Abstract
We chronically stimulated hippocampal networks in culture for either 0, 1 or 3 hr/day between 7 and 22 days in culture in an effort to increase spontaneous spike rates and to give these networks some portion of external stimuli that brain networks receive during their formation. Chronic electrical stimulation of hippocampal networks on multi-electrode arrays (MEAs) increased spike rates 2-fold after three weeks of culture compared to cultures that received no external stimulation prior to recording. More than 90% of the spikes for all experimental conditions occurred within bursts. The frequency of spikes within a burst increased with time of stimulation during culture up to 2-fold higher (90 Hz) compared to networks without chronic stimulation. However, spontaneous overall spike rates did not correlate well with the amount of stimulation either as hr/day or proximity to the limited number of stimulation sites due to shorter burst duration with 3 hr/day stimulation. The results suggest that chronic stimulation applied during network development recruits activity at 50% more electrodes and enables higher rates of spontaneous activity within bursts in cultured hippocampal networks.
Keywords: Electrode array, MEA, stimulation, network, action potential
1. Introduction
The overarching goal of in vitro networks of cultured neurons is to model network behaviour of the brain. In our systematic approach to improving live neuronal network activity on multi-electrode arrays (MEAs), we have shown that network responses can be improved by patterned substrates to more precisely locate neuronal soma over recording electrodes (Corey et al., 1991; Chang et al., 2006), added astroglia to improve network dynamics (Boehler et al., 2007) and a response-optimized, synapse-enhancing culture medium (Brewer et al., 2008; Brewer et al., 2009). Customarily, neural network cultures are placed in an incubator and allowed to develop spontaneous activity over a period of several weeks without external stimulation (Thomas et al., 1972; Gross, 1979; Pine, 1980; van Pelt et al., 2004; Chiappalone et al., 2006; Wagenaar et al. 2006; Brewer et al., 2009). A key reason for low spontaneous spike rates of such cultures may be caused by a sleeping or catatonic network which receives no external stimulation. Without external stimulation, Vogt et al. (2005) found that less than 10% of patched pairs of neurons were connected by excitatory transmission onto an excitatory neuron. During development, the brain receives large amounts of external stimulation critical to developing excitatory synapses (Turrigiano and Nelson, 2004). The effect of chronic electrical stimulation on spontaneous neuronal firing rates of dissociated hippocampal cultures during network development has not been widely studied, although directed training effects have been reported (Eytan et al., 2003; Stegenga et al., 2009).
This work was motivated by the network studies of Potter (Wagenaar et al., 2005) who showed that burst dynamics can be greatly modulated by repeated high frequency stimulation, but chronic stimulation over many days was not investigated. We chose a paired-pulse paradigm of stimulation with 50 ms interval for optimal presynaptic activation (Zucker & Regehr, 2002; Hama et al., 2004; Mori et al., 2004). We pose two alternative hypotheses applied to cultured networks. 1) Paired-pulse stimulation over long periods (chronic stimulation), will reduce spontaneous bursting, but increase overall spike rates similar to acute high frequency stimulation (Wagenaar et al., 2005). Or, 2) Chronic paired-pulse stimulation will increase spontaneous spike rates possibly within a burst. Here we report a major effect of chronic stimulation on spontaneous spike rates and burst dynamics during two-weeks of network development in vitro.
2. Methods
2.1 Neuron culture
E18 rat hippocampal cells were plated at 500 cells/mm2 on poly-D-lysine coated MEAs in NbActiv4™ medium (Brewer et al., 2008; Brewer et al., 2009) (BrainBits, Springfield, IL). Every 4–5 days, ½ of the culture medium was removed and replaced with the same volume of fresh medium up until the day of recording. At the time of recording at 3 weeks, the culture was comprised of about 67% neurons and 33% astroglia (Brewer et al., 2008). The results reported here were from 5 arrays for each of the 3 conditions of stimulation. The 5 arrays were the result of 4 different animal dissections/condition.
2.2 Multi-electrode arrays (MEA) and Recording
The MEA’s from Multichannel Systems (MCS, Reutlingen, Germany) consist of 59 TiN electrodes with diameters of 30 µm and spacing of 200 µm; one other electrode was used as ground. The spontaneous activity on the MEA’s was measured using an MCS 1100× amplifier at 40 kHz sampling with a hardware filter of 8–3000 Hz at 37°C under continuous flow of hydrated, sterile 5% CO2, 9% O2, balance N2 (AGA, Springfield, IL). Electrical stimuli were delivered by a stimulus generator (MCS STG2004). A Zebra strip (Fujipoly America, Carteret, NJ) was used on arrays to reduce noise and a Teflon membrane (ALA Scientific, Westbury, NY) was used to reduce evaporation and chances of contamination during stimulation. MCRack software was used to record for 3 min followed by a high-pass software filter of 200 Hz. Offline data analysis was performed in MATLAB (The Mathworks, Natick, MA). Spikes were analyzed within a 2 ms search window for their peak-to-peak amplitudes and detections were noted whenever the peak-to-peak amplitude exceeded 11 times the noise standard deviation (roughly equivalent to a threshold of 5.5 standard deviations above zero-to-peak amplitudes). Spikes occurring within a 1 ms refractory period were discarded. Both timestamp and amplitude were saved (Maccione et al., 2009).
