Abstract
Injury to the central nervous system often results in impairments that negatively affect walking function. Prior evidence suggests that vibration may improve walking function. The purpose of this study was to determine whether repeated use of whole-body vibration (WBV) is associated with improvements in walking function in individuals with spinal cord injury (SCI). Subjects were 17 individuals with chronic (≥ 1 year), motor-incomplete SCI. Subjects were tested before and after participation in a 12-session (3 days/week- for 4 weeks) intervention of WBV. We assessed change in walking function via 3D motion capture, with walking speed as the primary outcome measure. We also assessed the influence of the WBV intervention on secondary gait characteristics, including cadence, step length, and hip angle-to-knee angle intralimb coordination. Walking speed increased by a mean of 0.062 ± 0.011 m/s, a change that was statistically significant (p<0.001). The WBV intervention was also associated with statistically significant increases in cadence, and both the stronger and weaker legs exhibited increased step length and improved consistency of intralimb coordination. Changes in cadence and step length of the stronger leg were strongly correlated with improvements in walking speed. The improvement in walking speed observed with the WBV intervention was comparable to that reported in the literature in association with locomotor training. This magnitude of change has been identified as being clinically meaningful, even in non-clinical populations. These findings suggest WBV may be useful to improve walking function with effects that may persist for some time following the intervention.
Keywords: Gait, locomotor, afferent input, human movement system
Introduction
Loss of walking function is a common consequence of spinal cord injury (SCI), and for these individuals, regaining walking function is a high priority.1 Individuals with SCI, and other populations with disorders of the central nervous system, often have various impairments that negatively impact walking function. For example, muscle weakness and sensory impairment result in reduced levels of muscle activation and decreased walking speed.2 Spasticity may result in altered muscle timing and co-contraction associated with spastic gait patterns.2, 3 Decreased walking function results from any one, or a combination, of these deficits.2,4
In non-disabled (ND) individuals, vibration to the muscle body or tendon increases walking speed, depending on the combination of vibration placement and direction of progression.5 In individuals with Parkinson’s disease, vibration applied through the soles of the feet during walking increases walking distance, speed, stride length, and improves stride variability.6 Vibration may also excite spinal circuitry (i.e., locomotor pattern generators) involved in the production of locomotor output.7
Whole-body vibration (WBV) is increasingly being used in elderly individuals8, 9 and in clinical populations.10-14 In individuals with SCI, our studies offer preliminary evidence that a 12-session intervention of WBV decreases spasticity of the quadriceps muscles.15 The use of WBV has also been associated with changes in walking function.8, 10-12 Elderly individuals who received a 2-month WBV intervention in combination with balance, muscle strengthening, and walking exercises demonstrated increased walking speed and step length compared to individuals who did the same exercise program without WBV.8 In individuals with Parkinson’s disease, a 3-week intervention of WBV is associated with improvements in walking speed.11 In adults with spastic diplegia due to cerebral palsy, an 8-week intervention of WBV is associated with improvements in muscle strength and reductions in spasticity of the knee extensor muscles, but the distance walked in 6 minutes was unchanged.10 In individuals with SCI who were unable to stand without long-leg braces, a case series published in an abstract attributed the use of WBV with the progression of function from standing to walking.12 Evidence of the effects of WBV on walking function is limited in individuals with SCI.
The purpose of this study was to determine whether repeated use of WBV is associated with improvements in walking function, as defined by changes in walking speed, in individuals with chronic, motor-incomplete SCI. In individuals with SCI, increased walking speed is a standard benchmark for improvement in walking function.16-18 In addition, we assessed changes in secondary gait parameters including step length, cadence, and consistency of hip angle-to-knee angle intralimb coordination. Our prior studies of interventions to improve walking function in individuals with SCI have focused on locomotor training16,19 or nutrient supplementation.20 While there is preliminary evidence in that WBV may improve walking function in elderly individuals9 and individuals with Parkinson’s disease,11 there are no published studies related to the influence of WBV on walking function in individuals with SCI, many of whom have a limited ability to maintain standing. Based on the prior evidence, we hypothesized that the 12-session intervention of WBV would be associated with improvements in walking speed and the secondary gait parameters. In this consideration-of-concept study,21 our goal was to determine whether there was value in pursuing this line of study, and whether it was feasible to use WBV in individuals with SCI relative to subject tolerance and incidence of adverse events.
