Abstract
The amygdala plays key roles in emotion and social cognition, but how this translates to face-to-face interactions involving real people remains unknown. Here we found that a patient with complete amygdala lesions lacks any sense of personal space. Furthermore, healthy individuals showed amygdala activation to close personal proximity. The amygdala may be required to trigger the strong emotional reactions normally following personal space violations, thus regulating interpersonal distance in humans.
People automatically and reliably regulate the distance maintained between themselves and others during social interaction1. Personal space, defined as the area individuals maintain around themselves into which intrusion by others causes discomfort2, is one mechanism by which this automatic regulation of interpersonal distance is achieved. However, little is known regarding the neural substrates of personal space. One candidate brain region is the amygdala, since studies in nonhuman primates have found that this structure is involved in social approach and avoidance3-5. Here we show that one's sense of personal space is dependent on the amygdala.
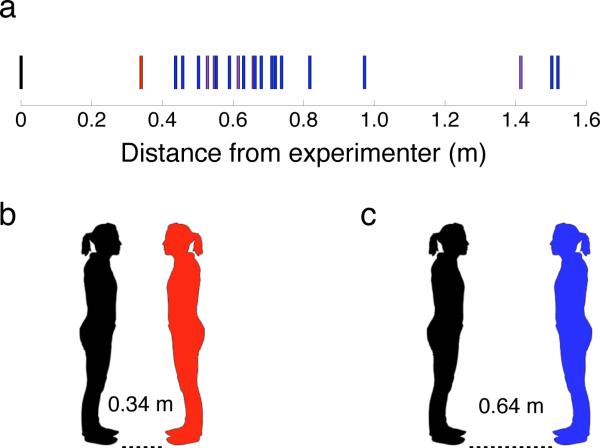
We studied a unique individual, patient SM, a 42-year-old woman with complete bilateral amygdala damage we have described extensively6,7. SM indicated the position at which she felt most comfortable as a female experimenter approached her from 4.7m across the room; chin-to-chin distance was recorded using a digital laser measurer. This procedure was repeated 4 times (counterbalanced with other trial types; see Supplementary Text). SM's preferred distance (0.34±0.02m; mean and standard deviation) was smaller than the smallest preferred distance on any trial of any comparison subject (0.76±0.34m, range = 0.44–1.52m, N=20; Fig. 1) and statistically significantly smaller than that of the comparison group (after excluding the 3 outliers with the largest distance preferences the mean comparison subject distance was 0.64±0.13m, Z=–2.20, p=0.014, one-tailed; with a modified t-test, t(16)= –2.14, p=0.024). This highly abnormal pattern was found reliably across a number of additional conditions (direct/averted gaze; who was walking; starting close or far; a total of 32 trials per subject; Z=–2.38, p=0.009, one-tailed; t(16)= –2.31, p=0.017, one-tailed, excluding 3 outliers), and when SM's distance preferences were compared to female controls alone (Z=–1.93, p=0.027 ; t(11)= –1.86, p=.045). Furthermore, it could not be accounted for by SM's degree of familiarity with the experimenter (see Supplementary Text for detailed results).
Figure 1.
Lesion Study: Mean preferred distances from the experimenter. (A) SM's (red) preference was the closest distance to the experimenter (black), compared to age-, gender-, race-, and education-matched controls (purple, n = 5), as well as general comparison subjects (blue, n=15). (B) SM's mean preferred distance away from the experimenter (image drawn to scale). (C) Control participants’ mean preferred distance away from the experimenter, excluding the 3 largest outliers (image drawn to scale).
Throughout the experiment, SM demonstrated a striking lack of discomfort at close distances. For example, on one trial she walked all the way toward the experimenter to the point of touching, and she repeatedly stated that any distance felt comfortable. We quantified this by asking her to rate her level of discomfort (1 = perfectly comfortable, 10 = extremely uncomfortable) while one of us stood facing her at various distances. Even when nose-to-nose with direct eye contact, SM rated the experience a 1. In a more natural and unexpected context, a completely unfamiliar male confederate stood abnormally close to her while engaging in conversation; SM again rated the experience a 1. By contrast, the confederate rated his experience a 7. While SM indicated afterward that she knew we were “up to something”, awareness that this was an experiment cannot explain her lack of discomfort, since the confederate had complete awareness yet still found the experience to be highly uncomfortable.
At a cognitive level, SM understood the concept of personal space. She spontaneously stated that she did not want to make the experimenter uncomfortable by standing too close, and also stated that she believed her personal space was smaller than most. Furthermore, we asked SM to position the experimenter at the distance she judged other people might feel most comfortable. While she considerably underestimated this distance (0.47±0.03m), her estimation was 38% greater than her own personal preference, thus demonstrating that she is aware that other people have personal space requirements different from her own. The fact that SM had a non-zero distance preference at all may simply reflect typical sensory processing constraints (e.g., too close makes it more difficult to focus on the person).