2.3 Chronic stimulation
Stimulation trains for chronic stimulation included groups of 30 μA paired pulses (50 ms ISI; biphasic, 100 µs/phase duration, positive first) with a wait of 5 seconds between pairs. This level consistently led to at or near 100% (maximal) activation (commonly 15 µA was the threshold) in related work in our laboratory with low density cultures and 30 µm diameter electrodes. This mode and level are consistent with reports in the literature (e.g. Eytan and Marom, 2006). Constant current stimulation has the advantage of consistent charge injection with variable electrode impedance, and stimulation is theoretically believed to be consistently correlated with current. We note, however, that there are competing reasons that lead others to find constant voltage stimulation preferable (Wagenaar et al., 2004). A paired pulse paradigm with delay of 50 ms was chosen as a minimum number of stimuli that could avoid short-term plasticity while enhancing the probability of postsynaptic potentials (Soleng et al., 2004). Wait times between pulses of 1 sec and 5 sec between 5 pairs of pulses were empirically chosen as minima that avoided desensitization. Arrays were chronically stimulated for either 0, 1 or 3 hour(s)/day at 7, 11, 12, 14, 18, 19, and 21 days in vitro. On the day of recording, arrays that did not receive any chronic stimulation were initially recorded for 3 minutes to obtain spontaneous activity. Arrays that had received chronic stimulation were chronically stimulated for 1h or 3h, then recorded for three minutes. An automatic stimulation program was created using Microsoft Visual C++ 2008 that stimulated the entire top half of the MEA (30 electrodes) in a pseudorandom sequence, the same for every array, every time; electrodes were not preselected for activity. Recorded arrays received 0, 2100, or 6300 stimuli/day × 8 days during culture development. The pseudorandom design avoided stimulation of any two adjacent electrodes consecutively. The bottom half of the array never received direct stimulation from an adjacent electrode and served as an unstimulated condition within the array. The experimental setup resulted in electrodes in the top half of the array being stimulated by one of 2, 3, 4, 5, 7, or 8 adjacent electrodes.
2.4 Burst detection
The activity of each electrode trace was searched for bursts using the method of Chiappalone et al. (2005). A burst was defined as a sequence of at least 5 spikes with inter-spike interval less than 100 ms. Burst analyses were conducted with MatLab software with a burst criterion of ≥ 0.4 bursts/min.
2.5 Statistics
Statistical analyses were performed by Student’s t-test with a cutoff at p<0.05 for significance. All graphs display means and S.E.
3. Results
3.1 Effect of chronic stimulation on spontaneous activity
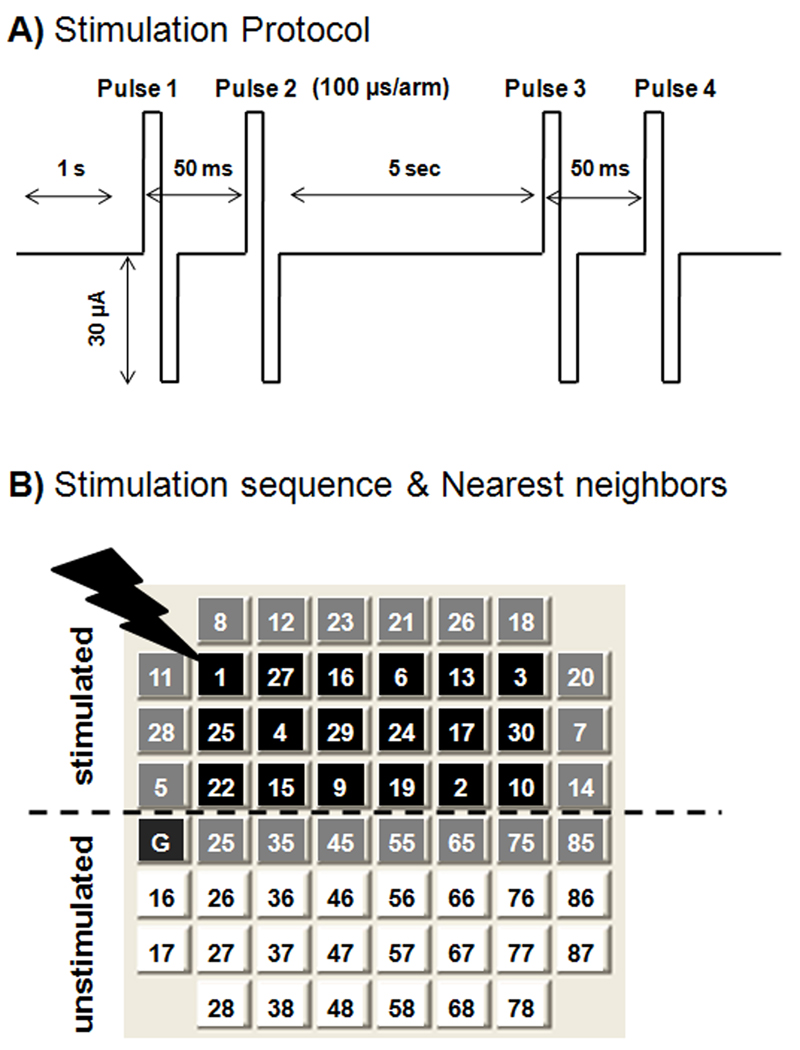
Figure 1A shows the experimental design for paired pulse stimulation. In contrast to high-frequency stimulation (Wagenaar et al., 2005), the 5 sec delay in this pulse sequence was devised to minimize immediate effects on activity. Figure 1B shows the pseudorandom sequence used during stimulation of the top half of the array. The numbering indicates the order in which electrodes received stimulation trains.