Subjects
Seventeen subjects (3 women, 14 men; age 28-65) with SCI enrolled in the study. All subjects underwent clinical examination prior to testing. Subject inclusion criteria was motor incomplete, chronic (≥ 1 year duration) SCI, and ability to rise from sitting to standing with no more than moderate assistance from one person, and ability to stand (using upper extremity support) for at least 1 minute. American Spinal Injury Association (ASIA) motor and sensory scores,22 and ASIA Impairment Scale (AIS) classification22 were evaluated by a physical therapist who was not otherwise involved in the study. All individuals had asymmetrical lower extremity motor scores that were used to identify the weaker and stronger extremity. Subject height was recorded for the purpose of normalizing gait parameters. Information about daily use of medications and assistive devices for mobility was also recorded.
Demographic data for the subjects is given in Table 1. All subjects gave written and verbal informed consent to participate in a protocol approved by the Human Subjects Research Office at the institution. All subjects were instructed to maintain their regular exercise and medication habits until completion of the study.
Table 1.
Demographics of participants; LEMS = lower extremity motor scores represent the sum of the motor scores for the 5 key muscles of both legs graded according to ASIA guidelines22 prior to the WBV intervention.
| Subject | Gender | Age | Injury Level | LEMS Scores | Antispastic Agents | Primary Daily Assistive Device |
|---|---|---|---|---|---|---|
| 1 | M | 41 | C5 | 25 | Tizanidine | Wheelchair |
| 2 | F | 42 | C4 | 23 | Baclofen | Wheelchair |
| 3 | M | 60 | C6 | 17 | Baclofen | Wheelchair |
| 4 | M | 48 | C5 | 33 | None | Wheelchair |
| 5 | M | 56 | C5 | 38 | Baclofen | Wheelchair |
| 6 | F | 60 | T4 | 34 | None | Wheelchair |
| 7 | M | 43 | T8 | 26 | None | Personal Transporter |
| 8 | M | 54 | T4 | 36 | None | Wheelchair |
| 9 | M | 34 | T4 | 29 | None | Wheelchair |
| 10 | M | 42 | C4 | 24 | Baclofen | Wheelchair |
| 11 | M | 41 | T6 | 23 | None | Wheelchair |
| 12 | M | 53 | T7 | 34 | None | Walker |
| 13 | M | 42 | C4 | 23 | Baclofen | Wheelchair |
| 14 | M | 43 | T7 | 42 | None | Wheelchair |
| 15 | M | 58 | C6 | 31 | None | None |
| 16 | M | 65 | C3 | 36 | None | Wheelchair |
| 17 | M | 28 | C4 | 39 | Baclofen | Wheelchair |
WBV Intervention
Subjects received an intervention consisting of WBV (Power Plate; Northbrook, IL) 3 days per week for 4 weeks. Each session included four 45-second bouts with one minute of seated rest without vibration between bouts according to previously published protocols (Figure 1).13, 14 During each WBV bout, subjects stood on the vibration platform with knees flexed approximately 30 degrees from anatomical neutral. Vibration was delivered at 50 Hz with a vertical displacement of 2-4 mm (depending on subject weight).
Figure 1.

Procedure for a single session of whole-body vibration (WBV). Each session of WBV consisted of 4 bouts of WBV at a frequency of 50 Hz and an intensity of 2-4 mm (depending on the subject weight). Each vibration bout was separated by a period of one minute seated rest.
Testing Procedure
The effects of the 12-session WBV intervention on walking function were quantified by comparing the kinematic data acquired prior to the intervention (initial test) to that collected within seven days of the last WBV session (final test). One subject returned an additional time at 5-weeks post WBV for a follow-up test session to allow assessment of persistence of intervention effects. An array of twenty-one reflective markers were placed bilaterally at the lateral malleoli, 5th ray metatarsal-phalangeal joints, heels, lateral knee joints, greater trochanters, anterior superior iliac spines, shoulders, elbows, and wrists as well as at C7, T10, and the sacrum. Kinematic data was collected using an 8-camera 3D motion capture system (Peak Motus® Software, Peak Performance, Centennial, CO). Recording of kinematic data was performed in a calibrated space with data captured at 60 Hz.
