Abstract
This review presents a novel conceptualization of addiction, integrating the concepts of interoception (i.e., the CNS representation of visceral feelings) and alliesthesia (i.e., that rewarding properties of stimuli are dependent on the internal state of the individual) with existing theories. It is argued that the body state, as defined by the integration of interoceptive information, is a crucial arbiter of the risk for initiation of and transition to compulsive use of addictive compounds. Overall, individuals at risk for drug dependence are characterized by an altered internal bodily state that leads to a change in hedonic and incentive motivational properties of addictive drugs. Specifically, drug dependent individuals experience alliesthesia of interoceptive processing, leading to increased incentive motivational properties of the drug over time and thereby increasing the probability of subsequent use. This extension of previous theories of addiction to include interoception and alliesthesia is based upon a clearly delineated set of neural substrates mediating interoception, key elements of which also recently have been implicated in drug addiction. The model thereby provides new potential targets for interventions that are aimed at changing the internal state that puts the individual at risk for continued substance use.
Keywords: Addiction, Insula, Interoception, Alliesthesia, Reward
In this review, we integrate approaches to develop a better understanding of the critical processes that go awry when individuals become addicted to drugs. First, allostasis and other current conceptualizations of addictive behaviors are reviewed, highlighting the importance of a balanced internal body state that is thought to be altered due to repeated administration of a drug. Second, the concept of interoception is introduced as the central nervous system representation of feelings originating from the interior of the body. Moreover, connections are drawn between interoception and reward, craving, and urge related behaviors. Third, we incorporate into addiction the construct of alliesthesia, which denotes that positive versus negative hedonic valuations of stimuli are dependent on the internal state of the individual. The fundamental notion proposed here is that addiction represents a disequilibrium of an internal state due to an altered body prediction error, i.e., the difference between the value of the anticipated/predicted and value of the current interoceptive state, which leads to inadequate regulatory mechanisms that critically involve the interoceptive system.
Adding interoception as a critical contributor to this process, we hypothesize that dysregulated insular cortex function in drug addicted individual results in an insufficient, instable or non-adaptive adjustment of the body prediction error. It is proposed that in addiction, a neural representation of an altered internal homeostatic state is implemented within the insular cortex, which contributes to the development of pathology via an altered body prediction error favoring the use of addictive substances. One possible scenario is that individuals who are beginning to use drugs are hypo-responsive to typically hedonic stimulation. Consequently, alternative stimuli are sought to establish an internal dynamic equilibrium. In comparison, in drug addicted individuals, the internally generated states are a result of long-term adjustments due to allostatic dysregulation, which renders the individual in an unstable and presumably aversive body state.
The extension of previous theories of addiction to include interoception and alliesthesia identifies a clearly delineated set of neural substrates that are important for visceral perception. The core neural substrate of this visceral neurocircuitry, the insular cortex, recently has been implicated in drug addiction (Naqvi et al. 2007). Additional focus on this neural system in future studies should provide new potential targets for intervention techniques that are aimed at changing the allostatic states to diminish the risk for continued substance use. Taken together, it is argued here that the body state, as defined by the integration of interoceptive information, is a crucial arbiter of the risk for initiation of and transition to compulsive use of drug of abuse.
1. Current Theories of Drug Addiction
Several theoretical approaches have been developed to understand some of the key characteristics of drug addiction: (1) a chronic disease process that develops into a relatively rigid pattern of behavior; (2) compulsive use of a substance; (3) difficulty in reducing or stopping use despite recognizing the harmful consequences; and (4) high probability of relapse (Koob and Le Moal 2008). Major exemplars of these theories are briefly reviewed below.
Anhedonia Model and Dopamine
As first observed in 1978 (Wise et al. 1978), Wise proposed that brain dopamine plays a critical role in the subjective pleasure associated with positive rewards and that drugs of abuse exert their powerful addictive effects by modulating the availability of dopamine (Wise 2008). This approach was further developed by a number of investigators to include other behavioral constructs such as incentive motivation.