Fig. 1.
Chronic stimulation protocol applied to determine effect of external stimuli on spike rates during time in culture. A) A train of two paired pulses, 50 ms between single pulses in a pair, 5 sec apart was delivered to a single electrode, followed by switching to a second electrode1 sec later. B) The switching was repeated to each of 30 electrodes in top half of array. This process was repeated for either 1 or 3 hr/day for a total of either 2100 or 6300 pulses per array/day over 8 days. The program switches channels in a pseudorandom sequence after each train to avoid stimulation of any two adjacent electrodes consecutively and to minimize short-term plasticity. Black squares represent electrodes with 5, 7, or 8 neighbours in close proximity during stimulation. Gray squares represent electrodes with 2, 3, or 4 neighbours in close proximity and white squares represent electrodes with zero neighbours in close proximity.
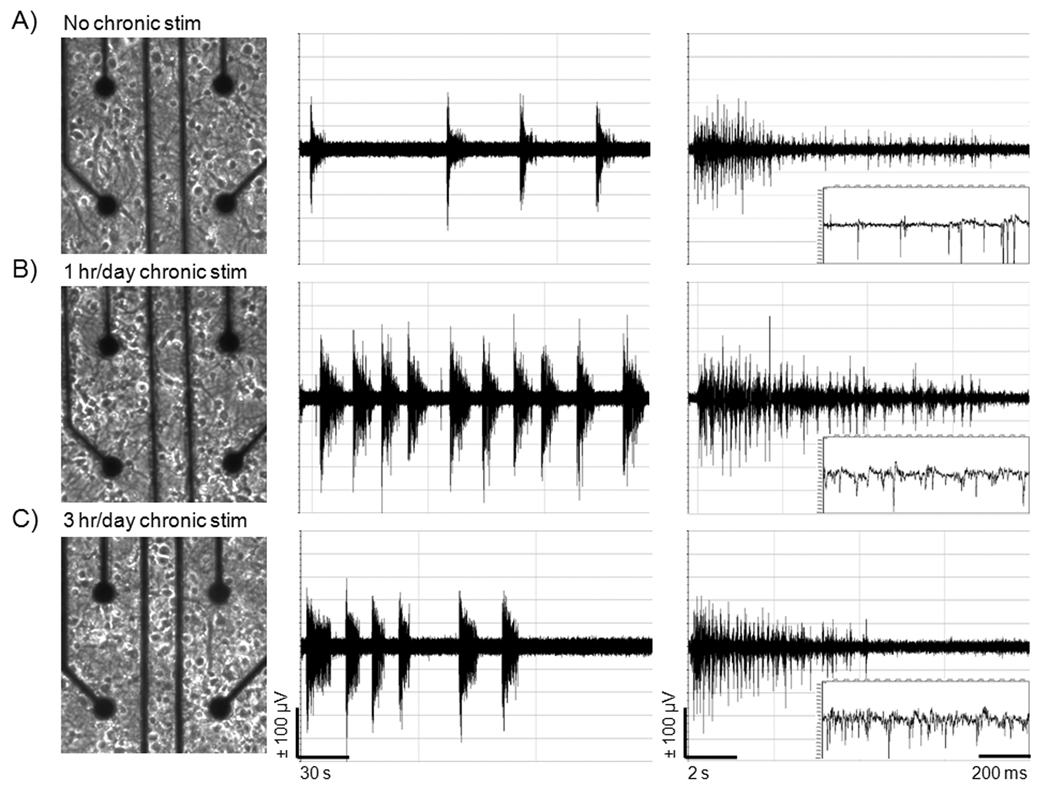
Figure 2 shows typical neurons evenly distributed on a multi-electrode array (MEA) after 21 days of culture in NbActiv4 medium. This appearance was not affected by time of chronic stimulation. Figure 2 also provides examples of spontaneous activity recorded after 3 weeks of culture of hippocampal networks. Compared to the customary procedure with no chronic stimulation during culture, chronic stimulation for 1 hr/day × 8 days resulted in higher overall spike rates, mostly in barrages of activity called bursts. As a consequence of chronic stimulation during culture 3 hr/day × 8 days, the spike rate within a burst was greater than either 0 or 1 hr/day chronic stimulation, but burst duration was shorter than that for 1 hr/day stimulation.
Fig. 2.
Chronic stimulation increases total bursts and burst frequency without morphological changes in neurons on MEAs compared to unstimulated conditions. A) Neuron distribution on an MEA after 21 days of culture in NbActiv4 for unstimulated condition, B) 1 hr/day chronic stimulation, and C) 3 hr/day chronic stimulation. Corresponding amplifier outputs are shown to the right. Frequency of bursts is highest with 1 hr/day chronic stimulation. Frequency of spikes within a burst is highest at 3 hr/day stimulation.