For each walking test session, subjects walked 5 times across a 10-meter walkway at their preferred walking speed; subjects were allowed to rest between walking tests. During each walking test, subjects were given 30 seconds to complete the 10-meter walk, and all outcome measures were extracted from data captured within the central 6 meters of the walkway. Subjects used assistive devices as required during testing. Fourteen subjects used a rolling walker, one subject did not have the necessary hand function to grip the handles of a standard rolling walker and performed the test using a rolling walker with bilateral forearm platform supports, and two subjects did not require assistive devices. If a subject required an assistive device, the same device was used both for the initial and final test sessions.
Data analysis and statistics
Statistical Analysis Software 9.1.3 (Cary, NC) was used for all statistical analyses. All data were checked and met the parametric assumptions of homogeneity (Levene’s test for equality of variances) and normality (Kolmogorov-Smirnov Z test). Significance was set at α=0.05. Walking speed (SPEED; meters/second; m/s) was calculated from the distance traversed in the direction of forward progression as measured from the sacral marker. A one-tailed, paired t-test was used to compare the mean speed of the five walking trials from the initial and final test sessions. A convenience sample of one participant (selected because he lived locally) returned for a follow-up walking test session 5-weeks after the last session of WBV. Walking speed for this subject was compared to both the initial and final tests to assess persistence of change in speed associated with the WBV intervention.
Gait parameters were calculated from the kinematic measures. Data were filtered using a Butterworth filter with a 6 Hz cutoff. Bilateral step lengths were extracted from the coordinates of the heel in the direction of progression. Step lengths (m) were determined by the distance between two consecutive contralateral heel strikes, and normalized to subject height. A one-tailed, paired t-test was used to compare the mean initial and final test values of the strong leg step length (SSL) and weak leg step length (WSL). Cadence (CAD; steps/minute) was calculated by dividing the total number of steps by the time need to complete the steps. A one-tailed, paired t-test was used to compare the mean CAD of the initial and final tests. The values of SSL, WSL and CAD are directly derived from a single reflective marker and may therefore be expected to be interrelated, therefore a Bonferroni correction was used to adjust the alpha level for 4 pair-wise (SPEED, CAD, SLL, WSL) comparisons resulting in a corrected value of α=0.0083.
Intralimb coordination was defined as the ability to produce a consistent relationship of hip-angle-to-knee-angle coupling over multiple step cycles.19, 23 The hip angle was defined by the trunk and thigh segments. The knee angle was defined by the thigh and shank segments. Vector coding was used to quantify intralimb coordination wherein the angular component of the coefficient of correspondence (ACC) represents the degree of consistency of the hip angle-to-knee angle relationship over multiple cycles.19, 23 It has been suggested that ACC values offer insights into the organization of control mechanisms (i.e., locomotor central pattern generators) underlying coordination of innate, cyclic behaviors.19, 23 In individuals with SCI, changes in the ACC value correlate well with changes in walking speed associated with locomotor training.19 ACC was calculated for both the strong ACC (SACC) and weak ACC (WACC) legs. An increase in ACC value from initial to final was interpreted as increased consistency of intralimb coordination. A one-tailed, paired t-test was used to compare the mean SACC and WACC values from the initial and final tests.
To determine which gait parameters were most closely associated with changes in walking speed, Pearson correlations where used to identify the relationship between SPEED and the parameters of CAD, SSL, WSL, SACC, and WACC. Pearson r values were interpreted as follows: 0.00-0.25 was considered little or no relationship, 0.25-0.50 was considered a fair relationship, 0.50-0.75 was considered a moderate relationship, and 0.75-1.0 was considered to be a good to excellent relationship.24
The pooled standard deviation and Cohen’s d was used to calculate the effect size of the change in SPEED associated with use of the WBV intervention.
Results
The group mean walking speed (SPEED) increased by 0.062 ± 0.011 (mean ± standard error) m/s (from 0.259 ± 0.248 m/s in the initial test to 0.321 ± 0.260 m/s in the final test), an increase that was statistically significant (p<0.001) but considered a small effect size (d=0.249).
Subject 16, who returned for a follow-up test 5-weeks after the last WBV session, had an initial SPEED of 0.128 m/s, a final SPEED of 0.215 m/s and a 5-week follow-up SPEED of 0.241 m/s. SPEED values of all subjects, group mean, and Subject 16 are illustrated in Figure 2.
