Abstract
Pulse oximetry is a common tool for detecting reduced pulmonary function in human interstitial lung diseases. It has not previously been used in a mouse model of interstitial lung disease. Further, Platelet Endothelial Cell Adhesion Molecule deficient mice rarely show symptoms until disease is advanced.
Using blood oxygen saturation, different stages of disease could be identified in a non-invasive manner. These stages could be correlated to pathology. Collagen deposition, using Picrosirius Red, did correlate with blood oxygen saturation. These studies are the first to show the use of an infrared pulse oximetry system to analyze the progression of a fibrotic interstitial lung disease in a mouse model of the human diseases. Further, these studies show that an early alveolar damage/enlargement event precedes the fibrosis in this mouse model, a stage that represents the best targets for disease analysis and prevention. This stage does not have extensive collagen deposition. Most importantly, targeting this earliest stage of disease for therapeutic intervention may lead to novel treatment for human disease.
Keywords: PECAM, lung, fibrosis, blood, oxygen
Pulse oximetry is a simple, widely used, non-invasive test that uses infrared sensing of hemoglobin emission and movement to determine many vital signs including heart rate and arterial blood oxygen saturation (1). In human studies of interstitial lung disease (ILDs), it has been shown that patients have a significant reduction in arterial blood oxygen saturation (SpO2) even while resting (2-4). In this study, a pulse oximetry system developed for rodents was successfully used to diagnose interstitial lung disease in mice.
Platelet Endothelial Cell Adhesion Molecule (PECAM) deficient mice in the FVB/n background spontaneously develop a chronic interstitial pneumonia that eventually leads to fibrosis (5). PECAM is used for leukocyte extravasation and has a role in apoptosis and cell signaling (6-14). Diseased mice exhibit many pathologic characteristics that resemble the human ILD known as Idiopathic Pulmonary Fibrosis (15), including fibroblastic hyperplasia, interstitial thickening, heterogeneous involvement of the lung, extensive airway expansion, and collagen deposition. There is no obvious cause of the murine disease and the phenotype is not fully penetrant, thus these mice represent a putative model of the human interstitial lung disease (5).
A major difficulty that we encountered was an inability to easily detect disease in mice until the disease had reached advanced stages. These mice were often in the last stages of disease, and were discovered hunching and rapidly breathing by animal care staff. Unfortunately, some would be found dead in the cage before any care or tissue collection could be done. Large scale culling of the population revealed that approximately 40% of the mice showed a range of disease, with small localized lesions to extensive fibrosis (5). Mice in the very earliest signs of disease do not exhibit any outward symptoms and this made any studies on disease progression difficult without randomly culling valuable mice. Using pulse oximetry, the entire colony can be screened weekly to diagnose disease at early time points. This will make it possible to identify cellular and molecular events early in the disease process.
Materials and Methods
Mice, Animal Use Protocol Approval, and Veterinary Care
All animals used were under the care and supervision of the Laboratory for Animal Research. FVB/n strain wild type and PECAM deficient mice (a generous gift of Dr. William A. Muller, Northwestern University) were raised in the same rooms. All procedures were reviewed and approved by the CSU research institutional Animal Care and User Committee.
Pulse Oximetry
This study used the MouseOx system (Starr Life Sciences, Oakmont, PA). Conscious (non-anesthetized) mice were used in all studies. Mice were held in one hand in dorsal recumbency. This was accomplished by scruffing at the neck with the thumb and forefinger and holding the tail between the fourth and fifth digits. The back of the hand was then rested on the table surface for optimal stability. The probe was then placed on the left rear foot of each mouse with the middle of the foot resting within the groove of the clamp. Care should be taken to exclude the hock, as well as to insure that the toes are extended as far out of the probe as possible. To ensure consistent readings, the black portion of the clamp was placed on the plantar surface at all times.
In addition to securing the mouse to minimize movements that could affect readings, it is important to secure the probe in such a way as to minimize pulling on the probe. The cord should not be left to dangle as it will pull on the probe and interfere with accurate measurements. Also, holding onto the probe, while it would seem to increase stability, actually interfered with measurements and rarely provided a clear signal. If the cord becomes twisted in any way, it can also apply torque to the foot. This is both a consideration both for obtaining measurements and the comfort of the mouse. If measurements are to be recorded in a lighted room, particularly with fluorescent lighting, it is essential that the light blocker be placed over the probe to block as much light as possible.