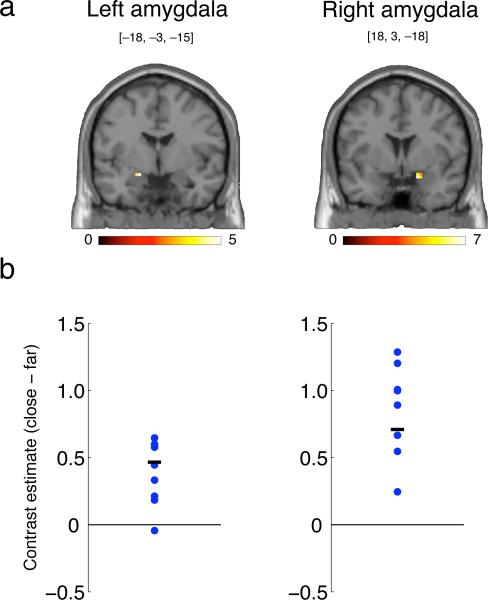
Our findings in SM make a clear prediction regarding the amygdala in healthy individuals: its activity should be modulated by interpersonal distance. As a preliminary test of this prediction, and to obtain corroborating evidence, we conducted a functional magnetic resonance imaging (fMRI) study in eight healthy participants. We found that the amygdala responded to a greater degree when the participants knew an experimenter was maintaining a close distance to them (standing immediately next to the scanner) compared to when they knew an experimenter was maintaining a far distance. This effect was statistically significant at the group level (Fig. 2; see Supplementary Text for details). While we did not collect ratings of subjective comfort from SM or control subjects on the protocol used in this fMRI study, our interpretation of the observed amygdala activation is that it reflects precisely the amygdala-dependent mechanism that comes into play when our personal space is noticeably violated.
Figure 2.
fMRI study: Activation of the amygdala by close (relative to far) interpersonal distance. (A) Coronal slices showing significantly activated voxels in the dorsal amygdala (cluster-level significance, p<0.05); scale shows t-value. (B) Contrast parameters (arbitrary units) for each of the eight subjects who participated in the experiment (extracted from and averaged across all significant voxels in (A); blue dots), along with the group mean (black line). Coordinates for the peak voxel are shown. Subjects were unable to see the position of the experimenter, but were informed of his location at all times. All experiments were approved by Caltech's Institutional Review Board, and informed written consent was obtained from all participants. See Supplementary Text for a detailed description of the experiment.
In sum, we found that the amygdala is differentially activated by proximity to another person, and that complete bilateral damage to this structure in SM results in no detectable personal space boundary and an abnormally small interpersonal distance preference. In various animal species, many social behaviors (including collective group organization and consensus decision-making) can be modeled as a balance between attractive and repulsive forces between individual members of a group8,9. Our findings suggest that the amygdala may mediate the repulsive force that helps to maintain a minimum distance between people. Further, our findings are consistent with those found in monkeys with bilateral amygdala lesions, who stay within closer proximity to other monkeys or people4,5, an effect we suggest arises from the absence of strong emotional responses to personal space violation.
One open question concerns how this mechanism might develop in infants and young children. It is possible that the amygdala is necessary for learning the association between close distances and aversive outcomes, rather than triggering innate emotional responses to close others. Since the developmental course of SM's lesion is unknown, her data cannot distinguish between these two possibilities. A second open question is how this mechanism can accommodate modulation by situational context, personal familiarity, and other factors2,10. Furthermore, there are variations in social distance between individuals, and gross dysregulation in disorders such as autism and Williams Syndrome. These effects could arise in part through modulation of the amygdala from the prefrontal cortex, an effect of considerable recent interest in explaining individual differences and psychiatric disease11.
Supplementary Material
Acknowledgements
We thank Catherine Holcomb for behavioral data collection, Remya Nair and Vikram Chib for help with the fMRI study, and Michael Spezio for helpful discussions. Supported by NIMH and the Simons Foundation (R.A.), and the Della Martin Foundation (D.P.K.).
References
- 1.Hall E. The Hidden Dimension. Doubleday; Garden City: 1966. [Google Scholar]
- 2.Hayduk LA. Psychol. Bull. 1978;85:117–134. [Google Scholar]
- 3.Kluver H, Bucy PC. Am. J. Physiology. 1937;119:352–353. [Google Scholar]
- 4.Emery NJ, et al. Behav. Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- 5.Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- 6.Adolphs R, Tranel D, Damasio AR. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TW, Tranel D, Adolphs R. In: The Human Amygdala. Whalen PJ, Phelps EA, editors. Oxford Univ. Press; New York: 2009. pp. 289–320. [Google Scholar]
- 8.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. J. Theor. Biol. 2002;218:1–11. doi: 10.1006/jtbi.2002.3065. [DOI] [PubMed] [Google Scholar]
- 9.Couzin ID, Krause J, Franks NR, Levin SA. Nature. 2005;433:513–516. doi: 10.1038/nature03236. [DOI] [PubMed] [Google Scholar]
- 10.Hayduk LA. Psychol. Bull. 1983;94:293–335. [Google Scholar]
- 11.Pezawas L, et al. Nat. Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.