Incentive Sensitization
First proposed by Robinson and Berridge in 1993, the incentive sensitization theory argues that compulsive use emerges as drug using individuals experience a sensitization or hypersensitivity to the incentive motivational effects of drugs and drug-associated stimuli, which has been paraphrased as increased “wanting” of drugs and drug-related stimuli (Robinson and Berridge 2008).
Allostatic Dysregulation and Negative Reinforcement Models
Koob and colleagues (Koob and Le Moal 2008) have developed a theoretical approach that is based on the notion of allostatically changed opponent processes and incorporates negative reinforcement principles to arrive at a conceptualization of drug addiction as a cyclical disorder comprising three distinct stages of preoccupation and anticipation, binge and intoxication, as well as withdrawal and negative affect (Koob 2008). The notion of allostasis originated as an extension of the stress regulation model (McEwen 1998) and was originally defined as an unstable state of stress involving chronic dysregulation of the brain, particularly the hypothalamic, pituitary, adrenal axis. Applying allostasis to addiction, Koob and colleagues (Koob and Le Moal 1997) proposed that repeated drug use promotes a transition of behavioral processes from an impulsive mode of action, which is driven by pleasure, relief, and gratification (i.e. maintained by positive reinforcement), towards a compulsive mode of action, which is driven by relief of anxiety or stress (i.e. maintained by negative reinforcement). This transition is thought to be due to allostatic dysregulation, i.e. a chronic deviation of the regulatory state from its homeostatic equilibrium (Koob 2008), of opponent processes (Solomon and Corbit 1974) that alter the subjectively experienced effect of drug administration. As a consequence the individual experiences an attenuation of the positive hedonic effects and but an exaggeration of the withdrawal effects of the drug. This physiological model of allostatic dysregulation is closely related to psychological models emphasizing negative reinforcement as dominant process underlying the development of drug addiction. Negative reinforcement is at work when an individual is experiencing or anticipates a negatively valued state and acts to terminate this state Reinforcers act via brain-related processes in several different ways by (a) activating neural substrates of approach or escape responses, (b) producing rewarding or aversive internal states, and (c) modulating information stored in memory (White 1996). Several investigators (Baker et al. 2004;Eissenberg 2004) have argued that addicted individuals take drugs to escape or avoid aversive states such as withdrawal or stress, and that these individuals learn to detect interoceptive cues predictive of such states. Sinha and colleagues have reported that aversive states are closely linked to craving, drug-taking behavior, and relapse (Sinha et al. 2005).
Habit Formation
As proposed by Robbins and Everitt, this model emphasizes the transition from goal-directed action to habitual behavior during the development of drug addiction (Robbins and Everitt 1999). This transition is proposed to be driven by instrumental learning via action-outcome contingencies that are due to interoceptive and exteroceptive states associated with drugs. More recently, these authors have proposed that impulsivity, defined as a behavioral tendency for premature responding, may play a critical role in the escalation of drug intake and the switch to compulsive drug taking behavior (Everitt et al. 2008)
Top-Down and Bottom-Up, Multi-Stage Failure Models
Redish (Redish et al. 2008) identifies a series of decision-related vulnerabilities that break down in addiction within a two-systems conceptualization of processing, consisting of a top-down cognitive planning system (presumed cortical), and an associative habit network (presumed striatal). Related models include (a) Kalivas and colleagues, in which addiction is viewed as exaggerated assignment of the salience to drug stimuli, which affects the neural regulation of behavioral output in response to those stimuli (Kalivas and Volkow 2005); and (b) Bechara and colleagues, who have conceptualized addiction as an unstable and disrupted equilibrium between a top-down control system and a bottom-up impulsive system leading to an increased propensity to engage in short-term reward-seeking behavior (Bechara 2005). Recently this group has integrated the notion of interoception and a role for the interoceptive cortex in regulating two types of dysfunction within the control and impulsive system. In their model, hyperactivity in the amygdala or impulsive system, which exaggerates the rewarding impact of available incentives, is combined with hypoactivity in the prefrontal cortex or reflective system, which forecasts the long-term consequences of a given action. Interoceptive signaling via the insula is thought to be particularly important for the physiological instantiation of the impulsive system (Naqvi and Bechara 2008;Verdejo-Garcia and Bechara 2008).