Pathology and Immunohistochemistry
Mice were euthanized and the pulmonary cavities were opened. The aorta was severed in the peritoneal cavity to minimize blood in the pulmonary cavity. To obtain lung samples for histology, the lungs were aseptically removed and fixed in IHC Zinc formalin-free Fixative (BD Pharmingen, San Diego, CA [note to reviewers, this is a proprietary formulation]) then left in fixative for 48 hours. The lungs were washed in PBS, embedded in paraffin, sectioned and the paraffin removed using EZ-DeWax solution (Biogenex Lab, San Ramon, CA). The slides were then rehydrated in an ethanol gradient by incubating for five minutes in 100%, 90%, 85%, and 70% ethanol solutions.
For H&E stained sections, following de-waxing and rehydration, the slides were stained with Hematoxylin (Surgipath, Richmond, IL) for 5 minutes. The slides were then washed with DI water, and incubated with a defining solution and bluing buffer (Surgipath). Following these steps the slides were washed with 96% ethanol for 1 minute and stained with Eosin Y (Surgipath) for 3 minutes. Finally, the slides were washed with 96% ethanol and mounted for microscopic observation.
Picrosirius Red Assessment of Collagen Deposition
In order to assess the extent of fibrosis, a protocol by J. A. Kiernan was used (16). Fixed and paraffin embedded lung tissue was sectioned on a standard microtome at the thickness of 5 um and mounted on Fisher adhesive slides. Slides were then baked on slide heater at 50 degrees C until adherence. Each slide was deparaffinized in two changes of Xylene for 3 minutes then rehydrated for three minutes in two changes of 100% isopropanol, followed by three minutes in two changes of 96% isopropanol, three minutes in one change of 70% isopropanol and finished for 3 minutes in distilled water. Slides were stained with Vector's formulation of Mayer's Iron Hematoxylin (catalog no. H-3404; Vector Laboratories, Burlingame, CA) for eight minutes at room temperature then rinsed under tap water until water ran clear. The slides were submerged in a bath of 0.1% Sirius Red in saturated Picric Acid (catalog no. 26357-02, Electron Microscopy Sciences, Hatfield, PA) in the chemical hood for 1 hour at room temperature. After the hour, slides were submerged for two minutes into two acidified water baths (5 ml Glacial Acetic Acid into 1 liter distilled water). Finally the slides were dehydrated with two minutes in distilled water, three minutes in 70% isopropanol, two changes of three minutes in 96% isopropanol, and two changes of three minutes in 100% isopropanol baths. The slides were cleared once in Xylene and mounted with Permount mounting media (Fisher Scientific, Pittshurgh, PA).
The sections were imaged using birefringence at 4X magnification on an Olympus IX-71 microscope equipped with two circular polarizing filters, and viewed with Q Capture Suite using a Q Color digital camera (Qimaging, Surrey, BC, Canada). The images were then analyzed with computer-assisted image analysis using Nikon NIS-Elements software (Nikon Instruments Inc., Melville, N.Y.). Each image was cropped to an area of 1,125,000 pixels. The thresholding function was used to assess the degree of Picrosirius staining on tissue contained within the cropped area, followed by the automeasure to assess the damage numerically. Data was exported in a spreadsheet for quantitation.
Statistics
All data was analyzed using the statistics software package JMP (SAS, Research Triangle, NC). Differences in means were tested using the Tukey-Kramer HSD (honestly significant difference) test between each group. Correlation, linear fit, and the Receiver Operating Characteristic tests were also done using JMP. Error bars shown are the standard error of the mean for each group.
