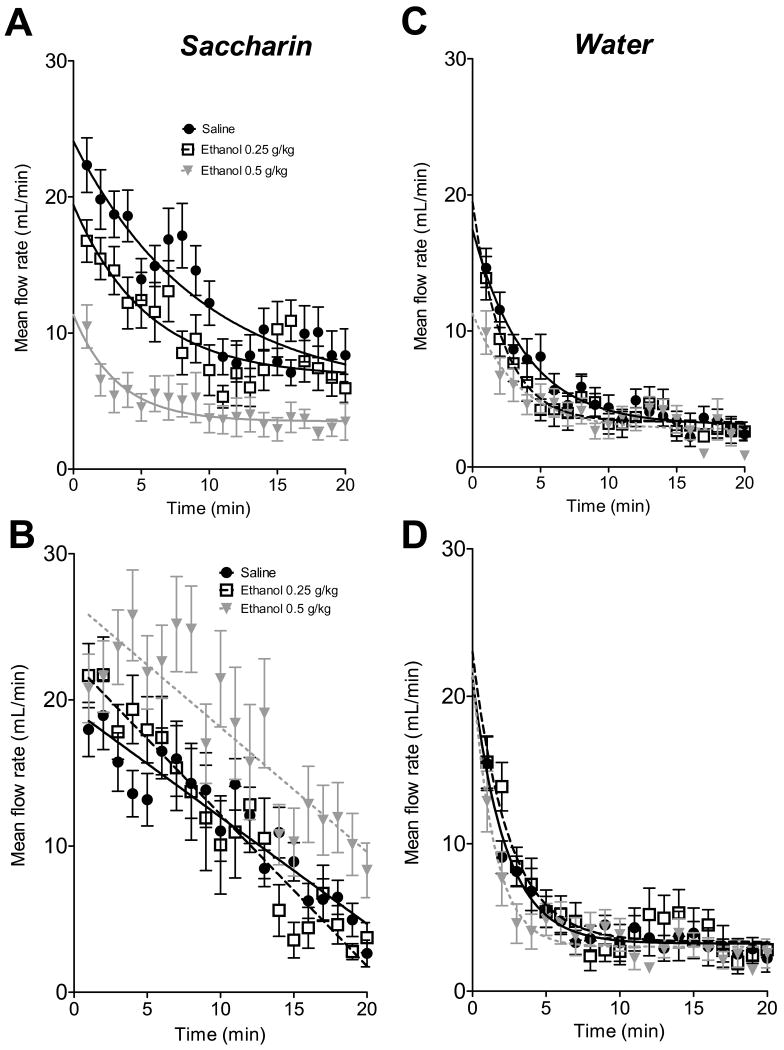
When rats were allowed to voluntarily extract fluids available from an intraoral cannula, flow rate gradually decayed with time (fig.3). We used nonlinear regression analysis (GraphPad Prism 5.0, Graph Pad Software, Inc., La Jolla, CA 92037 USA) to analyze changes of temporal flow trends between treatments and to compare best –fit values of flow curve parameters. It was found that for all treatment and age groups the best-fit model of temporal course of water flow was one-phase exponential decay (Y=(Y0 - A0)*exp(-K*t)+A0). The parameters of water flow decay (initial flow values Y0 and rate of decay, K) did not significantly differ between treatments in P12 animals, F(6,831)=2.17, p=0.71, (fig.3, right column). However, on P9 the rate of water flow decay was significantly lower in animals injected with 0.5g/kg of ethanol than in saline-injected controls, and the initial value of water flow rate was lower in animals treated with 0.5 g/kg of ethanol compared to 0.25 g/kg, F(6,831)=7.33, p<0.001.
Fig.3.
Temporal course of saccharin (A,B) and water (C,D) flow after i.p. administration of saline or two doses of ethanol in P9 (A,C) and P12 (B,D) pups. Data are presented as per minute mean flow rate ±SEM. Best-fit regression lines of flow trends were drawn for each treatment (injections of saline or 0.25g/kg, 0.5g/kg ethanol).
For temporal patterns of saccharin flow the best-fit models differed between P12 and P9 (fig.3, left column). On P9 the curves of saccharin flow were best approximated by one-phase exponential decay for all treatments, while on P12 the best-fit model of flow trend was linear (Y= Y0- B*t). Striking differences occurred during the initial period of fluid availability. On P9 administration of 0.5g/kg ethanol significantly decreased the initial value of exponential decay (Y0) compared to saline treatment, F(1,554)=3.87, p<0.05, (saline: 24.10 μl/min, confidence interval (CI): 20.13 -27.57, ethanol 0.5 g/kg: 11.34 μl/min, CI: 7.45 -15.43), whereas on P12 the intercept value of the flow regression line was significantly higher in pups injected with 0.5g/kg of ethanol than in saline treated animals (F(1,556)=22.59, p<0.01, salineY0: 19.29 μl/min; CI: 17.56 –20.91: ethanol Y0: 26.67 μl/min; CI: 24.10-29.22). Minute–per-minute comparisons between flow rates of P12 pups injected with saline vs. 0.5g/kg of ethanol revealed that flow rate under ethanol treatment significantly exceeded flow in saline pups on minute 2,3 and 4 but not on minute 1 of the test (fig.3B).
Temporal patterns of saccharin flow were also compared between males and females treated with saline, 0.25 or 0.5g/kg of ethanol. On P9 no sex differences in parameters of best-fit model (one-phase exponential decay) were found for all treatments. On P12 sex difference between best –fit parameters of saccharin flow curve was found only in animals treated with 0.5g/kg of ethanol: intercept value of the flow regression line was significantly higher in females than in males (F(1,277)=69.57, p<0.01, females: 30.00 μl/min; CI: 25.96 –34.05; males: 21.97 μl/min; CI: 18.97-24.86).