2. Interoception
Interoception comprises the sensing the physiological condition of the body, the conscious representation of the internal state within the context of ongoing activities, and the initiation of motivated action to homeostatically regulate the internal state (Craig 2007). The afferents that are involved in interoception can be divided based on the type of stimulus they respond to as mechanoreceptors, chemoreceptors, thermoreceptors, and osmoreceptors. As a consequence interoception includes a range of sensations such as, temperature, itch, tickle, sensual touch, muscle tension, air hunger, stomach pH, and intestinal tension, which together provide an integrated sense of the body’s physiological condition (for a recent review see (Craig 2009). These sensations travel via small-diameter C-fiber afferents, which are thought to comprise a cohesive system for homeostatic afferent activity that parallels the efferent sympathetic nervous system (Craig 2007), and terminate on projection neurons in the most superficial layer of the spinal dorsal horn. The modality-selective lamina I spinothalamic neurons project to a specific thalamo-cortical relay nucleus, which in turn projects to a discrete portion of dorsal posterior insular cortex. The posterior insula provides topographic and modality-specific interoceptive signals to the anterior insular cortex for integration.
The anterior insular cortex has bidirectional connections to: a) anterior cingulate cortex (Augustine 1996), which is important for cognitive control processes b) the amygdala (Jasmin et al. 2003), which is critical for processing stimulus salience (Paton et al. 2006); c) the ventral striatum (Reynolds and Zahm 2005), central for the incentive motivational aspects of rewarding stimuli (Robinson and Berridge 2008); and d) the orbitofrontal cortex (Ongur and Price 2000), which has been implicated in state-related valuation of external stimuli (Kringelbach 2005). Within the anterior insular cortex, a multi-dimensional representation and integration of the current and possibly the predicted (Paulus and Stein 2006) body state provides the individual with a representation of a “global moment in time” and the capacity to be aware of themselves, others and the environment (Craig 2009).
The columnar organization of the insular cortex shows a highly organized anterior-inferior to posterior-superior gradient (see (Mesulam and Mufson 1982)). This gradient is found in other parts of the brain wherever cortical representations are based on modulatory or selective feedback circuits (Shipp 2005). Therefore, it is not surprising that insular cortex has been reported across a wide range of processes, including pain (Tracey et al. 2000), interoceptive (Critchley et al. 2004), emotion related (Phan et al. 2002), cognitive (Huettel et al. 2004), and social functions (Eisenberger et al. 2003).
Two aspects of interoception are important for addiction. First, during interoceptive processing, the evaluation of the signal is highly dependent on the homeostatic state of the individual. For example, the same degree of heat (or cold) can be motivationally rewarding or punishing depending on the individual’s core body temperature. Thus, the absence of a drug in the bloodstream may enhance the aversive effects of stimuli in a drug-addicted individual. Second, interoceptive sensations are often associated with intense affective and motivational components. For example, interoceptive processing may result in increased propensity to continue in addictive behaviors, e.g. gambling individuals with relatively stronger activation of anterior insula to “near misses” had a higher urge to continue to gamble (Clark et al. 2009). On the other hand if the strong motivational aspect of drug-taking is lacking, individuals may be able to “quit smoking easily, immediately, without relapse, and without persistence of the urge to smoke” (Naqvi et al. 2007), which is precisely what was reported in individuals with lesions that included the anterior insular cortex.
3. Reward, Craving and Urges – Hedonic and Incentive Motivation
Reward
Reward is a complex construct that can be defined operationally as a stimulus that increases the frequency of a behavior, or experientially as an incident which induces a feeling of pleasure and an action towards obtaining it, and includes both hedonic and incentive motivational aspects (Robinson and Berridge 2008). Typically, the feeling is described as “pleasurable” or “positive” and the individual acts to approach the object associated with reward. In contrast, objects associated with the lack of reward result in an “aversive” experience and an avoidance action. It is not surprising that the internal state alters the degree of relative desirability of different stimuli (Loewenstein 1996). Consistent with this notion a neuroimaging study has shown that the repeated administration of an initially pleasantly valued stimulus (chocolate) resulted in increased satiety-related aversion, which was accompanied by increased insula activation (Small et al. 2001), demonstrating a role of the insula in modifying the degree of reward experience.