Results
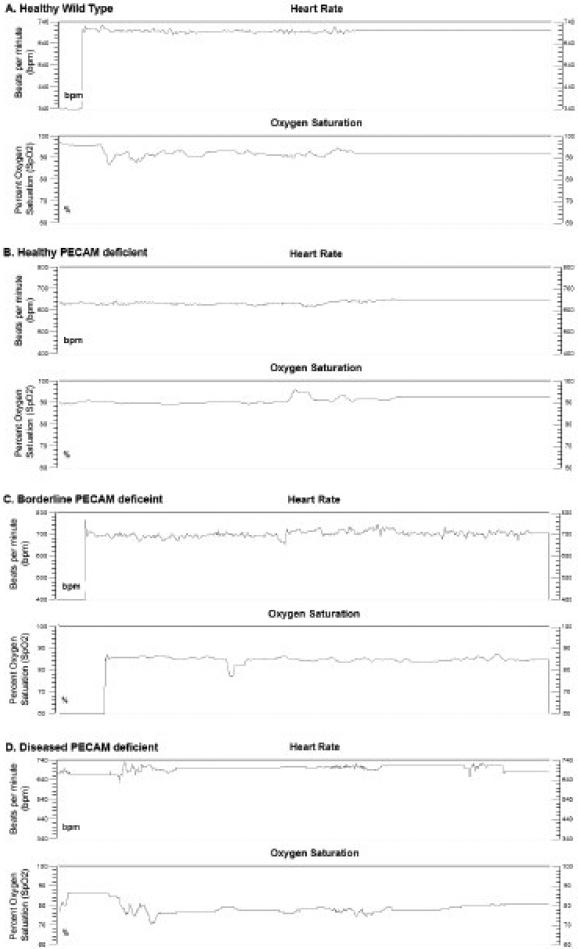
In order to identify diseased mice as early as possible, the pulse oximetry system was tested on conscious (not anesthetized) healthy wild type and PECAM deficient mice. Mice were monitored for times ranging between 30 seconds to two minutes to ensure several consistent measurements where the animal was calm and still (Figure 1). While attempts were made to take measurements from the ear and the tail, the pad of the foot was found to be the most reliable and was therefore used for all recorded values. The pulse oximetry system monitors pulse (beats per minute, bpm), arterial oxygen saturation (SpO2, % in Figure 1), pulse and breath distention, and breath rate. The latter parameters were found to be too difficult to accurately monitor on awake mice. We found the pulse rate of our mice to average about 650-700 bpm, probably due to some excitement during handling; published rates in resting mice are around 400-600 bpm (17, 18).
Figure 1.
Examples of typical output readings from the MouseOx system on awake mice. A. Wild type mouse, average SpO2 = 94% B. PECAM deficient healthy mouse, average SpO2 = 92%. C. PECAM deficient mouse, borderline, average SpO2 =87%. D. PECAM deficient mouse, diseased, average pO2 = 72%.
Examples of three data sets are shown in Figure 1. A wild type mouse (SpO2 average 92%, Fig. 1A), a healthy PECAM deficient mouse (SpO2 average 93%, Fig 1B), and two mice that were significantly below the average (SpO2 average 85%, Fig. 1C, average 77% Fig. 1D). The major requirement with this system is adequate time to acquire a signal, followed by signal optimization and then acquisition of usable data. One difficulty encountered was that some mice would continuously twitch and disrupt the sensor, so these animals would require longer sensing times.
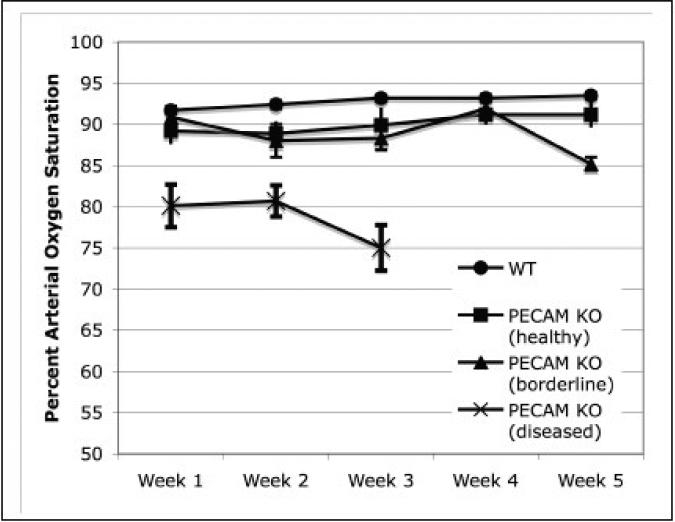
After weekly monitoring, the established baselines in wild type mice averaged from about 92% to 93% (Figure 2). Some wild type mice were as low as 87% and as high as 94%, but never for more than one observation point. The healthy PECAM deficient mice averaged about 89-91%, whereas the diseased mice averaged around 80%. Some of the mice with the lower values were classified as borderline for disease. On week three of the study, the diseased mice dropped further to 75% and were euthanized for humane care reasons and to examine the lung pathology. From the previously borderline PECAM deficient mice, several dropped to around 85% SpO2 and were euthanized to examine the lung pathology.
Figure 2.