3.1.3. Overall motor activity
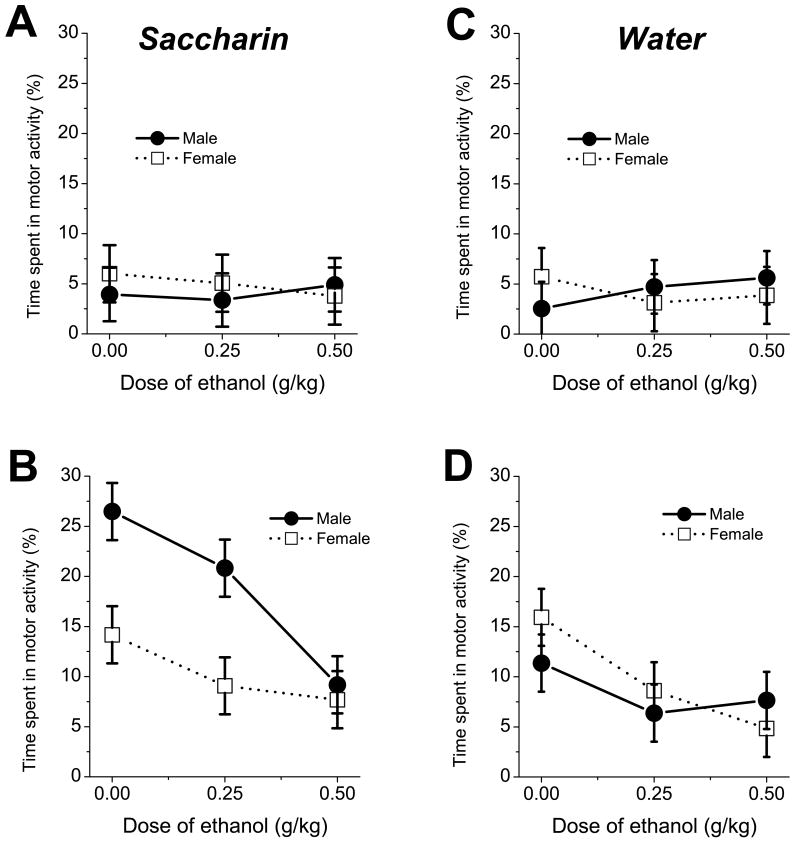
For time spent in motor activity the ANOVA revealed a significant main effect of age, F(1, 144)=10.37, p<0.01, with overall motor activity being higher on P12 than on P9 (fig.4). The main effect was significant for dose of ethanol, F(2, 144)=4.25, p<0.05, as were the interactions for age × dose, F(2, 144)=4.29, p<0.05 and age × fluid × sex F(1,144)=3.96, p<0.05. The highest level of motor activity was observed on P12 in saline-treated male pups ingesting saccharin. Males of this group spent significantly more time in motor activity than age-matched females and males ingesting water or females ingesting saccharin. The administration of 0.5 g/kg dose of ethanol significantly decreased non-ingestive motor activity of P12 animals but did not affect time spent in this type of activity in P9 rats (fig.4).
Fig.4.
Overall motor activity in P9 (A,C) and P12 (B,D) pups ingesting 0.1% saccharin (left) and water (right) as function of sex and dose of ethanol.
3.1.4. Myoclonic twitching
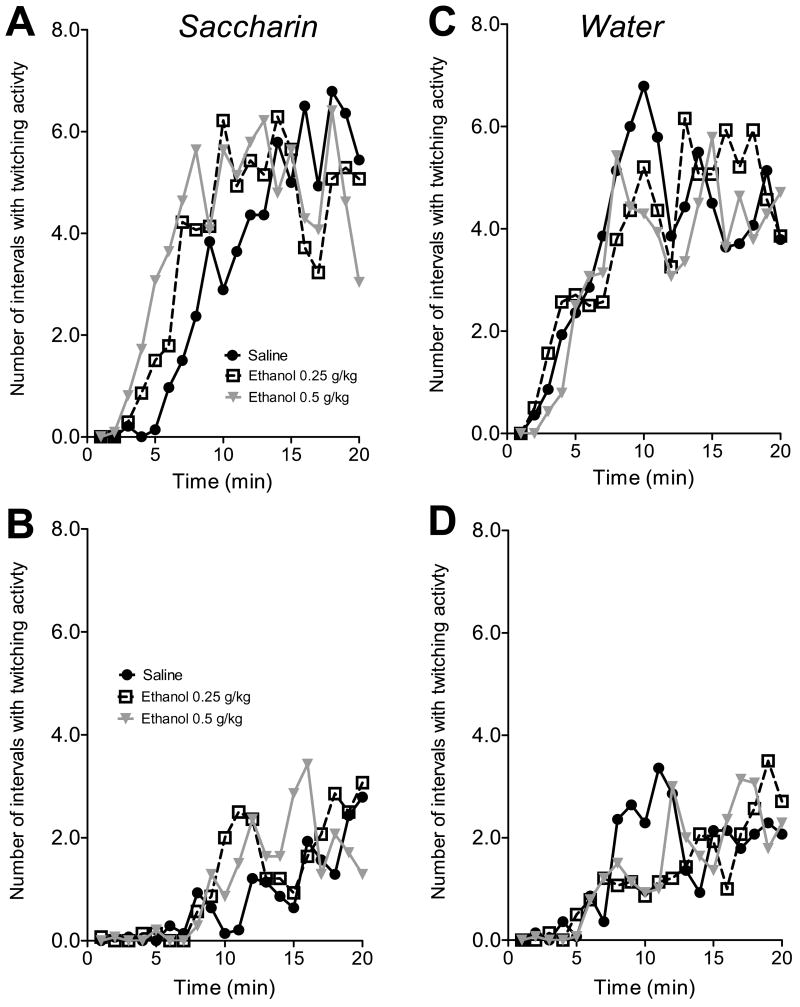
Time spent in myoclonic twitching was indexed as the number of 5-s intervals with at least 2 occurrences of myoclonic twitches and was computed for every minute of the test. A 2(fluid) ×2 (age) × 3 (dose) × 2(sex) ANOVA, with successive 1-min time intervals treated as a repeated measure, revealed a significant main effect of age, F(1, 144)=147.15, p<0.001, with total time spent in twitching activity being significantly higher on P9 than on P12. However, a total time spent in active sleep was not affected by either dose of ethanol in P9 or P12 pups.