Figure 2.
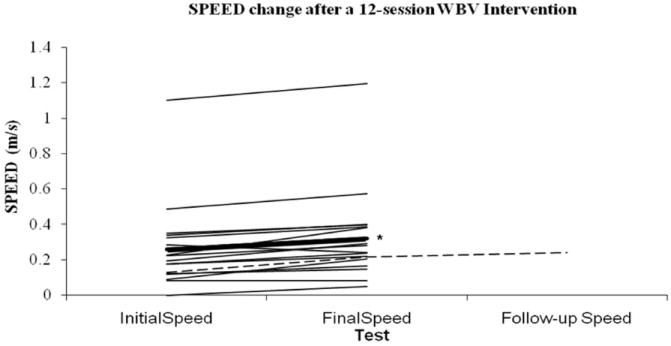
All subjects change in walking speed (SPEED; mean ± standard error) associated with 12-session whole-body vibration (WBV) intervention. Change in SPEED values for all subjects (thin lines) and group mean change (thick line). Group mean initial SPEED value (0.259 ± 0.248 m/s) and final SPEED value (0.321 ± 0.260 m/s) were significantly different (*). The y-axis has been broken from 0.6 m/s to 1 m/s because there were no SPEED values measured in this range. One subject returned for a follow-up test 5-weeks (dashed line) after the last WBV session, had an initial SPEED of 0.128 m/s, a final SPEED of 0.215 m/s and a 5-week follow-up SPEED of 0.241 m/s.
The 12-session intervention of WBV was also associated with a statistically significant increase in CAD (from 33 ± 5 step/min [initial] to 36 ± 5 steps/min [final]; p=0.002). SSL significantly increased (from 0.194 ± 0.090 m [initial] to 0.23 ± 0.063 m [final], p=0.006) and WSL significantly increased (from 0.180 ± .082 m [initial] to 0.212 ± 0.075 m [final], p=0.003). The relationship between change in SPEED to changes in, CAD, SSL, and WSL are illustrated in Figure 3.
Figure 3.
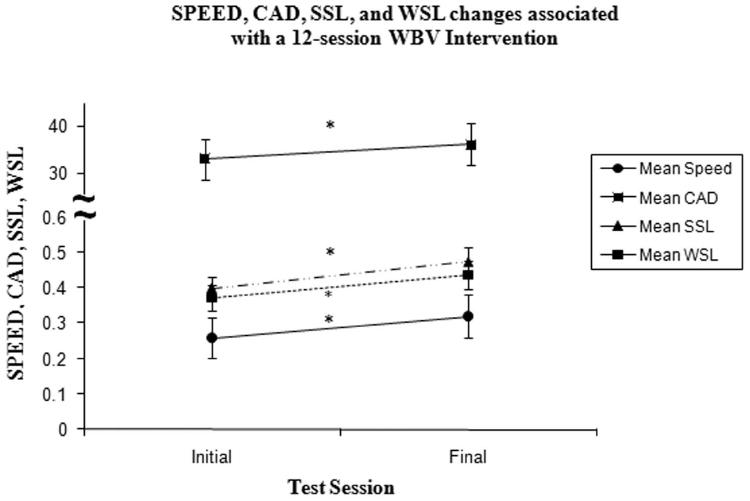
Group Mean Changes in walking speed (SPEED; m/s), change in walking cadence (CAD; steps/min), and change in weak (WSL) and strong (SSL) step lengths (m) after a 12-session whole-body vibration (WBV) intervention.
CAD, WSL and SSL significantly decreased (*) after the 12-session intervention of WBV. Theses gait parameters contributed to the increase in SPEED. The y-axis values are dependent on the reported measure and are denoted as group mean ± standard error bars. Note the break in the y-axis from 0.5 to 30 to accommodate for values of cadence.
There was a statistically significant change in SACC (from 0.68 ± 0.22 [initial] to 0.77 ± 0.12 [final], p=0.018) and in WACC (from 0.67 ± 0.22 [initial] to 0.74 ± 0.14 in [final], p=0.026). The relationship between change in SPEED to changes in SACC and WACC is illustrated in Figure 4.
Figure 4.
