Abstract
Organophosphorus agents cause cognitive deficits and depression in some people. We hypothesize that the mechanism by which organophosphorus agents cause these disorders is by modification of proteins in the brain. One such protein could be tubulin. Tubulin polymerizes to make the microtubules that transport cell components to nerve axons. The goal of the present work was to measure the effect of the organophosphorus agent chlorpyrifos oxon on tubulin polymerization. An additional goal was to identify the amino acids covalently modified by chlorpyrifos oxon in microtubule polymers and to compare them to the amino acids modified in unpolymerized tubulin dimers. Purified bovine tubulin (0.1 mM) was treated with 0.005-0.1 mM chlorpyrifos oxon for 30 min at room temperature and then polymerized by addition of 1 mM GTP to generate microtubules. Microtubules were visualized by atomic force microscopy. Chlorpyrifos oxon-modified residues were identified by tandem ion trap electrospray ionization and matrix-assisted laser desorption/ionization mass spectrometry of tryptic peptides. Nanoimaging showed that low concentrations (0.005 and 0.01 mM) of chlorpyrifos oxon yielded short, thin microtubules. A concentration of 0.025 mM stimulated polymerization, while high concentrations (0.05 and 0.1 mM) caused aggregation. Of the 17 tyrosines covalently modified by chlorpyrifos oxon in unpolymerized tubulin dimers, only 2 tyrosines were labeled in polymerized microtubules. The two labeled tyrosines in polymerized tubulin were Tyr 103 in EDAANNY*R of alpha tubulin, and Tyr 281 in GSQQY*R of beta tubulin. In conclusion, chlorpyrifos oxon binding to tubulin disrupts tubulin polymerization. These results may lead to an understanding of the neurotoxicity of organophosphorus agents.
Keywords: nanoimages, tubulin polymerization, chlorpyrifos oxon, mass spectrometry
Introduction
Exposure to organophosphorus pesticides has been linked to memory loss, learning disability, fatigue, depression, and Parkinson’s disease (Abou-Donia, 2003; Salvi et al., 2003; Beseler et al., 2006; Kamel et al., 2007; Hancock et al., 2008). These symptoms may appear after exposures too low to significantly inhibit acetylcholinesterase (Srivastava et al., 2000). A mechanism to explain the low dose toxicity of organophosphorus agents is needed. Tubulin has been implicated in neurodegenerative diseases (Gendron and Petrucelli, 2009) and may also have a role in low dose organophosphorus agent effects. Behavioral studies in rats treated with low doses of chlorpyrifos, followed by a wash out period before testing cognitive function in a water-maze hidden platform task, revealed memory impairment (Terry et al., 2007). In vitro tests of tubulin function in a turbidity assay showed that very low doses of chlorpyrifos oxon inhibited tubulin polymerization (Prendergast et al., 2007). Mice treated with a biotin labeled organophosphorus agent called FP-biotin have FP-biotin labeled tubulin in the brain (Grigoryan et al., 2009b). In vitro studies with purified bovine brain tubulin have identified tyrosine in tubulin as the site of covalent attachment of FP-biotin, chlorpyrifos oxon, soman, sarin, and diisopropylfluorophosphate (Grigoryan et al., 2008). One tyrosine is labeled rapidly in bovine tubulin, while an additional 16 tyrosines can be labeled at least partially with a 40-fold molar excess of chlorpyrifos oxon in a 24 h reaction at 37°C (Grigoryan et al., 2009b).
In the present study we tested the ability of tubulin to polymerize into microtubules after tubulin dimers had been treated with various concentrations of chlorpyrifos oxon. Microtubules are part of the cytoskeletal system that maintains the morphology of neurons including axonal and dendritic processes (Gendron and Petrucelli, 2009). Microtubules serve as highways for transport of mitochondria, synaptic vesicles, components of ion channels, receptors, and scaffolding proteins to and from synaptic sites. Synapses are highly vulnerable to impairments in transport. Perturbations to microtubule transport could cause malfunctions in neurotransmission and signal propagation and lead to synaptic degradation (Gendron and Petrucelli, 2009). Drugs that inhibit tubulin polymerization, for example colchicine, trigger apoptosis (Yeste-Velasco et al., 2008). Thus, the ability of tubulin to polymerize is very important for the life of a cell. We tested the hypothesis that modification of tubulin by chlorpyrifos oxon would affect tubulin polymerization. Tubulin polymers were visualized by atomic force microscopy. It was found that substoichiometric concentrations of chlorpyrifos oxon yielded short, thin microtubules. In contrast, stoichiometric concentrations of chlorpyrifos oxon resulted in clumping of tubulin. The nanoimaging study leads to the conclusion that chlorpyrifos oxon disrupts tubulin polymerization.