Reward processing as it relates to drug addiction is not restricted to the positive experience. Negative affective states (i.e., aversion, dysphoria, or pain) are as important an aspect of hedonic processing as positive affective states of pleasure/euphoria (Baker et al. 1986). As argued by Craig (Craig 2009), both feeling and action are intimately linked with the internal homeostatic state of the individual. The notion of feeling and action as critically dependent on the homeostatic state of the individual, within the context of drug-related urges, is well illustrated by the notion of “affective contrast” as postulated by Solomon some 30 years ago (Solomon 1980). Using heroin dependence as an example, repeated use often leads to development of substantial tolerance to the “pleasurable” effects of the drug, as evident in the chronic heroin addict’s dramatic escalation in dosage while chasing the memory of the initial euphoria produced by drug use). Although the absolute level of euphoria produced by a dose of heroin in the tolerant addict may be dramatically blunted relative to the euphoria of early use, based on Solomon’s view it is the relative contrast between the dysphoric state just prior to drug use and the blunted euphoria after use in the chronic user that is motivationally compelling in maintaining the urge to use. Thus, despite the development of tolerance to the absolute “pleasurable” effects of a drug, the relative perception of euphoria (feeling) and the urge to use (incentive motivation) may be magnified as a result of the homeostatic disequilibrium of withdrawal present at the time of use in the addict, as predicted by the alliesthesia construct elaborated below. Both positive (euphoric) experience of acute drug intoxication and negative (aversive, dysphoric) experience of drug withdrawal are closely linked to the internal state and, therefore, to interoception.
Craving
Craving has been described as an intense affect associated with a strong urge to act (Singleton and Gorelick 1998). Several craving models (for a review of these see (Tiffany 1999)) have been put forth to explain the psychological processes underlying the expression of craving. These include: a) the cognitive labeling model, which proposes that craving experiences arise from cognitive interpretations of conditioned reactions; b) the outcome expectancy model, which proposes that craving arises from expectations that predict contextually dependent outcomes; c) the dual-affect model, which proposes that competing belief systems trigger craving; d) the dynamic regulatory model, which presumes that craving arises from a combination of conditioned responses to cues, positive and negative affect, and the reinforcement of continuing drug use via a positive feedback loop; and e) the cognitive processing model, which proposes that craving is a combination of verbal, somato-visceral and behavioral responses that arise when automatized and stereotyped response sequences are blocked and cognitive control processes need to be initiated to take an action (Tiffany 1999). Others have suggested that craving arises from the competing expectancies of approach and avoidance behaviors, which activate non-automatic, cognitive control processes (Breiner et al. 1999). Regardless of the model one might favor, it is not difficult to see how the dynamic valuation and strong incentive properties of interoceptive stimuli make them essential elements to the craving experience, i.e. the hedonic component of the body state associated with the incentive to act is an important characteristic of craving. For example, an interoceptive response to conditioned cues may result in a strong craving sensation with the urge to act to receive the cue-predictive stimulus (the drug). Although simply recognizing the interoceptive aspect of craving does not specifically identify the psychological processes that are inherent in the generation of craving, by examining interoceptive processes in controlled behavioral experiments, one can evaluate the role of interoception in distinct components of the psychological models of craving outlined above.
Urges
Urges can be conceptualized as strong incentives to act. The processes underlying urges are complex. Some have suggested a stage approach that involves a motivation-to-action system (Baker et al. 1986). Initially, a stimulus triggers a neural cascade underlying the urge, i.e. the physical need to respond to the stimulus, which is then translated into a targeted goal. Next, actions are deployed to satisfy the urge-desire, and evidence is collected by neural systems to examine success of urge-related action. Finally, the sensory system determines whether the urge was satisfied (Davenport 2008). Urge-related processes are closely related to the incentive motivational aspects of reward because both focus on approach or avoidance behaviors that are initiated in response to a stimulus.