3.2 Burst analysis
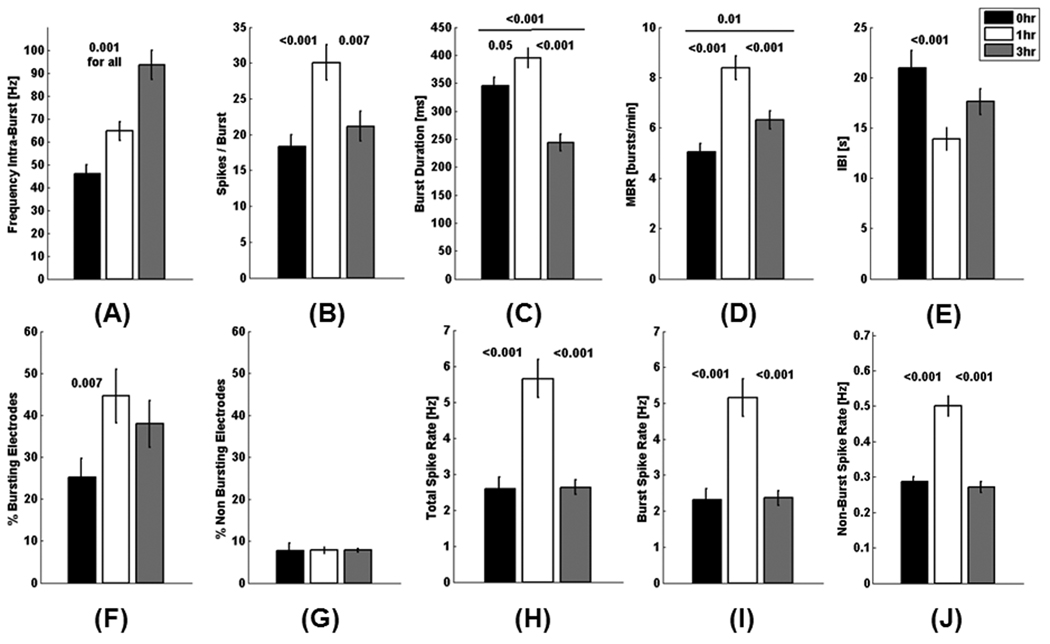
Variations in activity levels between the networks on arrays plated from the same animal dissection were similar to others from different dissections. Averaging the results from active electrodes (intra-network variability) from 4 separate network cultures on 5 arrays of each treatment, the frequency of spontaneous spikes occurring within a burst was seen to increase with duration of chronic stimulation (Fig. 3A). The intra-burst spiking frequency of 93 Hz in networks stimulated for 3 hr/day during culture development was 2-fold higher than networks without chronic stimulation at 45 Hz and a 1.4-fold higher than cultures chronically stimulated for 1 hr, 64 Hz. This was accompanied by an increase in spikes per burst for 1 hr/day chronic stimulation/day but a reduction for 3 hr/day stimulation (Fig. 3B). While the frequency of spikes within a burst was highest in cultures chronically stimulated for 3 hr, these networks had the lowest burst duration, about 100 ms less than cultures stimulated for 0 or 1 hr/day (Fig. 3C). Cultures chronically stimulated for 1h had the longest burst duration of 400 ms which was 50 ms longer than the unstimulated condition.
Fig. 3.
Chronic stimulation during culture increases burst spike frequency, spikes/burst, burst duration and total bursts compared to unstimulated condition after 3 weeks. Data represents analysis of channels with one or more bursts. The average response for each electrode from the active electrodes from 5 arrays/condition. p values represent student’s t-test (n=144, 254, and 216 active electrodes from 5 arrays/condition of 0, 1, and 3 hr/day stimulation). A) Spike frequency within a burst increases with duration of chronic stimulation. B) Spikes/ burst are highest with 1 hr/day chronic stimulation, but similar to control at 3 hr/day chronic stimulation. C) Burst duration is longest with 1 hr/day chronic stimulation and lowest with 3 hr/day chronic stimulation. D) 1 hr/day chronic stimulation produces a 2-fold increase in mean burst rate (bursts/ minute) above the unstimulated condition. E) Interburst interval (IBI) of 21 seconds for the unstimulated condition is 1-1/2 fold higher than the IBI of cultures chronically stimulated for 1 hr/day. F) Percentage of bursting electrodes nearly doubles with 1hr/day chronic stimulation and increases 50% with 3 hr/day chronic stimulation compared to unstimulated condition. G) Electrodes containing non-bursting spikes amounted to less than 10 percent of the total active electrodes for all conditions. H, I) Total spike rates and burst spike rates were more than 2-fold higher with 1 hr/day chronic stimulation compared to unstimulated and 3 hr/day stimulated conditions. J) Non-bursting spike rates were nearly 2-fold higher with 1 hr/day chronic stimulation compared to unstimulated and 3 hr/day stimulated conditions.