Materials and methods
Materials
Bovine brain tubulin (TL238) > 99% pure, purchased from Cytoskeleton, Inc (Denver, CO), contains dimers of alpha beta-tubulin. Chlorpyrifos oxon (MET-674B, Chem. Service Inc.,West Chester, PA) was diluted into dimethylsulfoxide and stored at -80°C. Guanosine 5′-triphosphate sodium salt hydrate (GTP, G8877) ≥95% HPLC pure powder; 1,4-piperazinediethanesulfonic acid (PIPES, P6757) > 99% pure powder; and colchicine (C9754) ≥95% HPLC pure powder were from Sigma (St Louise, MO). Sequencing grade modified porcine trypsin (V5113) was from Promega (Madison, WI). Slide-A-Lyzer 7K dialysis cassettes (No. 66370) were from Pierce Biotechnology Inc. (Rockford, IL). Alpha-cyano-4-hydroxycinnamic acid, and Cal Mix 5 were from Applied Biosystems (MDS Sciex, Foster City, CA). All other chemicals were of analytical grade.
Tubulin polymerization for nanoimaging
Tubulin was treated with chlorpyrifos oxon for 30 min before it was polymerized. Each tube contained 0.05 ml of 5 mg/ml tubulin (0.1 mM) in PEM buffer (80 mM PIPES, 2 mM magnesium chloride and 0.5 mM EGTA, pH 7.0) and 0, 0.005, 0.01, 0.025, 0.05, or 0.1 mM chlorpyrifos oxon. After 30 min at room temperature, samples were stored on ice for a brief period while GTP was added. Tubulin polymerization was initiated by addition of GTP to a concentration of 1 mM and continued for 2 h at 37°C. Samples were turbid after 2 h, confirming that tubulin had polymerized. A control to show the effect of inhibition of tubulin polymerization was tubulin incubated with 0.025 mM colchicine and 1 mM GTP (Skoufias and Wilson, 1992). A control to show the background from unpolymerized tubulin was tubulin in PEM buffer without GTP.
Nanoimages of microtubules
Samples for atomic force microscopy were fixed with 0.25% glutaraldehyde and diluted 10-fold before deposition of 5-7 μL onto freshly cleaved mica. The freshly cleaved mica had been treated with a 0.167 mM aqueous solution of 1-(3-aminopropyl) silatrane. This chemical treatment of the mica surface allows the sample to be deposited in a wide range of ionic strengths and pH. The negatively charged microtubules do not adsorb to untreated mica because untreated mica is negatively charged. After 2 min the samples were thoroughly rinsed with deionized water (Labconco Co., Kansas City, MO) and dried under argon gas flow. Images were acquired in air using a MFP-3D AFM microscope (Asylum Research, Santa Barbara, CA) operated in tapping mode. Pictures were acquired on average from 7 different 20 × 20 μm areas. Image processing and microtubule characterization were performed using Femtoscan software (Advanced Technology Center, Moscow, Russia) (Lushnikov et al., 2006). -
Mass spectrometry to identify sites modified by chlorpyrifos oxon
Bovine brain tubulin (0.5 mg/ml) in PEM buffer pH 7.0 containing 1 mM GTP and 20% glycerol was polymerized in an Eppendorf Thermomixer R thermoblock (Westbury, NY) for 3 h at 37°C. After three hours the sample was turbid, an indication that the tubules had polymerized. Chlorpyrifos oxon was added to a final concentration of 0.5 mM and incubation was continued at 37 °C for 24 h. Two control samples were 1) tubulin treated with chlorpyrifos oxon while the tubulin was an unpolymerized dimer, and 2) tubulin not treated with chlorpyrifos oxon. Protein was denatured by boiling in a water bath for 10 min. Excess chlorpyrifos oxon was removed by dialysis against 4 L of 10 mM ammonium bicarbonate (pH 8.3) at 4°C overnight. Tubulin (250 μg) was digested with 2.5 μg of Promega trypsin at 37°C overnight. One microliter of tryptic digest was spotted on a 384 well Opti-TOF sample plate (# 1016491, Applied Biosystems, Foster City, CA), air dried and overlaid with 1 μl of 10 mg/ml alpha-cyano-4-hydroxycinnamic acid dissolved in 50% acetonitrile, 0.1% trifluoroacetic acid. Mass spectra were acquired in positive ion reflector mode on a MALDI TOF TOF 4800 mass spectrometer (Applied Biosystems). The final spectrum was the average of 500 laser shots. The mass spectrometer was calibrated before each use with CalMix 5 (Applied Biosystems).