The degree of urge is an integral component of the current interoceptive state (Davenport 2008). In chronic smokers, situational pain increases urge ratings and produces shorter latencies to smoke, which may be related in part to pain-induced negative affect (Ditre and Brandon 2008). Exercise reduces alcohol urges, which re-emerge afterwards (Ussher et al. 2004). Even the abused substance itself, possibly via changing the internal state, can increase subsequent urges. For example, the urge to drink increases after alcohol but not placebo exposure (Rose and Grunsell 2008). Contextual conditioned cues can also provoke urges, as when cues predictive of smoking can elicit urges to smoke irrespective of whether cigarettes are available (Thewissen et al. 2005). Addicts report greater urges when presented with a context that increases the opportunity of using their drug of choice (Wertz and Sayette 2001). Taken together, the important constructs of reward, craving, and urges take into account implicitly or explicitly the internal state of the individual and therefore are inextricably linked to interoceptive processing.
4. Alliesthesia
Alliesthesia is a physiological construct introduced in 1973 to connect the stimulations that come from the “milieu exterieur” and affect the “milieu interieur” (Cabanac 1971). Alliesthesia critically links reward, which is typically ascribed to external stimuli, to the internal state of the individual as a result of interoception. Cabanac proposed that a given external stimulus can be perceived either as pleasant or unpleasant depending upon interoceptive signals (Cabanac 1971). In particular, negative alliesthesia refers to the notion that repeated administration of a stimulus will affect the internal milieu in such a way that the rewarding aspects of the stimulus are attenuated (e.g., the second or third piece of chocolate does not feel as good as the first). In contrast, positive alliesthesia is observed if the repeated administration (or omission) of a stimulus changes the internal milieu in such a way as to increase its rewarding aspects. For example, fasting can enhance the subjective pleasantness of food images (Stoeckel et al. 2007). These phenomena can also be observed in animals. Specifically, pre-feeding has been associated with negative alliesthesia (Zhao and Cabanac 1994), whereas food restriction was found to increase the sensory reward derived from alcohol, which is an example of positive alliesthesia (Soderpalm and Hansen 1999).
Based on the alliesthesia construct, some investigators proposed a dynamic, homeostatic hypothesis of pleasure regulation (Cabanac et al. 2002). This hypothesis proposes that: (1) we experience the intensity of a reward based on the evaluation of the stimulus relative to our internal state, and (2) we engage regulatory mechanisms to maximize the hedonic aspect of the internal state. This approach shares features with the notion of allostatic dysregulation as an underlying process of addiction as proposed by Koob and Le Moal (Le Moal and Koob 2007). Similar to the allostatic approach, the alliesthesia construct can be applied to both rewarding and aversive stimuli, but additionally the alliesthesia concept provides an explicit connection between the internal state (i.e. interoception) and rewarding or aversive stimuli in the environment. In particular, the reference to an internal state identifies additional circuitry, i.e. insular cortex and its connectivity to frontal cortical and extended amygdala circuits, which can be empirically tested for its role in processing appetitive or aversive stimuli.