Two separate 2(fluid) × 3(dose) × 20 (time) MANOVAs were performed to analyze the temporal changes in twitching activity on P9 and P12. The MANOVA revealed main effect of time in P9, F(19, 60)= 30.62, p<0.001, as well as in P12 pups, F(19, 60)= 6.05; p<0.001. The animals on P9 and P12 were always awake during the first few minutes of fluid exposure, and then the mean time spent in twitching activity gradually increased up to 30 s per minute (six 5s intervals per minute) in P9 pups and up to 12 s per minute in older animals (fig. 5). The mean number of intervals with twitching was comparable in saccharin and water groups. The MANOVA also revealed an interaction of time × fluid × dose on P9, F(38,120)= 1.89, p<0.05, but not on P12, F(38,120)= 1.38, p=0.096. A series of two-way (fluid × dose) ANOVAs conducted at each one-minute interval revealed significant effects on Min 4, 5, 7, 10 and on Min 20. Compared to saline-treated animals P9 pups injected with 0.5 g/kg of ethanol spent significantly more time in active sleep on Min 4, 5, 7, 10, but the reverse was true for Min 20 of the exposure.
Fig.5.
Temporal patterns of twitching activity in P9 (A,C) and P12 (B,D) pups exposed to 0.1% saccharin (left) or water (right) after treatment with saline or two doses of ethanol.
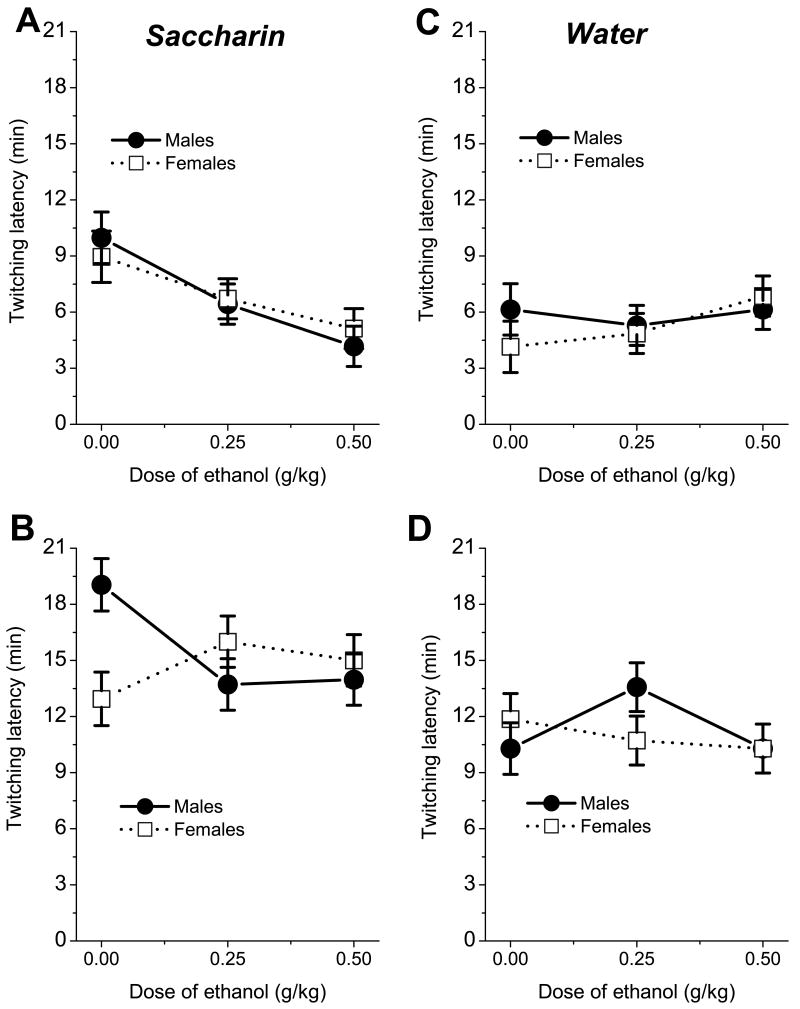
Pups injected with various doses of ethanol differed in the duration of their initial waking period. We analyzed the latency of the first occurrence of myoclonic twitching using a 2(fluid) × 2(age)× 3(dose) × 2(sex) ANOVA. Significant effects of age, F(1,144)=148.22, p<0.001, and fluid, F(1,144)=20.22, p<0.001 were found, with latency of twitching being delayed in P12 pups compared to P9 and in pups ingesting saccharin compared to animals exposed to water (fig.6). There were also significant interactions: age × fluid, F(1,144)=5.64, p<0.05, fluid × dose × sex, F(2,144)=3.44, p<0.05, and age × fluid × dose × sex, F(2,144)=3.83, p<0.05. On P9 the 0.5 g/kg ethanol dose significantly decreased latency of active sleep in males and females ingesting saccharin but did not affect the time of sleep onset during exposure to water. When pups were exposed to saccharin on P12, the latency of the first occurrence of myoclonic twitching was significantly higher in saline treated males than females. The injections of 0.25 and 0.5 g/kg of ethanol resulted in shorter latency to the first episode of myoclonic twitching in males but had no effect on latency of twitching in females. No significant differences between treatment groups were found for pups drinking water on P9 or on P12.
Fig.6.
Latency of first twitching episode in P9 (A,C) and P12 (B,D) pups exposed 0.1% saccharin (left) or water (right) as function of sex and dose of ethanol.
3.1.5. Saccharin intake and duration of sleep-wakefulness
The comparison of the temporal course of fluid flow and twitching activity showed that flow rate decreased when incidence of twitching activity increased (fig. 3 and fig.5). To assess the possibility that ethanol affected saccharin flow indirectly by altering the duration of waking we have computed a correlation between total flow intake of saccharin and two indices of sleep-waking states: (1) total time spent with myoclonic twitching (duration of active sleep) and (2) duration of initial waking period (latency of active sleep onset). Pooled within- group Pearson correlation coefficients are presented in Table 1. On P9 significant but modest positive correlation was found between total saccharin intake and duration of initial waking period (latency of twitching onset) in pups treated with saline (r=0.61; p=0.02) and 0.25 g/kg of ethanol (r=0.57; p=0.04). Significant negative correlation between saccharin intake and amount of time spent in active sleep was found in only one treatment group -- P9 pups injected with 0.5 g/kg ethanol (r=-0.68, p=0.01). On P12 the amount of saccharin consumed was uncorrelated with duration of initial waking period as well as with time spent in twitching activity in all treatment groups (Table. 1).