Tryptic digests were also analyzed by LCMSMS in a QTRAP 2000 tandem ion trap electrospray mass spectrometer (Applied Biosystems) as previously described (Lockridge et al., 2008).
Statistical analysis of microtubule parameters
The width of untreated and chlorpyrifos oxon treated microfibrils was measured using Femtoscan software (Advanced Technology Center, Moscow, Russia) (Lushnikov et al., 2006). On average 15-70 different fibrils from each sample were analyzed from at least 4-5 different areas. A one-way ANOVA was performed to determine statistical significance between the chlorpyrifos oxon treated groups compared to the control group. SPSS software (Microsoft Corp.) was used for this analysis.
Results
Concentration dependent effect of chlorpyrifos oxon on tubulin polymerization
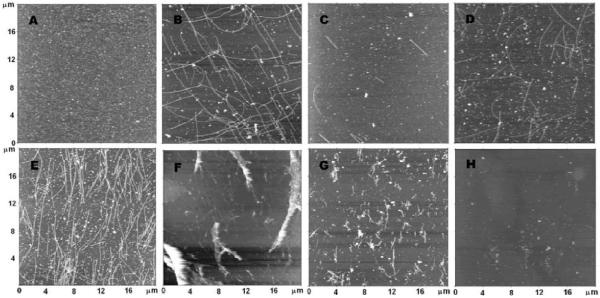
The effect of chlorpyrifos oxon on tubulin polymerization was evaluated by acquiring nanoimages of microtubules formed in the absence and in the presence of chlorpyrifos oxon. Five different concentrations of chlorpyrifos oxon were used. In addition, images were collected of unpolymerized tubulin and of tubules whose polymerization was inhibited by 0.025 mM colchicine. The results are illustrated at different magnifications in Figure 1 (20 × 20 μm) and Figure 2 (2 × 2 μm).
Figure 1.
Effect of chlorpyrifos oxon on tubulin polymerization visualized by atomic force microscopy. A) 0.1 mM bovine tubulin in PEM buffer, no GTP; B) 0.1 mM tubulin polymerized with 1 mM GTP, no chlorpyrifos oxon; C) 0.1 mM tubulin pretreated with 0.005 mM chlorpyrifos oxon before polymerization with GTP; D) pretreated with 0.010 mM chlorpyrifos oxon before polymerization; E) pretreated with 0.025 mM chlorpyrifos oxon before polymerization; F) pretreated with 0.050 mM chlorpyrifos oxon before polymerization; G) 0.1 mM tubulin pretreated with 0.1 mM chlorpyrifos oxon before polymerization; H) polymerized in the presence of 0.025 mM colchicine. The dimensions of each panel are 20 × 20 μm. Images are presented in height trace mode.
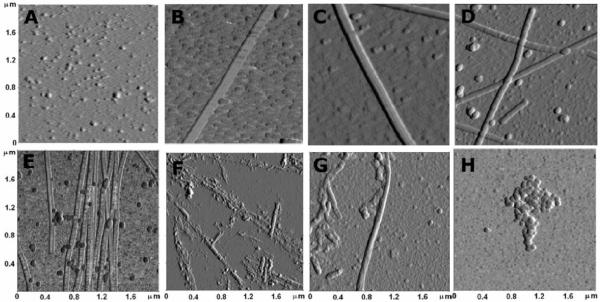
Figure 2.
Ten times magnified view of polymerized tubulin. Samples are the same as in Figure 1. A) no GTP, no chlorpyrifos oxon; B) control with GTP, no chlorpyrifos oxon; C) 0.005 mM chlorpyrifos oxon; D) 0.010 mM chlorpyrifos oxon; E) 0.025 mM chlorpyrifos oxon; F) 0.050 mM chlorpyrifos oxon; G) 0.100 mM chlorpyrifos oxon; H) 0.025 mM colchicine. The dimensions of each panel are 2 × 2 μm. Images are presented in phase trace mode.
Figure 1A shows unpolymerized tubulin, prepared in the absence of GTP. The background is full of small particles.
Figure 1B shows microtubules polymerized in the presence of 1 mM GTP without chlorpyrifos oxon. These microtubules are straight, well structured, and individually separated. They appear uniform in width and are long (average length 10.4± 1 μm). The background has fewer small particles than Figure 1A. The average number of microtubules in a 20 × 20 μm area was 60.7±6. See Table 1.
Table 1.