Percent arterial oxygen saturation data over time. Healthy wild type (circles) and PECAM deficient (PECAM KO) mice were measure over 3-5 weeks. Diseased mice (SpO2 < 85%) were sacrificed at week 3 due to humane care requirements. Borderline SpO2 (∼90%) mice showed no significant difference until week 5, and then showed a significant drop in SpO2 (Tukey-Kramer HSD, p < 0.01). All measurements of the diseased mice were significantly lower than both wild type and healthy PECAM-deficient mice (Tukey-Kramer HSD, p < 0.01). Wild type mice (n =24), healthy PECAM KO mice (n = 5), borderline PECAM KO mice (n = 4), diseased PECAM KO (n = 7). Error bars shown are the standard error of the mean.
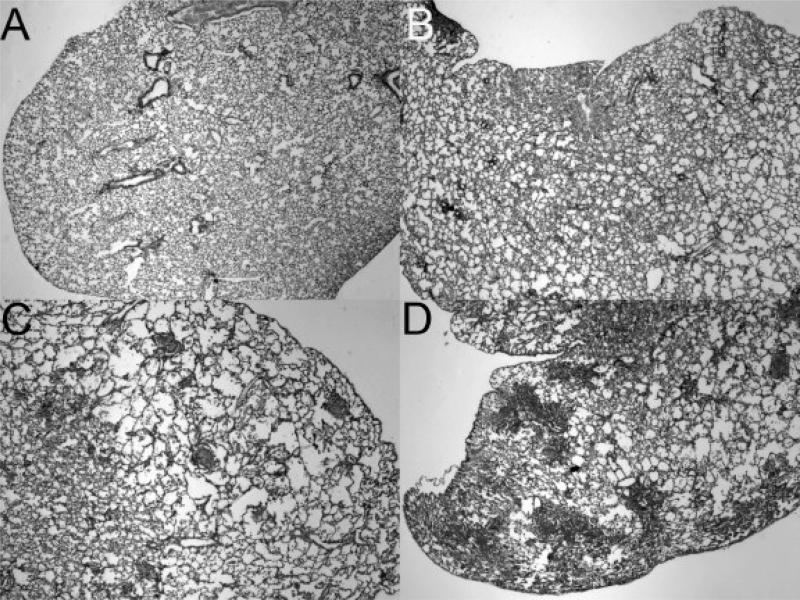
The primary goal of these studies is to test the sensitivity of the pulse oximetry in detecting the earliest forms of disease. Thirteen mice from each group, PECAM deficient mice and wild type controls, were included in the pathology examination (Fig. 3). The lungs in the wild type mice showed no signs of disease (Fig. 3A). As shown previously, a range of disease pathology was found in the PECAM deficient mice (Fig. 3B-D). In addition to fibroblast proliferation and collagen in the advanced lesions, moderate amounts of alveolar enlargement were found in some regions of PECAM deficient mice with SpO2 levels around 82% (Fig. 3B-C). PECAM deficient mice with SpO2 levels averaging just over 75% had more severe disease (Fig. 3D). In contrast with our earlier studies (5), mice with extensive fibrosis have not been used because we are sacrificing mice before the SpO2 levels drop below 75% for humane care reasons. Based on SpO2 levels and histology, a general scoring scheme can be used to define each group (Table 1). The comparison between SpO2 levels and histology will allow a more definitive examination of the cellular and molecular events during progression.
Figure 3.
Histology of lungs at different blood oxygen saturation levels Low magnification images showing disease in lungs A. Wild type lung, average SpO2 = 91% (2X magnification). B. PECAM deficient mouse, average SpO2 = 82% (2X magnification). C. PECAM deficient mouse, average SpO2 = 73% (2X magnification). D. PECAM deficient mouse, average SpO2 = 67%(2X magnification).
Table 1.