Table. 1. Correlation between saccharin intake and time spent in waking or sleep.
| Age | Ethanol (g/kg) |
Correlation between flow intake and | |||
|---|---|---|---|---|---|
| time in twitching | sleep latency | ||||
| r | p | r | p | ||
| P9 | 0 | 0.04 | 0.90 | 0.61* | 0.02 |
| P9 | 0.25 | -0.16 | 0.61 | 0.57* | 0.04 |
| P9 | 0.5 | -0.68* | 0.01 | 0.40 | 0.18 |
| P12 | 0 | -0.06 | 0.85 | 0.14 | 0.64 |
| P12 | 0.25 | -0.25 | 0.39 | 0.41 | 0.14 |
| P12 | 0.5 | -0.26 | 0.37 | -0.06 | 0.84 |
3.2. Experiment 2A: Taste reactivity to saccharin infused with low frequency
3.2.1. Flow reactions
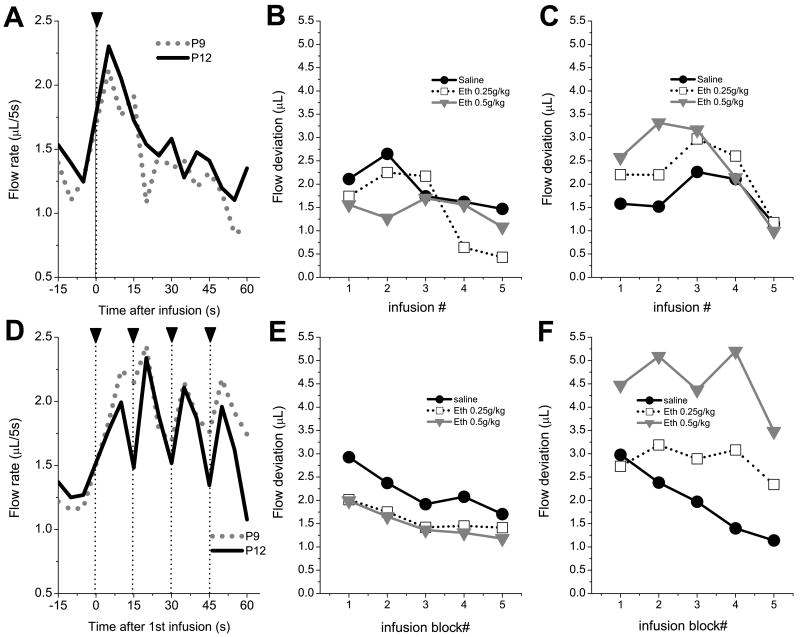
Pups on P9 and P12 responded to intraoral infusions of a small volume of saccharin by increasing water extraction from the cannula (fig. 7A). However, the increase of flow rate was transient, peaking at 10 sec after infusion and returning to baseline values 20 seconds after the infusion. The amplitude of flow reaction averaged across infusions was not affected by ethanol treatment, and there were no significant differences due to age or sex. Comparison of flow reactions to consecutive infusions with 2(age) × 3(dose) × 2(sex) × 5(infusion number) MANOVA revealed only a significant main effect of infusion number, F(4,57)=2.58, p<0.05, Wilks lambda=0.847. In general pups exhibited a biphasic pattern of flow response variations across 5 consecutive infusions with the mean flow rate being increased from first to third infusion and decreased thereafter (fig. 7B,C). However, this pattern was more pronounced on P12 than on P9. No significant main effects of dose and age or any effects of interactions between these factors were found.
Fig.7.
Flow reactions to infusions of 0.1% saccharin delivered at the slow (1/75 s) rate (A,B & C), and at the fast (1/15 s) rate (D,E & F). A and D show temporal patterns of water flow to intraoral infusions of 0.1% saccharin in animals treated with saline. The 60 s periods of flow time series were synchronously averaged against time of presentation of every saccharin infusion (A) or every 5th infusion (D). The 15 s pre-infusion interval is average of flow before all infusions (A) or before the first infusion of the series (D). Mean amplitude of flow reactions in P9 (B,E) and P12 (C,F) pups during 15 s post-infusion intervals across 5 consecutive infusions (B,C) or across 5 blocks of four infusions (E,F). The amplitude of flow was measured as deviation from baseline for 15 s interval.
3.2.2. Behavioral activity
Figure 8A shows the temporal patterns of non-ingestive motor activity and appetitive activity (paw liking and probing) to saccharin infusions in saline –treated animals. There is a clear temporal dissociation between flow reactions to saccharin (fig. 7A, top) and post infusion variations of behavioral activity (fig. 8A). Non-ingestive motor activity was low during the 15 s immediately following fluid infusion when the elevation of flow was observed. Saccharin infusion evoked paw licking and probing but onset of these activities was delayed against primary flow response.
Fig.8.
Overall motor activity (A,B & C) and appetitive taste-elicited behavior (D,E &F) to infusions of 0.1% saccharin. A and D show temporal patterns of behavioral activity during presentation of saccharin infusion at the slow (1/75 s) rate in animals treated with saline. Behavioral times series (measured as % time spent in activity for every 5 s bin) were synchronously averaged against time of presentation of saccharin infusion. B and E show percent time spent in behavioral activities after saccharin infusions delivered at slow (1/75 s) rate as function of age and dose of ethanol. C and F show percent time spent in behavioral activities after saccharin infusions delivered at the fast (1/15 s) rate. Behavior was scored during the whole inter infusion interval.
An effect of age was found for time spent in non-ingestive motor activity, F(1, 60)=9.96; p<0.01, as well as for paw licking and probing, F(1, 60)=5.29; p<0.05, with P12 pups showing a higher level of behavioral activities than P9 animals. The time spent in both types of behavior was not affected by either ethanol treatment or sex of the subject. However, it should be mentioned that pups on P12 spent significantly more time in non-ingestive motor activity than P9 animals after treatment with saline and 0.25 g/kg of ethanol, but after treatment with 0.5 g/kg of ethanol, the level of motor activity decreased in P12 pups and did not significantly differ from that of P9 pups (fig. 8B).