Cultures that were chronically stimulated for 1hr/day during culture development had the most bursts per minute (Fig. 3D) and a complementarily lower interburst interval (Fig. 3E) The percentage of electrodes with bursting activity nearly doubled in arrays chronically stimulated for 1 hr/day compared to the unstimulated condition (Fig. 3F). The majority of spikes occurred within bursts; electrodes with non-bursting spikes accounted for less than ten percent of the total active electrodes for all conditions (Fig. 3G). Total spike rates (Fig. 3H), burst spike rates (Fig. 3I) and non-burst spike rates (Fig. 3J) were nearly 2-fold higher for 1hr/day compared to the unstimulated condition and cultures stimulated 3 hr/day. Notice that the non-burst spike rates (3J) were consistently about 10% of the burst spike rates (3I), consistent with the percent non bursting electrodes (Fig. 3G).
3.3 Overall spike rates and active electrodes
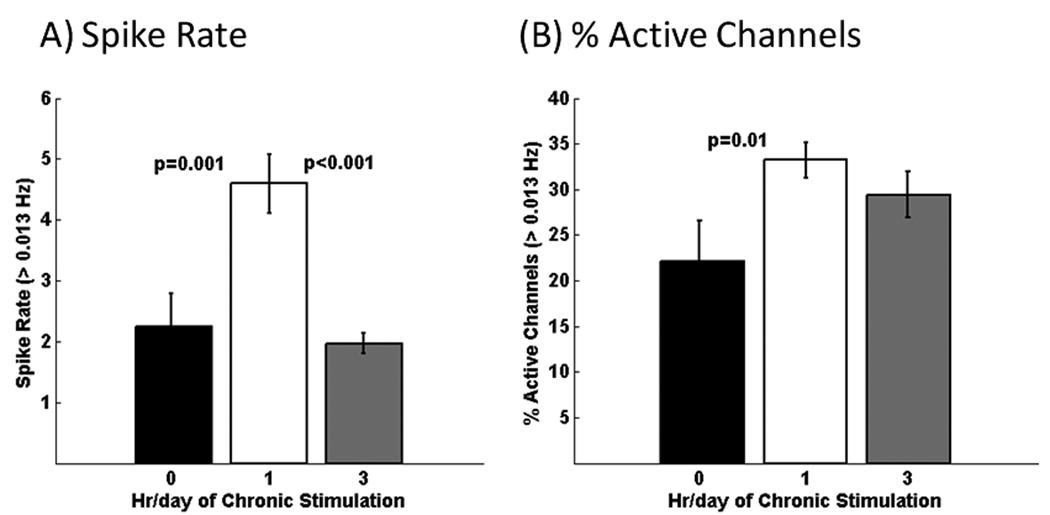
The overall spike rate of bursts and single spikes for cultures chronically stimulated for 1 hr/day was 2-fold higher than unstimulated cultures, but also higher than cultures stimulated for 3h (Fig. 4A). Cultures chronically stimulated for 1 or 3 hr/day also have 30–50% more active electrodes than the unstimulated condition (Fig. 4B). This suggests that chronic stimulation recruits more activity.
Fig. 4.
Considering all channels with >0.013 Hz activity, both bursting plus non-bursting, A) chronic stimulation for 1 hr/day produces 2-fold higher overall spike rates. B) Either chronic stimulation condition caused a 30–50% higher percentage of active electrodes per MEA compared to the unstimulated condition (n=221, 332, and 294 active electrodes from 5 arrays/condition of 0, 1, and 3 hr/day stimulation).
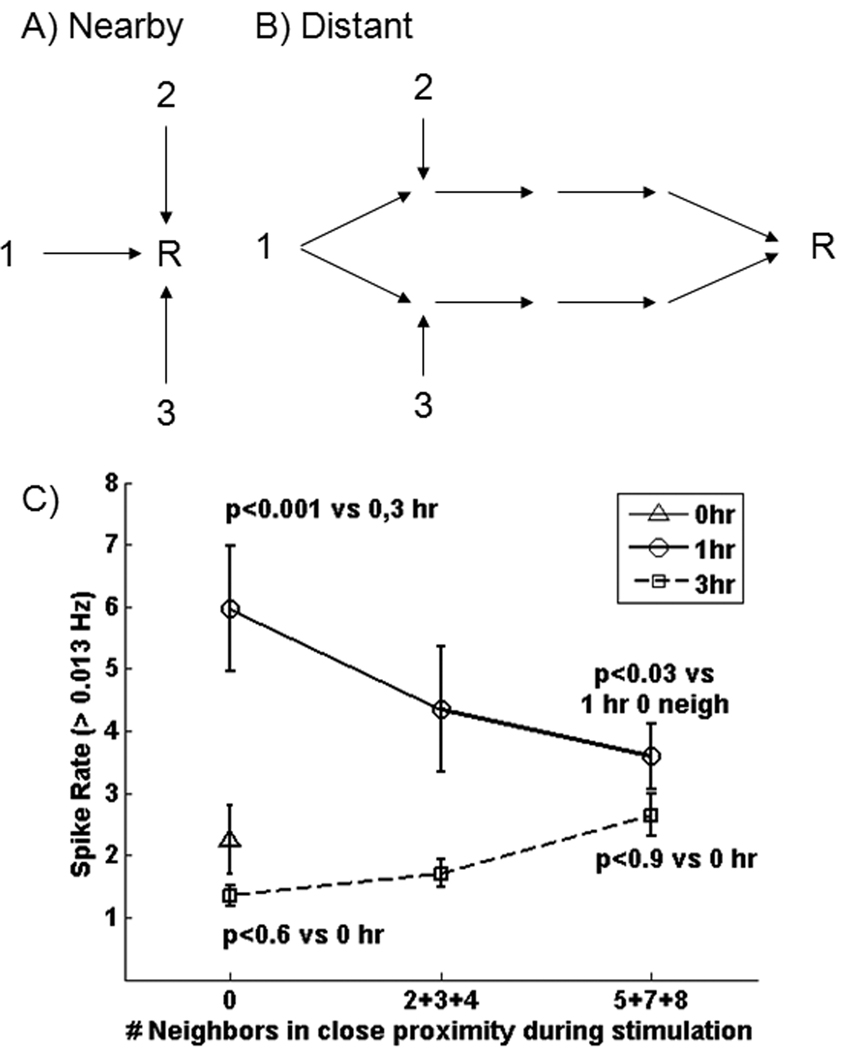
3.4 Impact of nearest neighbours
The experimental design enabled testing the hypothesis that chronic stimulation from a higher number of neighbouring electrodes (1, 2, 3, one at a time) produces higher spike rates at recording electrodes (R) (Fig. 5A). A second alternative is that distant electrodes fire at higher rates when they receive inputs from multiple sites through a branched route (5B), with branched routes less likely in close proximity to the stimulus. The null hypothesis is that spontaneous spike rate is not linearly related to duration of chronic stimulation. Note that electrodes in the bottom 3 rows of Fig. 1 do not receive any direct stimulation so they have zero nearest neighbours so that activity there is more likely to be the result of co-stimulation of multiple sites from a branched route. The interior electrodes in rows 2, 3 and 4 received stimulation from 5, 7 or 8 neighbours at close proximity. Fig. 5C shows the effect of the number of adjacent stimulation sites within one electrode perimeter of stimulation (horizontal or vertical 200 µm or diagonal 280 µm) in each array. Clearly, there is no simple, consistent relationship between spike rate and either stimulation proximity or duration of chronic stimulation. For 1 hour stimulation, activity decreased with the number of adjacent stimulation electrodes, consistent with the second hypothesis above. But with 3 hour stimulation, activity increased, albeit modestly, consistent with the first hypothesis.