Grouping of disease in PECAM deficient mice
| Group |
SpO2 range (3+ observations) |
Pathology scoring |
|---|---|---|
|
Healthy |
89-94 |
None |
|
Early/borderline |
88-85 |
Focal, small lesions and alveolar enlargement |
|
Moderate |
85-75 |
Many lesions, early fibrosis |
| Late | <75, often cyanotic | Large lesions, extensive fibrosis |
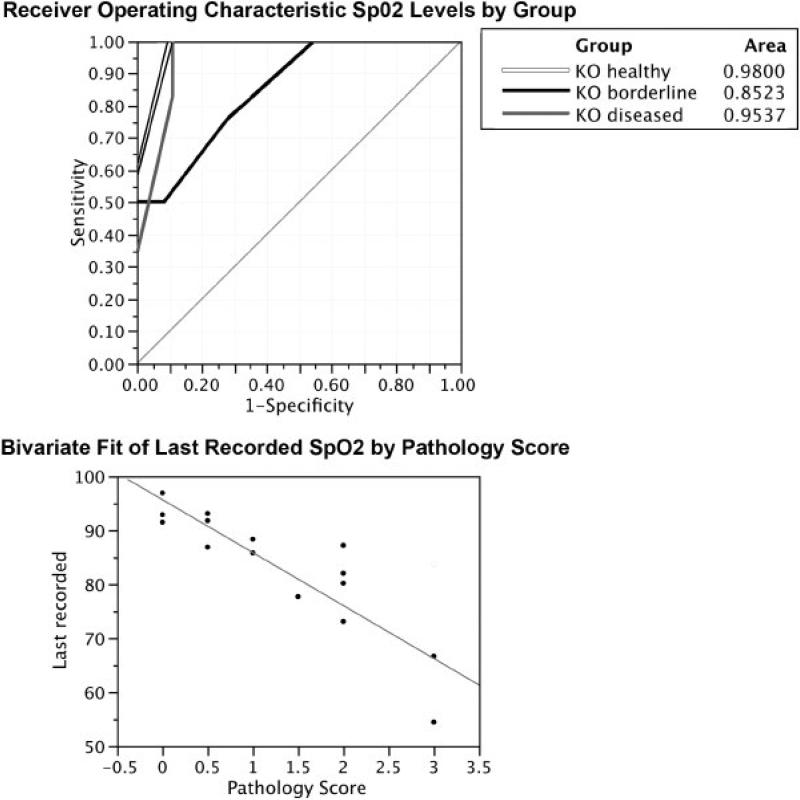
Based on SpO2 levels and histology, a general scoring scheme can be used to define each group (Table 1). Two statistical tests were performed to ascertain the usefulness of this improved scoring scheme, a simple correlation analysis between SpO2 levels and pathology and a test known as a Receiver Operating Characteristic (ROC) test (Figure 4). The ROC test is historically meant to identify the accuracy rate between false positives and false negatives in a scoring scheme. Based on this data alone, the correlation coefficient was 0.79 (Figure 4A). The ROC analysis also indicated that our scheme was sensitive and accurate, all three groups were correctly classified by testing SpO2 levels alone (Fig. 4B)
Figure 4.
Correlation and Receiver Operating Characteristic (ROC) tests on the data in this study. A. The correlation coefficient based on the linear fit was 0.79. B. The ROC analysis classified by testing SpO2 levels alone. The white line is mice classified as healthy, black line is mice classified as borderline, and grey is mice classified as diseased. In this analysis, the area of the curve should be greater than 0.5 and as close to 1.0 as possible.
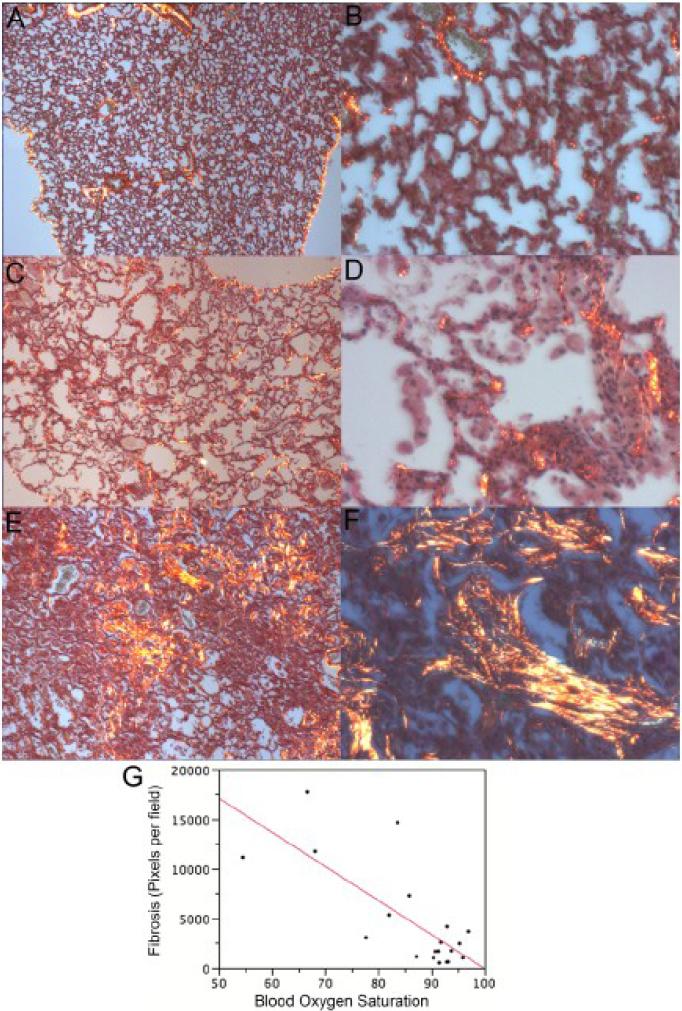
Collagen deposition is also a critical event in the disease process. Mason-Trichrome stain was used in our previous study (5). Its sensitivity is good but not useful for quantitation. The use of Picrosirius Red was recommended by Dr. Cory Hogaboam (19) as an alternative. As noted in the protocol (16), it is important to use circular polarizing filters to see all of the fibers. It is also important to use the same light intensity and the same parameters when scoring. The stain is suitable for quantitation using representative images captured with the 4X objective (Fig. 5). This correlated with blood oxygen saturation (R2 = 0.57 with wild type mice; N= 7 wild type mice and 13 PECAM deficient mice; ANOVA of linear regression, p < 0.0001). It is important to note that the earliest stage of disease, >85% SpO2 with alveolar enlargement, does not have a significantly higher amount of fibrosis in large regions of the lung (Fig. 5C). This is similar to the base line amount of collagen found around the blood vessels and bronchioles of wild type mice (Fig. 5A-B) but the developing lesions do contain collagen (Fig. 5D). It is only as lesions develop that fibrosis becomes more prominent (Fig. 5EF)and this leads to some of the variability in the linear regression. It is also important to note that the stain must be used at full strength and not re-used. Finally, scoring of all slides with NIS Elements should be done on the same day to minimize variation in thresholding.