3.3. Experiment 2B: Taste reactivity to saccharin infused with high frequency
3.3.1. Flow reactions
When saccharin was injected every 15 s the flow response to infusion was superimposed on the tail of the preceding flow reaction (fig. 7A bottom). We have split the whole series of 20 infusions into 5 blocks. The 4 flow reactions of every block were averaged and mean values were subjected to a 2(age) × 3(dose) × 2(sex) × 5 (block number) MANOVA with 5 blocks of saccharin infusions treated as repeated measure. The MANOVA revealed main effects of infusion block number, F(4,57)=8.25, p<0.001, Wilks lambda=0.633 (fig 7B,C). Significant univariate effects were also found-as main effects of age and an interaction age × dose, F(2,60)=7.51, p<.01. No main significant effect of sex as well as any significant interactions between sex and other factors were found. Flow reactions of P12 pups treated with 0.5g/kg of ethanol were significantly higher than flow reactions in control P12 animals (fig. 7C) and responses of P9 rats injected with 0.25 g/kg and 0.5 g/kg ethanol (fig. 7B).
As fig, 7B,C shows, flow reactions tended to decline across consecutive blocks of infusions. We applied linear regression analysis to assess the trends of flow reactions and to compare best fit parameters for flow curves between treatments. The regression analysis revealed that slope of flow decline significantly differed from zero in control animals on P9, (b=-0.49±0.16), F(1,58)=8.45, p<0.01, and on P12, (b=-0.54±0.16), F(1,58)=11.75, p<0.01. However, in P12 animals treated with 0.25g/kg or 0.5g/kg ethanol the amplitude of flow reactions did not decline across blocks of infusions -the slope of regression line did not differ from zero. Moreover, after treatment with 0.5g/kg ethanol initial values of flow reactions (intercept) were significantly higher on P12 than on P9, F(1,117)=46.33, p<0.01.
3.3.2. Behavioral activity
When infusions were delivered at a fast rate (Experiment 2B) pups spent less time in motor activity and appetitive behavioral reactions compared to the test with a low infusion presentation rate (Experiment 2A; unpaired t test; P <0.01, fig. 8B,C). However, the patterns of variations of behavioral reactions under different treatments were comparable between Experiments 2A and 2B.
3.4. Experiment 3: Blood ethanol analysis
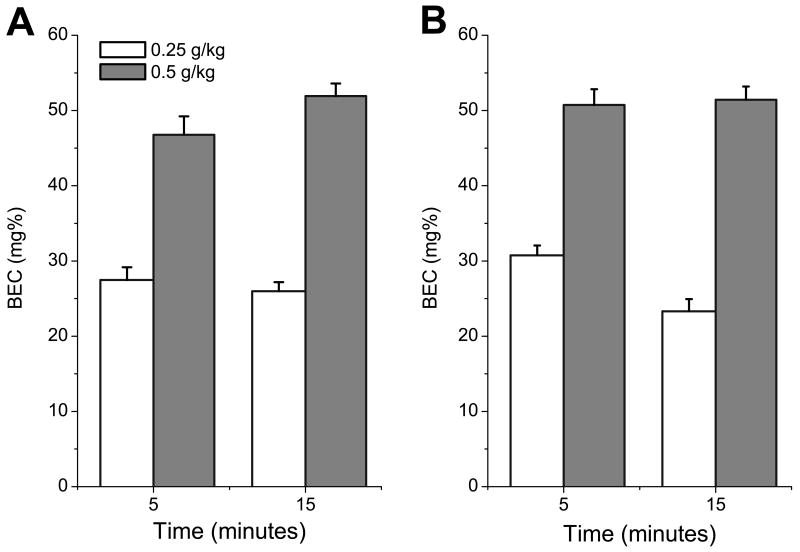
A 2(age) × 2(dose) × 2(time) × 2(sex) ANOVA revealed significant main effects of dose, F(1,144)=215.96; p<0.001 and an interaction of dose × post-administration time F(1,144)=4.27; p<0.05.
Administration of 0.5 g/kg ethanol resulted in significantly higher BEC (50.25±0.89 mg%) than injection of 0.25 g/kg (26.42±0.67 mg%). BEC significantly decreased between 5 and 15 minutes post- injection when animals were treated with 0.25 g/kg ethanol, but did not significantly change across post-administration times after injection of 0.5g/kg ethanol (fig.9). There were no significant effects of age or sex on BEC.
Fig.9.
Brain ethanol concentrations (BEC) following injections of 0.25g/kg and 0.5g/kg of ethanol in P9 (A) and P12 (B) pups as a function of post-injection time.
4. Discussion
The present experiments revealed an ontogenetic dissociation for acute ethanol effects on independent ingestive behavior of infant rats. The 0.5 g/kg dose of ethanol substantially decreased the intake of a palatable fluid (saccharin) in P9 pups, whereas the same dose injected in P12 rats resulted in elevated consumption of saccharin (with the latter effect being significant only in females). The ethanol dose of 0.25 g/kg did not affect intake of 0.1% saccharin, and consumption of water was not affected by either dose of ethanol. BEC measured in animals treated with 0.25 and 0.5 g/kg ethanol attained comparable values on P9 and P12 indicating that different absorption and metabolic utilization of ethanol is not responsible for age effects (fig.9).
The results of the present study suggest an important role of age and sex in ethanol's effects, since patterns of motor activity, ingestive behavior differed between nine-day and twelve- day old animals and between males and females on P12 due to ethanol dose. On P9 the pharmacological effects of ethanol were mostly suppressive (0.5 g/kg of ethanol resulted in 70% decrease of saccharin intake, earlier sleep onset and lower taste responsiveness comparative to saline-treated animals). In contrast, on P12 effects of acute ethanol were more diverse: intake of saccharin was enhanced by 0.5g/kg (however, only in females) even though non-ingestive motor activity was depressed by the same dose of ethanol.
The dissociation between ethanol effects on ingestion and overall motor activity emerging on P12 suggests that at this age ethanol's action on ingestive behavior is “decoupled” from the drug's effects on motor activity and control of sleep -waking. This is distinctly different from subjects receiving ethanol on P9. This “decoupling” indicates that control of ingestive behavior become differentiated from other behavioral mechanisms during the second postnatal week. A more complex pattern of ethanol effects on P12 may be in part due to developmental changes of some principle neurotransmitter systems that occurred between P9 and P12. For example, noradrenergic transmission in the locus coeruleus matures and begins to take on a more adult like pattern around P10 [26], [27]. Furthermore, GABA signaling switches from excitatory to inhibitory between P7 and P14 [28]. The developmental transformations in neurotransmitter operations enable brain networks to organize more selective and “shaped” responses to various types of stimulation.