As detailed above, interoception is strongly related to reward, urge, and craving, and alliesthesia emphasizes that through interoceptive processing these constructs are dependent on the pre-existing internal state of the individual, which may undergo positive/negative alliesthetic changes. Thus, three components appear critical for how an individual responds to drugs: (1) the internal or homeostatic state as signaled via afferents converging to the insular cortex; (2) the cognitive state of the individual, which includes a “contextualized” representation of the interoceptive state (Shipp 2005); and (3) the learned associations of the external stimulus (e.g. the drug of choice) with previous outcomes including predicted internal states. These components clearly differ across individuals who are initially exposed to drugs and those who have been taking drugs for years. In particular, as a person transitions from impulsive to compulsive use, predominantly positive reinforcement mechanisms (approach behaviors) are supplemented with negative reinforcement mechanisms (avoidance behaviors). In other words, as individuals progress from initial exposure to continued and compulsive use they may experience a progressive attenuation of the positive hedonic aspects of the drug (negative alliesthesia) and develop an increasing sensitization to the negative emotional processes (positive alliesthesia) associated with the withdrawal from the drug-induced state. This transition is consistent with the idea the sensitization of antireward systems aimed at opposing the positively valenced hedonic effects of drugs dominate the motivational state of the addicted individual (Koob and Le Moal 2008). The stimuli and associated interoceptive state predicting withdrawal are clearly distinct from the stimuli and interoceptive state predicting intoxication. Nevertheless, the interoceptive state is a crucial component determining whether an individual is likely to take a drug again or shows an inability to resist taking a drug despite the evidence of aversive consequences. Thus, adding interoception as a construct leads one to investigate a particular set of neural substrates as the neurobiological basis for “affective contrast” as postulated by Solomon (Solomon 1980), and provides a biological and conceptual underpinning for the altered hedonic experience of the heroin addict.
5. Proposed Model of Dysregulated Interoception in Drug Addiction
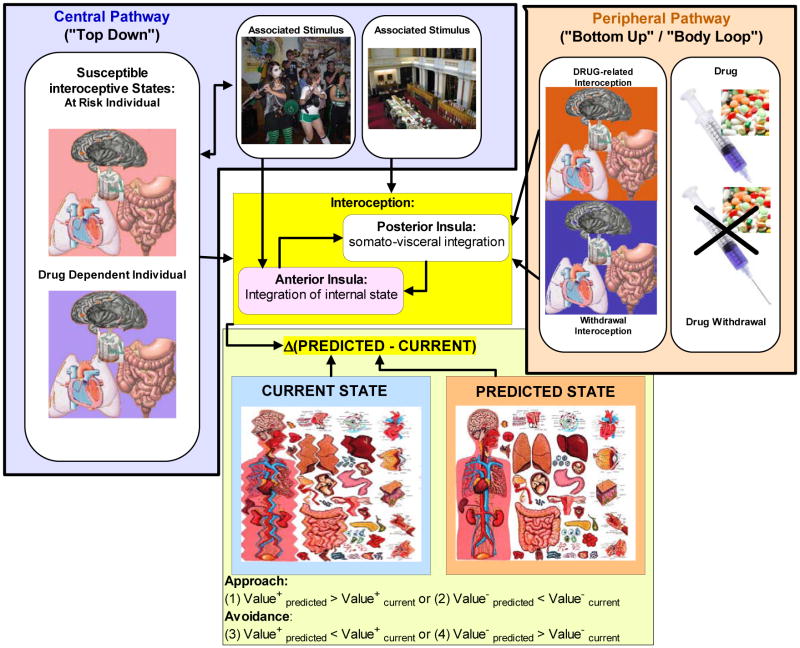
A neuroanatomical processing model is proposed as a heuristic guide to understand how interoceptive processing may contribute to the development and maintenance of drug addiction (see Figure 1). This model, consisting of four components, focuses on the notion of a body prediction error, i.e. the difference between the value of the anticipated/predicted and value of the current interoceptive state. First, information from peripheral receptors ascends via two different pathways, the A-β-fiber discriminative pathway that conveys precise information about the “what” and “where” of the stimulus impinging on the body, and the C-fiber pathway that conveys spatially- and time-integrated affective information (Craig 2007). These afferents converge via several midway and thalamic way stations to the sensory cortex and the posterior insular cortex to provide a sense of the current body state. Second, centrally generated interoceptive states (e.g., via contextual associations from memory) reach the insular cortex via temporal and parietal cortex to generate body states based on conditioned associations (Gray and Critchley 2007). Third, within the insular cortex there is a dorsal-posterior to ventral-anterior organization from granular to agranular, which provides an increasingly “contextualized” representation of the interoceptive state (Shipp 2005), irrespective of whether it is generated internally or via the periphery. These interoceptive states are made available to the orbitofrontal cortex for context-dependent valuation (Rolls 2004) and to the anterior cingulate cortex for error processing (Carter et al. 1998) and action valuation (Rushworth and Behrens 2008). Fourth, bidirectional connections to the basolateral amygdala (Jasmin et al. 2004) and striatum particularly the ventral striatum (Fudge et al. 2005), provide the circuitry to calculate a body prediction error (similar to reward prediction error (Schultz and Dickinson 2000)), and provide a neural signal for salience and learning. The insular cortex relays information to other brain systems to initiate motivated action to achieve a steady state (Craig 2007) by minimizing the body state prediction error.