Figure 5.
Picrosirius Red Stain to quantitate fibrosis. A. Wild type lung (4X magnification). C, PECAM deficient mice with borderline SpO2 (84%; 4X magnification). D. PECAM deficient mice with borderline SpO2 (84%; 40X magnification) E, PECAM deficient mouse with average SpO2 = 68%. (4X). F. PECAM deficient mouse with average SpO2 = 68%. (40X) F. Multivariate analysis between blood oxygen saturation and stain intensity.
Discussion
The most important finding of this study is that the earliest stage of disease in PECAM deficient mice has an alveolar damage/enlargement event that precedes lesion formation and fibrosis. There is some evidence of a similar stage of disease in human familial idiopathic pulmonary fibrosis (20). Although it is still unclear what causes the loss of alveolar structure in both humans and PECAM deficient mice, this is clearly a critical event in the disease process. The cellular and molecular events that occur at this stage are the focus of our current studies. This highlights the value of complimentary animal models of disease because it is not feasible to study these early events in humans.
The studies here show how the MouseOx system was used to accurately identify decreased arterial oxygen saturation in diseased mice and correlates with the extent of disease with SpO2. This system is sensitive enough to identify mice at the earliest stages of disease and appears to be the first studies using this system in the context of disease progression in a murine model of interstitial lung disease. In pulse oximetry studies of human interstitial lung disease, SpO2 levels that drop below 90% during exercise are considered at highest risk (2-4). As the mice in this study are not truly ”resting” inasmuch as a mouse under observation is rarely resting compared to humans, the substitution of this method as a surrogate of moderate exercise has plenty of predictive value. It might be necessary to actually place mice in active exercise routines similar to the step tests in human medicine, but the time and effort to do these tests may outweigh any benefit when screening a moderately large mouse colony. The data here show sufficient sensitivity for the needs of this project at this time.
This assay can be done with minimal stress to the animal, provides a means for researchers and animal care givers to recognize the need for medical and supportive care early in the disease, and spares experimental animals from unnecessary suffering. Other animal models of cardiovascular disease may also benefit from the use of these systems. Most important, the staging of disease is vital for precise cellular and molecular analysis during disease progression.