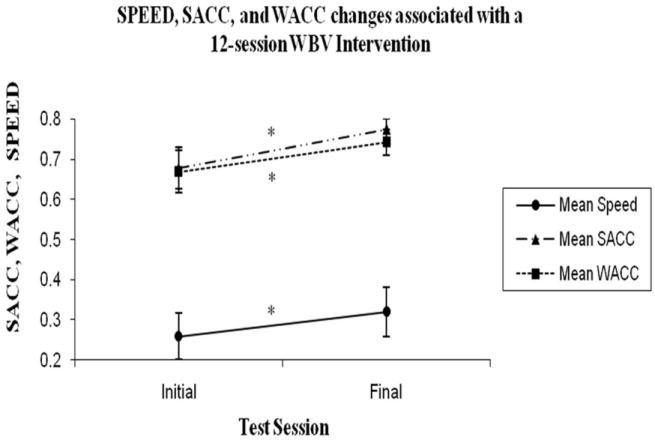
Group Mean Changes in walking speed (SPEED; m/s) and the change in strong (SACC) and weak (WACC) coefficients of correspondence (ACC) after a 12-session whole-body vibration (WBV) intervention. The y-axis values are dependent on the reported measure and are denoted as group mean ± standard error bars. Note the break in the y-axis from 0.4 to 0.7 because no values were reported in this range. SACC and WACC significantly decreased (*) after the 12-session intervention of WBV.
Changes in SPEED had a significant, moderate, direct correlation with CAD (r=0.528, p=0.029), and a non-significant, fair, direct relationship with SSL (r=0.344, p=0.176). Changes in SPEED had a non-significant, weak, direct correlation with WSL (r=0.154, p=0.554), SACC (r=0.018, p=0.946), and WACC (r=0.204, p=0.433).
All subjects tolerated the 12-sessions of WBV, were able to maintain the standing posture for the 45-sec bouts of WBV, and reported no adverse effects. These results suggest that it is feasible to use WBV as an intervention in future randomized, controlled studies that assess interventions to improve walking function in individuals SCI. All subjects adhered to the study protocol and completed the intervention; many of these individuals indicated they would like to continue with these sessions and would be interested in participating in future studies involving WBV.
Discussion
Our results indicate that consistent use of WBV is associated with an increase in walking function, as defined by walking speed, in individuals with SCI who have some ability to maintain voluntary standing. While the lack of a control group, the inclusion of individuals with varying degrees of walking ability, and the fact that it was not possible to blind subjects to the intervention limits the conclusions that can be drawn from these findings, the fact remains that the observed changes in walking speed associated with WBV were equivalent to those reported for studies of locomotor training.16 In individuals with SCI who participated in a 3-month 5-day/week locomotor training intervention, walking speed changes of 0.023 m/s, 0.05 m/s, 0.05 m/s, and -0.005 m/s were associated with manually assisted treadmill training, stimulation-assisted treadmill training, overground training, and robotic-assisted treadmill training, respectively. It is possible that participation in a study made the subjects more conscientious of their walking performance or that the pre-, post-test design increased familiarity with the test, resulting in an improvement. However, we feel these explanations are unlikely, especially in light of comparisons to the locomotor training literature.16 In healthy elders, a change in walking speed of 0.05 m/s (with a similar effect size [d= 0.2]) has been judged to be a clinically meaningful change.25 As illustrated in Figure 2, 10 out of 17 of the subjects exceeded a change of 0.05 m/s after the 12-session intervention of WBV. Subject 13 was unable to take a single step in the initial test. After the 12-session intervention of WBV, the subject was able to take 4 steps. It could be argued that this magnitude of change may be even more meaningful in individuals with SCI than it is in healthy elders. Our findings are consistent with improvements in walking function observed in elderly individuals8 and in individuals with Parkinson’s disease who received a WBV intervention,11 and therefore provide preliminary evidence that regular use of WBV may be a potent intervention for improving walking function in individuals with SCI.
In the single subject who performed a follow-up test session 5 weeks after the WBV intervention, the effects on walking speed not only persisted, but increased 5 weeks after the WBV intervention. These are similar to findings in individuals with Parkinson’s disease who had a persistent change in walking speed 4 weeks after receiving a 3-week WBV intervention.11 The use of afferent input to induce positive plastic effects in the nervous system has received considerable attention in the recent research literature.26-28 These plastic changes are most meaningful when they are associated with a lasting improvement in function.11, 26 Future studies should assess the persistence of effects of WBV through follow-up testing performed some time after the final intervention.