Fig. 5.
Impact of stimulation proximity on spike rate. A) Recording sites at close proximity to simulating sites 1, 2, or 3 condition these sites for higher spontaneous spike rates. Alternatively, B) pathways that get recruited by chronic stimulation more often converge at a distant electrode to increase spike rates. C) Measures of electrodes on MEA cultures receiving 1 hr/day (circle) of chronic stimulation produce 3-fold higher spike rates for recording sites distant in proximity to the stimulating electrode (0 nearby stimuli), compared to unstimulated or 3 hr/day chronically stimulated cultures. Spike rates decrease for electrodes with more frequent adjacent stimulation. For 3 hr/day stimulation (squares), the spike rate was similar to all sites on arrays without chronic stimulation (triangle), but tended to increase for sites that received close-proximity stimuli.
4. Discussion/Conclusion
Motivated by the work of Potter, we first reasoned that neuron networks that develop in vitro from embryonic neurons were deprived of their natural stimuli during development. We hypothesized that chronic stimulation would train the network for more spontaneous communication within such an in vitro hippocampal system. The results clearly show an increase in spike frequency within bursts that depended on the duration of chronic stimulation over the course of culture time, as well as a 50% increase in active electrodes with chronic stimulation. The bursts per minute also increased with chronic stimulation, although not monotonically.
Most paradoxical in our results is the increase in several measures with 1 hr/day of stimulation and a reduction at 3 hr/day. It is tempting to compare these results to findings in the literature for denser, unstimulated cultures in which bursting activity gradually develops, firing rates increase, and intense superbursts occur (Wagenaar et al., 2006). By following burst dynamics over 6 weeks of development, van Pelt et al. (2004) found the temporal onset and offset of the bursts first broadened to a peak at 3 weeks, then sharpened with shorter overall lengths. These may be analogous to the changes evident in Figure 2 and in the statistics in Figure 3. The argument is that the stimulation paradigms have accelerated the natural phases of increasing then decreasing and sharpened bursts, with higher spike rates within the burst. Still, it is only speculation that our stimulation paradigms, chosen for convenience, may have accentuated the cultures’ development.
We hypothesized a simple concept of greater induction or strengthening of spontaneous activity from stimuli delivered adjacent to the recording electrode. Instead, we observed no simple relationship between chronic stimulation for 0, 1 and 3 hr/day and spike rate on the scale of minutes. However, examination of Fig. 3A shows clearly a direct relationship between chronic stimulation and spike frequency within a burst. These somewhat discrepant results are reconciled by observing that Fig. 3B, C, D show a non-linear relationship of burst duration with chronic stimulation. These results indicate that chronic stimulation increases spike rate within a burst, but not necessarily overall burst rate. Higher spike rate within bursts may indicate stronger information transfer evoked by chronic stimulation.