Microtubule length, density, and width as a function of chlorpyrifos oxon (CPO) concentration during polymerization of 0.1 mM bovine tubulin
| [CPO], mM | length, μm | number of tubules in 20 × 20 μm area | width, nm |
|---|---|---|---|
| 0 (control) | 10.4±1 | 60.7±6 | 84.2±20 |
| 0.005 | 4.3±0.4 | 8.3±0.8 | 75.2±7 |
| 0.010 | 6±0.6 | 49.6±5 | 72.1±8 |
| 0.025 | >20 | 135 | 65.1±4.9 |
| 0.050 | clumps | clumps | clumps |
| 0.100 | clumps | clumps | clumps |
A one-way ANOVA on the width of fibrils at each concentration of chlorpyrifos oxon demonstrated statistically significant differences p - 0.04 for the 0.01 mM treated group and p = 0.002 for the 0.025 mM group compared to control.
Figure 1C shows the effect of pretreatment of 0.1 mM tubulin with 0.005 mM chlorpyrifos oxon. There is a drastic reduction in the number of microtubules, and in their length (average length 4.3±0.4 μm). The average number of tubules in a 20 × 20 μm area was 8.3±0.8, indicating that 0.005 mM chlorpyrifos oxon inhibited tubulin polymerization 7.3-fold.
Figure 1D shows the effect of pretreatment of 0.1 mM tubulin with 0.010 mM chlorpyrifos oxon. Microtubule density and length are reduced compared to Figure 1B where tubulin had not been treated with chlorpyrifos oxon. However, there are more microtubules than were found after treatment with 0.005 mM chlorpyrifos oxon, and they are longer (average length 6±0.6 μm). The average number of tubules in a 20 × 20 μm area was 49.6±5 indicating 0.010 mM chlorpyrifos oxon inhibited polymerization only 1.2-fold.
Figure 1E shows that pretreatment with 0.025 mM chlorpyrifos oxon stimulates microtubule polymerization. The density of microtubules is 2-fold greater than in Figure 1B, with an average of 135 tubules in a 20 × 20 μm area. Microtubules were not well separated, they were clustered, and they were longer than those formed in the absence of chlorpyrifos oxon, on average being longer than 20 μm.
Figure 1F shows that pretreatment with 0.050 mM chlorpyrifos oxon causes microtubules to clump. The structures also seem to be more fragile, with the blurred images likely being the result of deformation induced by the atomic force microscope probe.
Figure 1G shows that pretreatment of 0.1 mM tubulin with 0.1 mM chlorpyrifos oxon causes further degradation of the microtubules.
Figure 1H shows a nearly blank background for microtubules polymerized in the presence of 0.025 mM colchicine. Colchicine is a potent inhibitor of tubulin polymerization (Skoufias and Wilson, 1992).
Figure 2 shows images comparable to those in Figure 1, after 10-fold magnification. Such magnification provides better images for analysis of detailed structural differences, including measurement of width. It is tempting to propose that the globules in Figure 2A are tubulin dimers. This interpretation is based on the similarity of the size of these particles to those in Figure 2H, where polymerization has been precluded by the presence of colchicine.
The control microtubules in Figure 2B show an average width of 84.2±20 nm. The average width of microtubules prepared from tubules pretreated with 0.005 mM chlorpyrifos oxon was 75.2±7 nm (Figure 2C), and those pretreated with 0.010 mM chlorpyrifos oxon was 72.1±8 nm (Figure 2D). Pretreatment of 0.1 mM tubulin with 0.025 mM chlorpyrifos oxon resulted in abnormal polymerization (Figure 2E) and a further reduction in the average width to 65.1±4.9 nm. Thus pretreatment of tubulin with chlorpyrifos oxon reduced the width of microtubules compared to that for control microtubules.
The picture dramatically changed when 0.1 mM tubulin was incubated with 0.05 and 0.10 mM chlorpyrifos oxon (Figure 2F and 2G). Under those conditions, the most prominent structures are large aggregates. In addition there are fine elongated shapes that suggest narrow microtubules may have been formed, but then have been degraded during sample preparation. In Figure 2G the tubules are fragmented. The widths of clumps and fragments of tubules were not measured.
No microtubules are present in Figure 2H for the sample treated with 0.025 mM colchicine. Only small aggregates and particles are present. The nanoimages confirm that colchicine is a potent inhibitor of tubulin polymerization.
Figures 1 and 2 define a triphasic process which implies the binding of at least three molecules of chlorpyrifos oxon to tubulin. The initial binding results in a decrease in polymerization, the second binding causes an increase, and the third results in destruction of the microtubules. We have previously demonstrated that as many as 17 tubulin tyrosines can be labeled by chlorpyrifos oxon.