A recent advance in the hardware used to obtain the blood oxygen saturation is a collar-based system. This collar, which is a very small and gentle clamp, measures blood oxygen from the carotid artery. It unfortunately came out too recently to be included in this study, but so far has superior sensitivity to the foot/tail clamp method we used here. In head to head comparisons, we found that both the foot/tail clamp and the collar provided nearly identical data. The collar has the advantage of allowing the mouse to freely roam while measurements are being taken, further decreasing the stress of the animal and improving the chances that a measurement is accurate. However, on obese mice such as older single males, the foot or tail clamp was the only way to measure the blood oxygen saturation as the neck was too large to allow the clamp to hold on.
We have shown that Picrosirius red stain can be used as an independent quantitative analysis of later stages of disease but this earliest stage precedes collagen deposition. The molecular and cellular events at the earliest stages of disease will be the focus of future studies now that SpO2 levels can be used to identify mice early in the process. It should now be feasible to select mice as they transition from health to disease and establish a clinical scoring system based on both SpO2 and pathology. This is critical for identifying the events that lead to interstitial lung disease in our mouse model and for development of therapeutic strategies to combat similar human diseases.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants HL-064774 and AI-44072. The authors want to thank Bianca Junge and Elisa French (Colorado State University) for expert animal care, Dr. Anne Lenaerts and Dr. Gavin Ryan for access to histology instruments, and Jamie S. Schenkel for critical reading of the manuscript.
Supported by Colorado State University new faculty funding and NIH HL064774
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelleher JF. Pulse oximetry. J Clin Monit. 1989;5:37–62. doi: 10.1007/BF01618369. [DOI] [PubMed] [Google Scholar]
- 2.Zisman DA, Ross DJ, Belperio JA, Saggar R, Lynch JP, 3rd, Ardehali A, Karlamangla AS. Prediction of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2007;101:2153–2159. doi: 10.1016/j.rmed.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP, 3rd, Martinez FJ. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 4.Stephan S, de Castro Pereira CA, Coletta EM, Ferreira RG, Otta JS, Nery LE. Oxygen desaturation during a 4-minute step test: predicting survival in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:70–76. [PubMed] [Google Scholar]
- 5.Schenkel AR, Chew TW, Chlipala E, Harbord MW, Muller WA. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp Mol Pathol. 2006;81:23–30. doi: 10.1016/j.yexmp.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller WA. The role of PECAM-1 [CD31] in leukocyte emigration: Studies in vitro and in vivo. Journal of Leukocyte Biology. 1995;57:523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- 7.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 8.Newton-Nash DK, Newman PJ. A new role for platelet-endthelial cell adhesion molecule-1 (CD31): Inhibition of TCR-mediated signal transduction. J. Immunol. 1999;163:682–688. [PubMed] [Google Scholar]
- 9.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 10.Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, Salmon M, Buckley CD. Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J Cell Sci. 1999;112(Pt 12):1989–1997. doi: 10.1242/jcs.112.12.1989. [DOI] [PubMed] [Google Scholar]
- 11.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184–193. doi: 10.1182/blood-2002-01-0027. [DOI] [PubMed] [Google Scholar]
- 13.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–179. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- 14.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch JP, 3rd, Thannickal VJ. Idiopathic Pulmonary Fibrosis. In: Baughman RP, du Bois RM, Lynch JP 3rd, Wells AU, editors. Diffuse Lung Disease. A Practical Approach. Arnold; London, U. K.: 2004. pp. 130–151. [Google Scholar]
- 16.Kiernan JA. Sirius Red Staining Protocol for Collagen. 2007.
- 17.Chu V, Otero JM, Lopez O, Morgan JP, Amende I, Hampton TG. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001;1:6. doi: 10.1186/1472-6793-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 19.Murray LA, Knight DA, McAlonan L, Argentieri R, Joshi A, Shaheen F, Cunningham M, Alexopolou L, Flavell RA, Sarisky RT, Hogaboam CM. Deleterious role of TLR3 during hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2008;178:1227–1237. doi: 10.1164/rccm.200807-1020OC. [DOI] [PubMed] [Google Scholar]
- 20.Chibbar R, Shih F, Baga M, Torlakovic E, Ramlall K, Skomro R, Cockcroft DW, Lemire EG. Nonspecific interstitial pneumonia and usual interstitial pneumonia with mutation in surfactant protein C in familial pulmonary fibrosis. Mod Pathol. 2004;17:973–980. doi: 10.1038/modpathol.3800149. [DOI] [PubMed] [Google Scholar]