The chronic stimulation protocol and measures of responses certainly have limitations. We did not optimize the stimulation train, or the number of hours/day or days of chronic stimulation; these parameters were chosen for convenience. We also wanted to avoid over-stimulation and neuron death. It is likely that the spike frequency within bursts has not reached the maximum with 3 hr/day stimulation. The percent active electrodes could surely be increased by increasing the density of plated neurons or by the addition of extra astroglia (Boehler et al., 2007). Although we stimulated with a paired-pulse stimulus, other stimuli at higher frequency are likely to affect the burst characteristics (Wagenaar et al., 2005).
In the absence of external stimulation, cultured networks develop spontaneous activity in vitro (Thomas et al., 1972; Gross, 1979; Pine, 1980). But the nature of this activity may be different from in vivo. In vitro networks have a smaller than expected proportion of excitatory connections (Vogt et al., 2005) and a high fraction of inhibitory GABAergic synapses (Liu et al., 2000; Robain et al., 1987; Rozenberg et al., 1989). The decline of these GABAergic synapses and the rise of glutamatergic transmission in vivo may require the external stimulation that neurons receive in the brain. Conversely, synaptic development and transmission is compromised if external stimulation is blocked during development in vivo (Turrigiano and Nelson, 2004). Therefore, the synaptic scaling of excitatory and inhibitory receptors is likely to be different in neuronal networks in vitro with or without external stimuli during development. In comparison to spike rate measures also made at 3 weeks in rat neurons, but in cortical neurons in Neurobasal/B27, Chiappalone et al. (2006) found a spontaneous spike rate of 2.2 Hz that compares well with our unstimulated rate of 2.6 Hz. Their work and ours both found a burst rate of 5 bursts/min. However, their threshold for spike detection of 7× S.D. was much less than ours of 11.
Finally, these results raise interesting questions about the nature of brain network communication and bursts. Since burst activity represents 90% of the total activity that we observe under all conditions, can we reasonably conclude that information coding occurs in bursts and less so in individual or smaller groups of action potentials? Others have observed these high levels of bursting activity and used short term stimulation to modulate patterns of activity (Maeda et al., 1995; Maeda et al., 1998; Stegenga et al., 2008). Certainly bursting activity occurs during development in vivo (Kim and McCormick, 1998; Leinekugel et al., 2002) and is different from epileptiform activity. Our finding that the frequency within a burst of spontaneous activity increases with prior chronic stimulation suggests that the prior stimulation increased the synaptic drive of the network in order to produce faster firing rates or lowered inhibitory connections. To establish this conjecture, we will need to compare synaptic densities, resting membrane potentials and inhibitory to excitatory inputs in the unstimulated and chronically stimulated networks. Also, a resting membrane potential that is more depolarized with chronic stimulation (closer to threshold) could explain higher spontaneous spike rates within bursts from a higher ratio of excitatory to inhibitory inputs (Li et al., 1998; Kim and McCormick, 1998).
In conclusion, chronic stimulation of planar hippocampal networks on MEAs during the course of culture results in more spontaneous activity than unstimulated cultures, with higher spike rates within bursts. Thus, chronic stimulation appears to change the dynamics of the network so that proximity to stimulus is no longer the most important determinant of spike rate.
Acknowledgements
This work was supported in part by NIH NS052233 from the National Institute of Neurological Sciences. We thank Professor Martinoia’s laboratory for providing the spike/burst detection algorithm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boehler MD, Wheeler BC, Brewer GJ. Added astroglia promote greater synapse density and higher activity in neuronal networks. Neuron Glia Biol. 2007;3:127–140. doi: 10.1017/S1740925X07000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Boehler MD, Jones TT, Wheeler BC. NbActiv4 medium improvement to Neurobasal/B27 increases neuron synapse densities and network spike rates on multielectrode arrays. J. Neurosci. Meth. 2008a;170:181–187. doi: 10.1016/j.jneumeth.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Boehler MD, Pearson RA, Demaris AA, Ide AN, Wheeler BC. Neuron network activity scales exponentially with synapse density. J. Neural Eng. 2009;6:14001. doi: 10.1088/1741-2560/6/1/014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Brewer GJ, Wheeler BC. Neuronal network structuring induces greater neuronal activity through enhanced astroglial development. J. Neural Eng. 2006;3:217–226. doi: 10.1088/1741-2560/3/3/004. [DOI] [PubMed] [Google Scholar]
- Chiappalone M, Novellino A, Vajda I, Vato A, Martinoia S, van Pelt J. Burst detection algorithms for the analysis of spatio-temporal patterns in cortical networks of neurons. Neurocomputing. 2005;65–66:653–662. [Google Scholar]