Sites in polymerized microtubules modified by chlorpyrifos oxon
The above nanoimaging study showed that chlorpyrifos oxon binding to tubulin interfered with tubulin polymerization. In previous work we had identified 17 tyrosines in the unpolymerized tubulin αβ-dimer that are covalently modified by treatment with a 40-fold molar excess of chlorpyrifos oxon (Grigoryan et al., 2008; Grigoryan et al., 2009b). Those labeled sites are listed in Table 2. In the present study we tested the hypothesis that some of these chlorpyrifos oxon binding sites would be blocked in polymerized tubulin and therefore would be unavailable for reaction with chlorpyrifos oxon.
Table 2.
Chlorpyrifos oxon-labeled tryptic peptides from bovine alpha1 and beta2 tubulin identified by mass spectrometry
| Tubulin chain | peptide | CPO labeled tyrosine in tubulin dimer | CPO labeled tyrosine in polymerized microtubules | contact type in crystal structure |
|---|---|---|---|---|
| alpha | TGTY*R | 83 | ||
| EDAANNY*AR | 103 | 103 | ||
| GHY*TIGK | 108 | |||
| LSVDY*GK | 161 | lateral | ||
| NLDIERPTY*TNLNR | 224 | longitudinal GTP binding | ||
| FDGALNVDLTEFQTNLVPY*PR | 262 | longitudinal | ||
| IHFPLATY*APVISAEK | 272 | |||
| VGINY*QPPTVVPGGDLAK | 357 | |||
| FDLMY*AK | 399 | longitudinal | ||
| beta | INVY*YNEATGGK | 50 | lateral | |
| INVYY*NEATGGK | 51 | lateral | ||
| Y*VPR | 59 | |||
| GHY*TEGAELVDSVLDVVR | 106 | |||
| EEY*PDR | 159 | lateral | ||
| GSQQY*R | 281 | 281 | lateral | |
| Y*LTVAAVFR | 310 | |||
| NSSY*FVEWIPNNVK | 340 |
Numbering of the organophosphorylated residues corresponds to accession numbers gi 15988311 for alpha and gi 15988312 for beta tubulin. The crystal structure of the bovine tubulin heterodimer (Protein Data Bank code 1jff) identifies residues involved in contacts in the microtubule (Nogales et al., 1999). Chlorpyrifos oxon (CPO) labeled residues in the tubulin dimer were identified by mass spectrometry in previous publications (Grigoryan et al., 2008; Grigoryan et al., 2009), while those in polymerized microtubules are reported in the present work.
The polymerized microtubules were incubated with 0.5 mM chlorpyrifos oxon at 37°C for 24 h. To show the difference in site availability, tubulin that had not been polymerized was also treated with 0.5 mM chlorpyrifos oxon. Another control was tubulin not treated with chlorpyrifos oxon and not polymerized.
Covalent binding of chlorpyrifos oxon to tubulin was determined in two ways. First, the masses of tryptic peptides from the tubulin chlorpyrifos oxon reaction mixture were compared to masses of tryptic peptides from unreacted tubulin using the MALDI TOF TOF 4800 mass spectrometer. Labeled peptides gained 136 amu following reaction with chlorpyrifos oxon due to formation of a diethoxyphosphate adduct. MALDI analysis was performed to obtain an estimate of the extent of labeling. The relative intensities of the labeled and unlabeled peptides were taken as a measure of the extent of labeling. This estimate assumes that the diethoxyphosphate label did not significantly affect the ionization properties of the peptide. Second, the labeled peptides were isolated and fragmented in the QTRAP 2000 mass spectrometer after online liquid chromatography separation. We have found that such LCMSMS analysis with the QTRAP mass spectrometer revealed more labeled peptides than the MALDI, and that the fragmentation spectra were more readily analyzed. Fragmentation spectra were manually analyzed to confirm the sequence of the peptide and to identify the labeled amino acid.