At the behavioral level more specific and adaptive responding to environmental challenges also emerge during the second postnatal week. According to Hall [29] generalized and non-directed behavioral excitation elicited by milk infusions in 1-, 3- and 6 day old pups changes to more ingestive-specific activation on postnatal days 11 and 12 [20]. Mechanisms of orosensory positive feedback that facilitate intake of sweet solutions also mature during the second postnatal week [30]. In agreement with the these findings, the present experiment showed that specialized appetitive behaviors like probing and paw licking were more prominently exhibited on P12 than on P9 (fig.8, bottom) and these reactions were a bit less sensitive to the sedative effects of ethanol than overall motor activity.
The P9 and P12 animals also differed in baseline levels of arousal. Saline treated animals on P12 showed higher levels of motor activity, longer periods of initial wakefulness and less time spent in active sleep than their younger counterparts. The level of wakefulness affected the efficacy of saccharin consumption because in the present free intake paradigm pups might need to be awake and behaviorally active to extract fluid from a cannula. However, significant correlations between saccharin intake and duration of initial waking period was found only in P9 (r=0.61 and r=0.57 for animals treated with saline and .25 g/kg ethanol) but not in P12 subjects, indicating that ingestive behavior of P9 pups was more dependent on waking state than those of P12 animals. Actually, attenuation of saccharin intake was observed in parallel with decreased latency of sleep onset in ethanol treated P9 animals (compare fig 1 and fig 5) suggesting sedation may be at least partly responsible for this decrease. In contrast, 0.5 g/kg ethanol exerted sedative effects on general waking activity on P12 (expressed as motor suppression and decreased sleep latency) but stimulatory effects on saccharin intake. Therefore, stimulation of saccharin ingestion by ethanol in P12 animals cannot be solely explained by their higher resistance to the sedative effects of ethanol. We suggest that treatment with 0.5 g/kg of ethanol also increased the rewarding properties of the sweet tastant. However, given that increased consumption of saccharin was found only in ethanol-treated P12 females showing little signs of sedation, it could be argued that waking activity should be normal or unsuppressed for the stimulatory effects of ethanol on ingestion to appear. It is important to note, however, that the level of activity for P12 male subjects treated with 0.5 g/kg ethanol was identical to those of females treated with the same dose of the drug. The decrease in male activity due to ethanol treatment was a decrease in comparison to baseline activity which was higher in males than in females (fig. 4A).
One possible explanation for the pattern of ethanol effects observed in P12 pups (increase of saccharin consumption and reduction overall motor activity) could be the anxiolytic properties of the ethanol. During testing the rat pup is isolated from littermates and this is an anxiogenic situation for the infant rat ([31]; [32], [33]). The reduction of isolation-induced stress by ethanol may be responsible for the pups increased ingestion of saccharin in comparison to saline treated controls. Future research will investigate this possibility by explicitly controlling stress levels during the experimental procedure.
As has been shown elsewhere [34], [35] the sweetness of saccharin is not monotonic function of concentration with higher saccharin concentrations being able to elicit aversive reactions. Accordingly, elevated saccharin intake in animals treated with 0.5 g/kg ethanol could be a consequence of amelioration of the aversiveness of saccharin or an increase of its palatability. However, according to our recent data [17] aversive responsiveness of P12 rats to the concentration of saccharin solution (0.1%) used in the present experiments is quite low. Therefore, while the ameliorating effects of ethanol on responsiveness to aversive taste stimulation play an important role in some experimental situations [36], we suggest that in the present experiment ethanol affected intake in P12 animals by increasing their appetitive reactions to saccharin exposure.
According to the parameter estimations of best fit models for the temporal course of saccharin flow, ethanol treatment affected the initial level of saccharin flow rate on P12 as well as on P9. One possible explanation for this effect is changes in perception and acceptance of sweet solutions. Results of the taste reactivity test suggest that orosensory properties of saccharin were affected by treatment with ethanol (fig. 7B,C, bottom). That is, administration of 0.5 g/kg ethanol increased short-term ingestive reactions to very small saccharin infusions on P12 and changed them very little on P9. These changes of flow rate to saccharin infusions, which seem to be primarily sensory driven, indicate that ethanol (0.5 g/kg) may have increased the palatability of saccharin making it more attractive to P12 pups.
Interestingly, that the ethanol –mediated enhancement of oral response to sweet stimulation occurred when the appetitive taste stimulus was delivered at a high rate (one per 15 seconds) with no changes being revealed during presentation of saccharin at the slow rate of delivery (one per 75 seconds). Moreover, under high rates of infusions ethanol-treated animals not only increased the amplitude of flow responses to infusions but also maintained a high magnitude of responding over time (for at least 5 minutes). Given that control animals showed habituation of flow responding to repeated presentation of a taste stimulus (fig. 7, bottom) the sustained flow response over a series of infusions in P12 subjects treated with ethanol may represent ethanol-induced sensitization of taste-elicited-reactions. In other words, 0.5g/kg ethanol may have increased the efficacy (“gain”) of positive orosensory feedback to sweet taste stimulation in P12 pups. Sensitization of mouthing activity to infusions of 10% sucrose has been shown in preweanling rats [20]. The temporal course of this sensitization is strongly affected by rate of taste stimulation, concentration of sweet solution and the deprivation state of the animal. Moreover, sensitization of oral activity interacts in complex ways with sensory habituation, competing behavioral activities and baseline levels of arousal. Comparison between of the present Experiments 2A and 2B (fig. 7, top and bottom) indicates that when pups were exposed to 0.1% saccharin temporal summation of oral responses did not occur if the interval between successive taste stimuli was long enough (about 75 seconds).
When P12 pups voluntarily ingested saccharin through an intraoral cannula the rate of oral contact with the taste stimulus may have been optimal for sensitization of taste stimulation for this particular age and fluid. Actually, during the intake test on P12 the rate of saccharin extraction from the cannula gradually increased from min 1 to min 4 of the intake test (fig. 3B,) after injection of 0.5g/kg ethanol. This rise in flow rate was not observed in saline- treated animals or following an injection of 0.25 g/kg ethanol. Thus, data on the temporal course of flow combined with the results of the taste reactivity experiment suggest that 0.5g/kg ethanol stimulated intake of 0.1% saccharin by making this solution more palatable and by promoting sensitization of ingestive responses to taste stimulation. Due in part to a complex interaction between habituation, sensitization and development of sleep, the temporal pattern of the stimulatory effect of ethanol on saccharin intake is age dependent.