- Chiappalone M, Bove M, Vato A, Tedesco M, Martinoia S. Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res. 2006;1093:41–53. doi: 10.1016/j.brainres.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Corey JM, Wheeler BC, Brewer GJ. Compliance of hippocampal neurons to patterned substrate networks. J. Neurosci. Res. 1991;30:300–307. doi: 10.1002/jnr.490300204. [DOI] [PubMed] [Google Scholar]
- Droge MH, Gross GW, Hightower MH, Czisny LE. Multielectrode analysis of coordinated, multisite, rhythmic bursting in cultured CNS monolayer networks. J. Neurosci. 1986;6:1583–1592. doi: 10.1523/JNEUROSCI.06-06-01583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan D, Brenner N, Marom S. Selective adaptation in networks of cortical neurons. J. Neurosci. 2003;23:9349–9356. doi: 10.1523/JNEUROSCI.23-28-09349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan D, Marom S. Dynamics and effective topology underlying synchronization in networks of cortical neurons. J. Neurosci. 2006;26:8465–8476. doi: 10.1523/JNEUROSCI.1627-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GW. Simultaneous single unit recording in vitro with a photoetched laser deinsulated gold multimicroelectrode surface. IEEE Trans. Biomed. Eng. 1979;26:273–279. doi: 10.1109/tbme.1979.326402. [DOI] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC Signaling Mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- Kim U, McCormick DA. The functional influence of burst and tonic firing mode on synaptic interactions in the thalamus. J. Neurosci. 1998;18:9500–9516. doi: 10.1523/JNEUROSCI.18-22-09500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Li YX, Schaffner AE, Walton MK, Barker JL. Astrocytes regulate developmental changes in the chloride ion gradient of embryonic rat ventral spinal cord neurons in culture. J. Physiol. 1998;509(Pt 3):847–858. doi: 10.1111/j.1469-7793.1998.847bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Coulombe M, Dumm J, Shaffer KM, Schaffner AE, Barker JL, Pancrazio JJ, Stenger DA, Ma W. Synaptic connectivity in hippocampal neuronal networks cultured on micropatterned surfaces. Dev. Brain Res. 2000;120:223–231. doi: 10.1016/s0165-3806(00)00014-6. [DOI] [PubMed] [Google Scholar]
- Maccione A, Gandolfo M, Massobrio P, Novellino A, Martinoia S, Chiappalone M. A novel algorithm for precise identification of spikes in extracellularly recorded neuronal signals. J. Neurosci. Meth. 2009;177:241–249. doi: 10.1016/j.jneumeth.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Maeda E, Kuroda Y, Robinson HP, Kawana A. Modification of parallel activity elicited by propagating bursts in developing networks of rat cortical neurones. Eur. J. Neurosci. 1998;10:488–496. doi: 10.1046/j.1460-9568.1998.00062.x. [DOI] [PubMed] [Google Scholar]
- Maeda E, Robinson HP, Kawana A. The mechanisms of generation and propagation of synchronized bursting in developing networks of cortical neurons. J. Neurosci. 1995;15:6834–6845. doi: 10.1523/JNEUROSCI.15-10-06834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Abegg MH, Gahwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- Pine J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Meth. 1980;2:19–31. doi: 10.1016/0165-0270(80)90042-4. [DOI] [PubMed] [Google Scholar]
- Robain O, Barbin G, Ben-Ari Y, Rozenberg F, Prochiantz A. Gabaergic neurons of the hippocampus: development in homotopic grafts and in dissociated cell cultures. Neuroscience. 1987;23:73–86. doi: 10.1016/0306-4522(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Rozenberg F, Robain O, Jardin L, Ben-Ari Y. Distribution of GABAergic neurons in late fetal and early postnatal rat hippocampus. Brain Res. Dev. Brain Res. 1989;50:177–187. doi: 10.1016/0165-3806(89)90193-4. [DOI] [PubMed] [Google Scholar]
- Soleng AF, Baginskas A, Andersen P, Raastad M. Activity-dependent excitability changes in hippocampal CA3 cell Schaffer axons. J. Physiol. 2004;560:491–503. doi: 10.1113/jphysiol.2004.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegenga J, le Feber J, Marani E, Rutten W. The effect of learning on Bursting. IEEE Trans Biomed. Eng. 2009;56:1220–1227. doi: 10.1109/TBME.2008.2006856. [DOI] [PubMed] [Google Scholar]
- Thomas CA, Jr., Springer PA, Loeb GE, Berwald-Netter Y, Okun LM. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972;74:61–66. doi: 10.1016/0014-4827(72)90481-8. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Van Pelt J, Wolters PS, Corner MA, Rutten WL, Ramakers GJ. Long-term characterization of firing dynamics of spontaneous bursts in cultured neural networks. IEEE Trans. Biomed. Eng. 2004;51:2051–2062. doi: 10.1109/TBME.2004.827936. [DOI] [PubMed] [Google Scholar]
- Vogt AK, Brewer GJ, Decker T, Bocker-Meffert S, Jacobsen V, Kreiter M, Knoll W, Offenhausser A. Independence of synaptic specificity from neuritic guidance. Neuroscience. 2005;134:783–790. doi: 10.1016/j.neuroscience.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Wagenaar DA, Madhavan R, Pine J, Potter SM. Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J. Neurosci. 2005;25:680–688. doi: 10.1523/JNEUROSCI.4209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar DA, Pine J, Potter SM. Effective parameters for stimulation of dissociated cultures using multi-electrode arrays. J. Neurosci. Meth. 2004;138:27–37. doi: 10.1016/j.jneumeth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Wagenaar DA, Pine J, Potter SM. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006;7:11–17. doi: 10.1186/1471-2202-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]