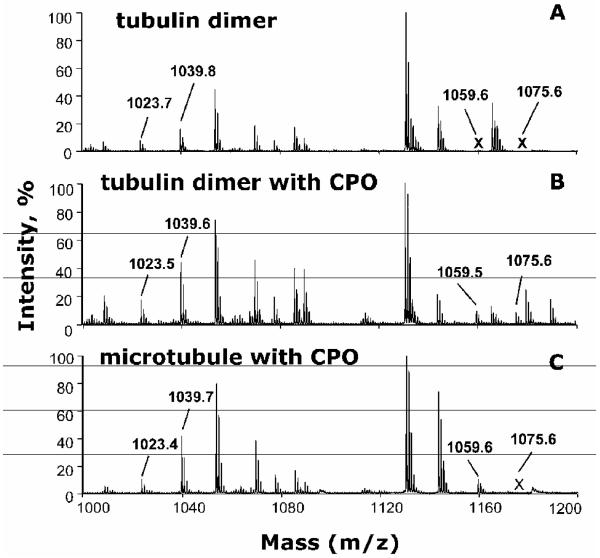
An example of the MALDI TOF TOF analysis is given in Figure 3. Figure 3A shows the MALDI mass spectrum of tubulin that has not been treated with chlorpyrifos oxon. The peak at 1023.5 amu is consistent with the unlabeled peptide EDAANNYAR of alpha tubulin. The peak at 1039.6 amu is consistent with the unlabeled YLTVAAVFR from beta tubulin. Figure 3B shows the MALDI mass spectrum of tubulin treated with chlorpyrifos oxon, but not polymerized. The unlabeled peaks at 1023.5 and 1039.6 amu are present, but in addition two new peaks have appeared. The new peaks at 1059.6 and 1075.6 amu correspond to chlorpyrifos oxon labeled EDAANNY*AR and Y*LTVAAVFR peptides. The added mass of +136 amu from chlorpyrifos oxon was shown to be on the tyrosine (Y*), indicated by an asterisk, by subsequent fragmentation in the QTRAP mass spectrometer. Comparison of the relative peak heights for the labeled and unlabeled peptides suggests that 30% of peptide EDAANNYAR and 15% of peptide YLTVAAVFR were labeled. Figure 3C shows the MS spectrum of microtubules that had been treated with chlorpyrifos oxon after the tubulin had polymerized. The unlabeled peaks at 1023.5 and 1039.6 amu are present in Figure 3C. One labeled peak, at 1059.6 amu is present, but there is no peak at 1075.6 amu. This shows that tyrosine 103 in peptide EDAANNY*AR was labeled in polymerized microtubules, but that tyrosine 310 in peptide YLTVAAVFR was not. The relative peak heights of the unlabeled and labeled EDAANNY*AR are similar, suggesting that 50% of peptide EDAANNYAR was labeled.
Figure 3.
MS spectra showing chlorpyrifos oxon-labeled and unlabeled tubulin tryptic peptides. A) control tubulin dimer without chlorpyrifos oxon (CPO); B) tubulin dimer treated with 0.5 mM CPO; C) polymerized microtubules treated with 0.5 mM CPO. Peaks at 1023.5 and 1039.6 amu correspond to unlabeled EDAANNYAR and YLTVAAVFR peptides from alpha and beta tubulin, respectively. Peaks at 1059.6 and 1075.6 amu correspond to CPO labeled (+136 amu added mass from CPO) EDAANNY*AR and Y*LTVAAVFR peptides. The labeled peak at 1059.5 amu is present in panels B and C. The labeled peak at 1075.6 amu is present only in panel B. The letter X in panels A and C indicates absent peaks.
The identity of the labeled residue was determined from tandem MSMS spectra acquired in the QTRAP 2000 mass spectrometer. Peptides were partially separated by liquid chromatography on a C18 reverse phase column before being electrosprayed into the mass spectrometer. This method confirmed the list of 17 labeled peptides given in Table 2 for tubulin treated with chlorpyrifos oxon before polymerization (Grigoryan et al., 2008; Grigoryan et al., 2009b). Only 2 labeled peptides were identified when polymerized tubulin was treated with chlorpyrifos oxon. The first, EDAANNY*AR, was described above in the section on MALDI analysis. The second was GSQQY*R where tyrosine 281 had an added mass of +136 (Table 2). MSMS spectra for each labeled residue can be found in previous publications (Grigoryan et al., 2008; Grigoryan et al., 2009b).
Table 2 indicates residues involved in contacts between protofilaments in the crystal structure (Nogales et al., 1999). Eight of the chlorpyrifos oxon labeled tyrosines are involved in contacts, suggesting that modification of these tyrosines alters the conformation of tubulin dimers and therefore accounts for the abnormal polymer structures observed in Figure 1. Only 2 of the 17 tyrosines were available for covalent binding by chlorpyrifos oxon when polymerized microtubules were treated with chlorpyrifos oxon, suggesting that the other 15 tyrosines were buried in the polymer structure and therefore involved in microtubule contacts.
Discussion
Nanoimaging to measure effect of organophosphorus agents on tubulin polymerization
The classical way to measure tubulin polymerization is by increase in turbidity. This method was not satisfactory for measurement of the effect of chlorpyrifos oxon because all concentrations of chlorpyrifos oxon showed similar increases in turbidity. A lack of concentration-dependence has been reported by others (Prendergast et al., 2007). However, nanoimaging revealed pronounced concentration-dependent effects of chlorpyrifos oxon on tubulin polymerization. This is the first report to use atomic force microscopy to measure the effect of an organophosphorus agent on tubulin polymerization.