The present data suggest that a relatively low dose of acute ethanol (0.5 g/kg) potentiates ingestion of saccharin by sensitizing the response of P12 females to oral sweet stimulation. This result is in stark contrast to the P12 male subjects, whose intake of saccharin does not change, and the younger (P9) subjects which reduce their intake with increasing ethanol doses. The latter effect seems to correlate with the sedation seen in these young animals. However, the older (P12) animals drink the same (males) or more (females) saccharin even though their motor activity levels are similarly reduced.
Therefore, it would seem that the effects of ethanol on saccharin intake are not only age dependent but also sex dependent, with females significantly more affected by ethanol than their male counterparts. This difference in saccharin intake is seen even at baseline levels with saline treated females ingesting slightly more saccharin than males. One possible explanation for this difference in baseline flow between male and female rat pups (P12) is sex-based sensitivity to sweet taste. Certainly sex differences in preference for salty as well as sweet tastes have been described in adult rats. Female rats have a higher affinity for sweet taste and for higher concentrations of sweet solutions than males [37]. This same pattern was seen in the current experiments albeit surprisingly early in ontogeny. It is possible that ethanol increases the perception of the sweetness of the saccharin solution and female rats are more inclined to increase intake of this extra sweetened substance than males.
Another possible explanation for these results could be patterns of sexual differentiation in the brain of the growing rat pup [38]. Sex differences derived from developmental changes of GABA-related neurotransmission could be responsible for the sex differences in ethanol effects. It has been shown [39] that the excitatory-to-inhibitory switch in GABA signaling occurs later in males (around P17) than in females (around P10). On P12, when the sex related differences appeared in the present experiment, more GABA dependent networks supposedly switched to an adult mode of signaling in females than in males. Given the role of GABAergic neurotransmission for many behavioral actions of ethanol in adults [40], [41] it could be hypothesized that the slower transition from immature to adult types of GABA signaling in males is responsible for sex differences seen on P12. Considering differential sensitivity of males and females to pharmacological treatments in broader context it could be also suggested that sex differences emerge as result of heterochronous maturation of several brain and neurochemical systems in males and females. If that true, then some sex effects could be linked to specific ontogenetic period and they might disappear during development. To test this hypothesis more age groups should be included in further research of pharmacological effects of ethanol in infant rat.
Endogenous opioids and neuropeptides represent another possible neurochemical mechanism that mediates the stimulatory effects of ethanol on ingestion. Although ethanol can stimulate the expression and production of orexigenic peptides [42], [43] it is unlikely that feeding-stimulatory peptides are responsible for ethanol dependent enhancement of saccharin consumption in the present experiments. The increase of orexigenic peptides has been observed at much longer delays after ethanol administration (3 hours and longer) than the enhancement of saccharin intake seen in the present studies (5-7 minutes after i.p. injection). The effects of orexigenic peptides also have been seen at higher doses (1-2.5 g/kg) than those used here. Finally, orexigenic peptides predominantly increase consumption of caloric diets [30], [45], whereas saccharin is devoid of any caloric value.
Numerous studies have shown opioid involvement in the appetitive effects of ethanol in adult animals [46], [47] as well as in infant rats [11]. Endogenous opioids have also been implicated in the hedonic evaluation of food and palatability [48], [49]. Pharmacological blockade of opioid receptors eliminates appetitive effects of acute ethanol in developing rats [11] and similarly, opioid antagonists reduce the palatability of some alcohol solutions in adults [50], [51]. These data combine with the findings of the present study to suggest that opioids could represent a neurochemical link connecting acute effects of ethanol with sensitization of orosensory positive feedback during ethanol consumption in the infant rat.
In our previous study [17] intake of 10% ethanol increased from P9 to P12 in naïve untreated animals while the opposite was found for intake of 0.1% saccharin. This pattern of ontogenetic changes taken together with the present data indicate that the processes implicated in the stimulatory effects of 0.5 g/kg ethanol on saccharin intake of P12 animals may also be responsible for increased consumption of 10% ethanol by P12 naïve untreated pups. Comparison between temporal patterns of saccharin flow (ingestion) in ethanol-treated P12 pups in the present study and those of 10% ethanol flow in previous experiments (fig 6 in [17], suggests that the greater increase in rate of 10% ethanol intake between 7 and 10 minutes of the session may be due to pharmacological effects of ethanol ingested by P12 animals.
In summary, the present data together with results of our previous studies [11], [15], [19] suggest a substantial role of pharmacological effects of ethanol (positive, stimulatory and sedative, inhibitory) in age-related differences of ethanol acceptance and indicates that sensitivity of ingestive behavior to the pharmacological effects of ethanol changes between postnatal days 9 and 12.