We observed improvements in cadence, step length of the strong and weak legs, and consistency of hip angle-to-knee angle intralimb coordination after a 12-session intervention of WBV. Furthermore, changes in walking speed and cadence were moderately correlated suggesting the increase in walking speed associated with the WBV intervention is related to an increase in the number of steps taken per minute. In addition to an increase in cadence, changes in walking speed also had a fair relationship with changes in the stronger step length, suggesting that increased stronger leg step lengths may also contributed to the improvements in walking speed. The improvements in walking speed and step length are consistent with increases in stride length reported in individuals with Parkinson’s disease during vibration.6 However, this is contrary to evidence in ND individuals wherein no change in stride length during walking was observed with vibration applied to tibialis anterior, triceps surae, biceps femoris, rectus femoris, or quadriceps femoris.29 Changes in stride length may not be evident in ND individuals because their strides are relatively longer compared to individuals with a neuropathology. For this reason, individuals with neuropathology may have a larger margin for vibration-induced improvement of spatial walking characteristics compared to ND individuals.
The degree of consistency of the hip-knee intralimb coordination of both legs improved, and the improvement was comparable to those observed in a 3-month locomotor training wherein the ACC values improved from 0.56 before to 0.65 after locomotor training.19 However, unlike that prior study wherein the change in intralimb coordination was associated with the change in walking speed, in the present study the change walking speed was only weakly correlated with the change intralimb coordination. Contrary to our findings, in ND individuals, bilateral Achilles tendon vibration did not change walking speed or leg inter-segmentalcoordination30 Also in ND individuals, changes in intralimb ankle-knee coordination were not found with vibration applied to tibialis anterior, triceps surae, biceps femoris, rectus femoris, or quadriceps femoris.29 Our findings and other evidence in individuals with SCI19 suggest that the consistent use of afferent input improves the motor output of the control mechanisms that have been impaired after a SCI.
Implications for function
These results provide preliminary support for the use of WBV as an intervention to improve walking function in individuals with SCI, with changes that appear to be comparable to those achieved with some forms of locomotor training. With a 12-session intervention of WBV, we found significant improvements in walking speed, cadence, step lengths, and intralimb coordination over multiple steps. Furthermore, the effect of WBV on walking function may continue to improve walking function even after the use of WBV has ended. However, this was assessed in a single subject and requires further investigation.
Acknowledgments
We gratefully acknowledge the technical contributions of Stephen Lindley and Saumtra Sinha Ray. This study was supported by The Miami Project to Cure Paralysis and the NIH grant #HD667647.
Footnotes
Conflict of Interest
There is no conflict of interest with any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46(7):500–506. doi: 10.1038/sj.sc.3102172. [DOI] [PubMed] [Google Scholar]
- 2.Perry J. Pathological Mechanisms. Gait Analysis Normal and Pathological Function. 1992:169–182. [Google Scholar]
- 3.Krawetz P, Nance P. Gait analysis of spinal cord injured subjects: effects of injury level and spasticity. Arch Phys Med Rehabil. 1996;77(7):635–638. doi: 10.1016/s0003-9993(96)90000-3. [DOI] [PubMed] [Google Scholar]
- 4.Stein RB, Chong S, James KBCJWM. Devices for Improved Mobility After Spinal Cord Injury and Stroke. Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 1998;20:2297–2300. [Google Scholar]
- 5.Ivanenko YP, Grasso R, Lacquaniti F. Influence of leg muscle vibration on human walking. J Neurophysiol. 2000;84(4):1737–1747. doi: 10.1152/jn.2000.84.4.1737. [DOI] [PubMed] [Google Scholar]