Thin, short microtubules
Microtubules grow by elongation of open sheets that later close into a cylinder. Lateral interactions between adjacent protofilaments govern the stability of microtubules. The finding of thin microtubules in chlorpyrifos oxon treated samples suggests that protofilaments were lost. A normal microtubule contains 13 protofilaments, but the thin microtubules probably contain fewer. This explanation was offered for the thin microtubules observed in mice deficient in crystallins chaperones (Xi et al., 2006), and seems applicable to the thin microtubules observed in our study.
The width of wild-type mouse microtubules measured by electron microscopy is 27±3 nm (Xi et al., 2006) and the width of bovine microtubules measured in fluid tapping mode atomic force microscopy is also 27±3 nm (Wagner et al., 2004). In contrast, the width of wild-type bovine microtubules measured by atomic force microscopy of dried samples in our experiments was 84 nm. The width of porcine microtubules measured by scanning force microscopy was 70-100 nm (Vater et al., 1995), similar to the values we obtained. The larger widths of microtubules in the latter experiments are explained by collapse and flattening of the hollow tube structure during drying of the sample.
Abnormally short microtubules, such as those observed after treatment with 0.005 or 0.01 mM chlorpyrifos oxon, suggest that these chlorpyrifos oxon-modified microtubules may be fragile and break along their length. Alternatively, adducts formed with chlorpyrifos oxon may partially block polymerization. At 0.01 mM chlorpyrifos oxon, the only tyrosine found to be significantly labeled was Tyr 83, in the alpha tubulin peptide TGTY*R. About 12% of Tyr 83 was covalently modified (Grigoryan et al., 2009b). Tyrosine 83 is not known to be involved in lateral or longitudinal contacts in the crystal structure (Nogales et al., 1999), but it did not appear to be labeled in microtubules. This observation suggests that its accessibility is changed upon polymerization and that labeling this residue might alter the capacity of tubulin to change shape during polymerization, thereby inhibiting polymerization. Alternatively, since excess chlorpyrifos oxon was not removed before polymerization, the possibility cannot be ruled out that the short microtubules that are generated upon treatment with 0.005 and 0.01 mM chlorpyrifos oxon are due to noncovalent binding of chlorpyrifos oxon to tubulin. Excess chlorpyrifos oxon was not removed prior to polymerization because dialyzed tubulin did not polymerize.
Stimulation of polymerization
Pretreatment of 0.1 mM tubulin with 0.025 mM chlorpyrifos oxon, yielded a higher than normal number of microtubules. This is opposite to the effect seen at lower concentrations of chlorpyrifos oxon. We speculate that the additional sites bound by chlorpyrifos oxon at the higher concentration had a stabilizing effect on microtubule structure, similar to that of taxol (Derry et al., 1995).
Aggregation
When 0.1 mM tubulin was pretreated with 0.05 and 0.1 mM chlorpyrifos oxon, the microtubules clumped into aggregates. At these concentrations of chlorpyrifos oxon, alpha tubulin is covalently modified on about 40% of Tyr 83, 7% of Tyr 224, and 5% of Tyr 272, while beta tubulin is covalently modified on 10% of Tyr 59, 2% of Tyr 106, and 20% of Tyr 281 (Grigoryan et al., 2009). Some of these residues are involved in contacts between protofilaments and in GTP binding (Table 1), suggesting that their modification resulted in structurally altered polymers. The nanoimages suggest that binding of chlorpyrifos oxon destabilized the structure of the tubules.
These high concentrations of chlorpyrifos oxon are not going to be found in living mammalian species. High concentrations are mainly useful for finding labeled peptides in the mass spectrometer.
Defective tubulin assembly in neurodegenerative diseases
Disruption of microtubule polymerization in neuronal axons has been implicated as a mechanism leading to neurodegenerative diseases (Gendron and Petrucelli, 2009). Alzheimer disease brains have defective microtubule assembly (Iqbal et al., 1986). We hypothesize that the neurotoxicity of organophosphorus agents may be linked to disruption in microtubule function.
Modification of microtubule associated proteins
Covalent binding of organophosphorus agents to tyrosine in a variety of proteins has been found (Grigoryan et al., 2009a). Therefore reaction of chlorpyrifos oxon with other proteins involved in tubulin polymerization may also occur. Prendergast et al have shown that chlorpyrifos oxon treatment of rat brain slices causes a marked decrease in the concentration of microtubule associated protein-2 and results in neuron injury (Prendergast et al., 2007). Since this protein promotes tubulin polymerization, it is reasonable to propose that microtubule associated protein-2, as well as other microtubule associated proteins such as kinesin (Gearhart et al., 2007) may be modified by chlorpyrifos oxon. Thus, disruption of microtubule function may involve chlorpyrifos oxon modification of several proteins including tubulin and proteins associated with microtubules.