Acknowledgments
The research presented in this paper was supported by NIAAA grants R01AA11960, R01AA015992 and R01AA13098 to Norman E. Spear
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: Effects on suckling plasticity. Behav Neurosci. 2001;115:1318–31. [PubMed] [Google Scholar]
- 2.Lee JS, Crawford J, Spear NE. Characteristics and consequences of free-feeding ethanol ingestion during the first two postnatal weeks of the rat. Alcohol Clin Exp Res. 1998;22:1615–1622. [PubMed] [Google Scholar]
- 3.McKinzie DL, Cox R, Murphy JM, Li TK, Lumeng L, McBride WJ. Voluntary ethanol drinking during the first three postnatal weeks in lines of rats selectively bred for divergent ethanol preference. Alcohol Clin Exp Res. 1999;23:1892–1897. [PubMed] [Google Scholar]
- 4.Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–58. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 5.Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: Ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28:1200–11. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- 6.Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: A theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez HD, Bocco G, Chotro MG, Spear NE, Molina JC. Operant responding controlled by milk or milk contaminated with alcohol as positive reinforcers in infant rats. Pharmacol Biochem Behav. 1993;44:403–9. doi: 10.1016/0091-3057(93)90482-9. [DOI] [PubMed] [Google Scholar]
- 8.Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–19. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–79. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 10.Kiefer SW. Alcohol, palatability, and taste reactivity. Neurosci Biobehav Rev. 1995;19:133–41. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- 11.Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: Involvement of the opioid system. Behav Neurosci. 2006;120:267–80. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- 12.Nizhnikov ME, Varlinskaya EI, Spear NE. Reinforcing effects of central ethanol injections in newborn rat pups. Alcohol Clin Exp Res. 2006;30:2089–96. doi: 10.1111/j.1530-0277.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- 13.Nizhnikov ME, Molina JC, Spear NE. Central reinforcing effects of ethanol are blocked by catalase inhibition. Alcohol (Fayetteville, N.Y.) 2007;41:525–34. doi: 10.1016/j.alcohol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nizhnikov ME, Petrov ES, Varlinskaya EI, Spear NE. Newborn rats' first suckling experience: Taste differentiation and suckling plasticity. Physiol Behav. 2002;76:181–98. doi: 10.1016/s0031-9384(01)00672-2. [DOI] [PubMed] [Google Scholar]
- 15.Cheslock SJ, Varlinskaya EI, Silveri MM, Petrov ES, Spear LP, Spear NE. Acute effects of ethanol and the first suckling episode in the newborn rat. Alcohol Clin Exp Res. 2000;24:996–1002. [PubMed] [Google Scholar]
- 16.Petrov ES, Nizhnikov ME, Varlinskaya EI, Spear NE. Dynorphin a (1-13) and responsiveness of the newborn rat to a surrogate nipple: Immediate behavioral consequences and reinforcement effects in conditioning. Behav Brain Res. 2006;170:1–14. doi: 10.1016/j.bbr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Kozlov AP, Varlinskaya EI, Spear NE. Ethanol, saccharin, and quinine: Early ontogeny of taste responsiveness and intake. Alcohol Clin Exp Res. 2008;32:294–305. doi: 10.1111/j.1530-0277.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheslock SJ, Varlinskaya EI, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn's first suckling experience. Alcohol Clin Exp Res. 2001;25:391–402. [PubMed] [Google Scholar]
- 19.Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–91. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- 20.Myers KP, Arnold HM, Hall WG. Sensitization of ingestive responding in preweanling rats: Time course and specificity. Behav Neurosci. 1997;111:413–23. doi: 10.1037//0735-7044.111.2.413. [DOI] [PubMed] [Google Scholar]
- 21.Swithers-Mulvey SE, Miller GL, Hall WG. Habituation of oromotor responding to oral infusions in rat pups. Appetite. 1991;17:55–67. doi: 10.1016/0195-6663(91)90084-6. [DOI] [PubMed] [Google Scholar]
- 22.Swithers-Mulvey SE, Hall WG. Control of ingestion by oral habituation in rat pups. Behav Neurosci. 1992;106:710–7. doi: 10.1037//0735-7044.106.4.710. [DOI] [PubMed] [Google Scholar]
- 23.Kozlov AP, Petrov ES, Varlinskaya EI, Spear NE. Taste differentiation in the context of suckling and independent, adultlike ingestive behavior. Dev Psychobiol. 2006;48:134–145. doi: 10.1002/dev.20123. [DOI] [PubMed] [Google Scholar]
- 24.Gramsbergen A, Schwartze P, Prechtl HF. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson KA, Blumberg MS. The union of the state: Myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–7. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- 26.Moriceau S, Sullivan RM. Neurobiology of infant attachment. Dev Psychobiol. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura F, Nakamura S. Postnatal development of alpha-adrenoceptor-mediated autoinhibition in the locus coeruleus. Brain Res. 1987;432:21–6. doi: 10.1016/0165-3806(87)90004-6. [DOI] [PubMed] [Google Scholar]
- 28.Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in gabaergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- 29.Hall WG. The ontogeny of feeding in rats: I. Ingestive and behavioral responses to oral infusions. Journal of Comparative & Physiological Psychology. 1979;93:977–1000. doi: 10.1037/h0077771. [DOI] [PubMed] [Google Scholar]
- 30.Smith GP. Ontogeny of ingestive behavior. Developmental Psychobiology. 2006;48:345–359. doi: 10.1002/dev.20145. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin GA, Barr GA. Evidence for opioid and nonopioid processes mediating adaptive responses of infant rats that are repeatedly isolated. Dev Psychobiol. 1997;31:217–27. doi: 10.1002/(sici)1098-2302(199711)31:3<217::aid-dev6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: Reversal of stress with maternal stimuli. Dev Psychobiol. 1986;19:385–98. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- 33.Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Dev Psychobiol. 2003;42:206–22. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- 34.Dess NK. Saccharin's aversive taste in rats: Evidence and implications. Neurosci Biobehav Rev. 1993;17:359–72. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin FL, Amit Z. Relative taste thresholds for ethanol, saccharin, and quinine solutions in three strains of rats nonselected for ethanol: A comparative study. Exp Clin Psychopharmacol. 2000;8:216–24. doi: 10.1037//1064-1297.8.2.216. [DOI] [PubMed] [Google Scholar]
- 36.Pautassi RM, Sanders S, Miller S, Spear N, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30:448–59. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- 37.Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science (New York, N.Y.) 1967;156:942–3. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- 38.Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction (Cambridge, England) 2007;133:331–59. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- 39.Kyrozis A, Chudomel O, Moshé SL, Galanopoulou AS. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci Lett. 2006;398:1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–44. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA a receptor subtypes. Pharmacol & Therapeutics. 2006;112:513–28. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leibowitz SF, Avena NM, Chang GQ, Karatayev O, Chau DT, Hoebel BG. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiology & Behavior. 2003;79:103–11. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 43.Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: Relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31:249–59. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 44.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 46.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. Prog Neuro-Psychopharmacol Biol Psychiatry. 1999;23:1171–212. doi: 10.1016/s0278-5846(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 47.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology & Behavior. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 48.Berridge KC. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 49.Ferraro FM, Hill KG, Kaczmarek HJ, Coonfield DL, Kiefer SW. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol (Fayetteville, N.Y.) 2002;27:107–14. doi: 10.1016/s0741-8329(02)00220-3. [DOI] [PubMed] [Google Scholar]
- 50.Coonfield DL, Kiefer SW, Ferraro FM, 3rd, Sinclair JD. Ethanol palatability and consumption by high ethanol-drinking rats: Manipulation of the opioid system with naltrexone. Behav Neurosci. 2004;118:1089–96. doi: 10.1037/0735-7044.118.5.1089. [DOI] [PubMed] [Google Scholar]