- 6.Novak P, Novak V. Effect of step-synchronized vibration stimulation of soles on gait in Parkinson’s disease: a pilot study. J Neuroeng Rehabil. 2006;3:9. doi: 10.1186/1743-0003-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA. Locomotor-like movements evoked by leg muscle vibration in humans. Eur J Neurosci. 1998;10(5):1608–1612. doi: 10.1046/j.1460-9568.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- 8.Kawanabe K, Kawashima A, Sashimoto I, Takeda T, Sato Y, Iwamoto J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med. 2007;56(1):28–33. doi: 10.2302/kjm.56.28. [DOI] [PubMed] [Google Scholar]
- 9.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–908. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006;38(5):302–308. doi: 10.1080/16501970600680262. [DOI] [PubMed] [Google Scholar]
- 11.Ebersbach G, Edler D, Kaufhold O, Wissel J. Whole body vibration versus conventional physiotherapy to improve balance and gait in Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(3):399–403. doi: 10.1016/j.apmr.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Gianutsos J, Oakes LC, Siasoco V, Appelblatt S, Hamel J, Gold J. Motor Rehabilitation of Spinal Cord Dysfunction by Means of Whole Body Vibration. Abstract presented at 3rd Mediterranean Congress of Physical Medicine and Rehabilitation; Athens, Greece. 9-4-2000. [Google Scholar]
- 13.van Nes I, Geurts AC, Hendricks HT, Duysens J. Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: preliminary evidence. Am J Phys Med Rehabil. 2004;83(11):867–873. doi: 10.1097/01.phm.0000140801.23135.09. [DOI] [PubMed] [Google Scholar]
- 14.van Nes I, Latour H, Schils F, Meijer R, van KA, Geurts AC. Long-term effects of 6-week whole-body vibration on balance recovery and activities of daily living in the postacute phase of stroke: a randomized, controlled trial. Stroke. 2006;37(9):2331–2335. doi: 10.1161/01.STR.0000236494.62957.f3. [DOI] [PubMed] [Google Scholar]
- 15.Ness LL, Field-Fote EC. Effects of whole body vibration on spinal reflex activity and walking function in individuals with chronic, motor incomplete spinal cord injury. Society for Neuroscience. 2008 [Google Scholar]
- 16.Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther. 2005;29(3):127–137. doi: 10.1097/01.npt.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- 17.Lapointe R, Lajoie Y, Serresse O, Barbeau H. Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord. 2001;39(6):327–335. doi: 10.1038/sj.sc.3101167. [DOI] [PubMed] [Google Scholar]
- 18.Scivoletto G, Romanelli A, Mariotti A, et al. Clinical factors that affect walking level and performance in chronic spinal cord lesion patients. Spine. 2008;33(3):259–264. doi: 10.1097/BRS.0b013e3181626ab0. [DOI] [PubMed] [Google Scholar]
- 19.Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82(7):707–715. [PubMed] [Google Scholar]
- 20.Nash MS, Meltzer NM, Martins SC, Burns PA, Lindley SD, Field-Fote EC. Nutrient supplementation post ambulation in persons with incomplete spinal cord injuries: a randomized, double-blinded, placebo-controlled case series. Arch Phys Med Rehabil. 2007;88(2):228–233. doi: 10.1016/j.apmr.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Dobkin BH. Progressive Staging of Pilot Studies to Improve Phase III Trials for Motor Interventions. Neurorehabil Neural Repair. 2009;23(3):197–206. doi: 10.1177/1545968309331863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 23.Tepavac D, Field-Fote EC. Vector Coding: A Technique for Quantification of Intersegemental Coupling in Multicycle Behaviors. Journal of Applied Biomechanics. 2001;17:259–270. [Google Scholar]
- 24.Portney LG, Watkins MP. Foundations of Clinical Research. 3. 2008. Correlation. [Google Scholar]
- 25.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 26.Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51(1):122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther. 2007;87(2):208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- 28.Rosenkranz K, Rothwell JC. Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci. 2006;23(3):822–829. doi: 10.1111/j.1460-9568.2006.04605.x. [DOI] [PubMed] [Google Scholar]
- 29.Verschueren SM, Swinnen SP, Desloovere K, Duysens J. Effects of tendon vibration on the spatiotemporal characteristics of human locomotion. Exp Brain Res. 2002;143(2):231–239. doi: 10.1007/s00221-001-0987-3. [DOI] [PubMed] [Google Scholar]
- 30.Courtine G, Pozzo T, Lucas B, Schieppati M. Continuous, bilateral Achilles’ tendon vibration is not detrimental to human walk. Brain Res Bull. 2001;55(1):107–115. doi: 10.1016/s0361-9230(01)00504-4. [DOI] [PubMed] [Google Scholar]