Acknowledgement
Nanoimages were obtained with the support of Dr. Luda S. Shlyakhtenko, co-director, and Dr. Yuri L. Lyubchenko, director of the Nanoimaging Core Facility at the University of Nebraska Medical Center in the Department of Pharmaceutical Sciences. The Nanoimaging Core Facility is supported by grants from NIH (SIG program), the UNMC Program of Excellence (POE) and the Nebraska Research Initiative (NRI). Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center. We thank Ellen G. Duysen for help with statistical analysis. Supported by the U.S. Army Medical Research and Materiel Command W81XWH-07-2-0034, the National Institutes of Health U01 NS058056 and P30CA36727, and DGA/PEA grants 08co501 and ANR-06-BLAN-0163.
Abbreviations
- CPO
chlorpyrifos oxon
- PEM buffer
80 mM PIPES, 2 mM magnesium chloride and 0.5 mM EGTA, pH 7.0
- MS
mass spectrometry
- MALDI TOF TOF
matrix-assisted laser desorption ionization time of flight mass spectrometry
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–497. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- Beseler C, Stallones L, Hoppin JA, Alavanja MC, Blair A, Keefe T, Kamel F. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the agricultural health study cohort. J Occup Environ Med. 2006;48:1005–1013. doi: 10.1097/01.jom.0000235938.70212.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry WB, Wilson L, Jordan MA. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr. Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Jiang W, Schopfer LM, Peeples ES, Duysen EG, Thompson CM, Lockridge O. Mass spectrometry identifies multiple organophosphorylated sites in tubulin: dynamics of chorpyrifos oxon binding. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: A potential mechanism of long term toxicity by organophosphorus agents. Chem Biol Interact. 2008;175:180–186. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B. Defective brain microtubule assembly in Alzheimer’s disease. Lancet. 1986;2:421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Xue W, Gaydess A, Grigoryan H, Ding SJ, Schopfer LM, Hinrichs SH, Masson P. Pseudo-esterase activity of human albumin: slow turnover on tyrosine 411 and stable acetylation of 82 residues including 59 lysines. J Biol Chem. 2008;283:22582–22590. doi: 10.1074/jbc.M802555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushnikov AY, Potaman VN, Oussatcheva EA, Sinden RR, Lyubchenko YL. DNA strand arrangement within the SfiI-DNA complex: atomic force microscopy analysis. Biochemistry. 2006;45:152–158. doi: 10.1021/bi051767c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr. Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146:330–339. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- Skoufias DA, Wilson L. Mechanism of inhibition of microtubule polymerization by colchicine: inhibitory potencies of unliganded colchicine and tubulin-colchicine complexes. Biochemistry. 1992;31:738–746. doi: 10.1021/bi00118a015. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Gupta BN, Bihari V, Mathur N, Srivastava LP, Pangtey BS, Bharti RS, Kumar P. Clinical, biochemical and neurobehavioural studies of workers engaged in the manufacture of quinalphos. Food Chem Toxicol. 2000;38:65–69. doi: 10.1016/s0278-6915(99)00123-4. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Gearhart DA, Beck WD, Jr., Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther. 2007;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Vater W, Fritzsche W, Schaper A, Bohm KJ, Unger E, Jovin TM. Scanning force microscopy of microtubules and polymorphic tubulin assemblies in air and in liquid. J Cell Sci. 1995;108(Pt 3):1063–1069. doi: 10.1242/jcs.108.3.1063. [DOI] [PubMed] [Google Scholar]
- Wagner OI, Ascano J, Tokito M, Leterrier JF, Janmey PA, Holzbaur EL. The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell. 2004;15:5092–5100. doi: 10.1091/mbc.E04-05-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi JH, Bai F, McGaha R, Andley UP. Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. Faseb J. 2006;20:846–857. doi: 10.1096/fj.05-5532com. [DOI] [PubMed] [Google Scholar]
- Yeste-Velasco M, Alvira D, Sureda FX, Rimbau V, Forsby A, Pallas M, Camins A, Folch J. DNA low-density array analysis of colchicine neurotoxicity in rat cerebellar granular neurons. Neurotoxicology. 2008;29:309–317. doi: 10.1016/j.neuro.2007.11.007. [DOI] [PubMed] [Google Scholar]