Figure 1. Conceptual model of interoceptive dysregulation in addiction.
This figure conceptualizes the proposed alteration in interoceptive processing in drug addiction. Drug-use itself can be seen as a repeated perturbation of the current body state, which becomes associated with conditioned stimuli that contribute to the sensitization of the body prediction error. In drug dependent individuals, the internally generated states are a result of long-term adjustments due to allostatic dysregulation, which renders the individual in an unstable and presumably aversive body state. It is proposed that addicted individuals exhibit two types of abnormalities: (1) an increase in body prediction error and insula response to anticipating and experiencing aversive events, potentially due to allostatic dysregulation; and (2) an increased decay of the body prediction error and insula activity following the experience of an aversive event, which results in a decreased flexibility to adjust behavior when anticipating or experiencing an aversive event.
Drugs of abuse exert their effects via the peripheral pathway by altering the body state of the individual, and by directly affecting the centrally generated internal states via direct neurotransmitter-modulating properties (Everitt et al. 2001). One possible scenario is that individuals who are beginning to use drugs and are at risk for drug dependence exhibit different sensitivity to naturally rewarding stimuli, possibly due to pre-existing traits. As a consequence, alternative stimuli are sought to establish an internal dynamic equilibrium. As described above, there is evidence for the internal-state dependent valuation of external stimuli, i.e. alliesthesia (Cabanac 1971). Moreover, drug use can be seen as a repeated perturbation of the current body state, which becomes associated with conditioned stimuli that may ultimately contribute to the sensitization of the body prediction error. In drug addicted individuals the internally generated states are a result of long-term adjustments due to allostatic dysregulation, which renders the individual in an unstable and presumably aversive body state. It is proposed that addicted individuals exhibit two types of abnormalities: (1) an increase in body prediction error and insula response to anticipating and experiencing aversive events potentially due to allostatic dysregulation; and (2) an increased decay of the body prediction error over time and insula activity following the experience of an aversive event, which results in a decreased flexibility to adjust their behavior when anticipating or experiencing an aversive event, and may be linked to the transition from goal-directed to habitual behavior (Robbins and Everitt 1999). Thus, there is an inability of the predicted aversive body states to appropriately influence cognitive control mechanisms (Botvinick et al. 2004), e.g. via anterior cingulate (Brown and Braver 2005), which may result in response patterns that are primarily driven by striatal responses, which are associated with the habit system (Everitt and Robbins 2005). In both cases, we hypothesize that inadequate insular cortex function in individuals who use or are dependent on drugs results in an insufficient, instable, or non-adaptive adjustment of the body prediction error.
The notion of a dynamically perturbed homeostatic steady state is consistent with that of the allostatic dysregulation model proposed by Koob and colleagues (Koob and Le Moal 2008). The proposed model inserts the body prediction error as a key variable into existing top-down (peripheral) and bottom up (central) regulatory models, and is additional consistent with predictions of Incentive Sensitization and Habit Formation Models described above. Other modulatory brain areas may amplify the difference between the predicted and observed body state, and therefore enhance or increase use of this information to generate approach or avoidance actions. Alternatively, these modulatory areas may dampen this difference and enable cognitive control structures to plan and initiate actions, and help to suppress habitual striatal responses to the perceived body state differences. In essence, cortical afferents to the interoceptive system act as differential amplifiers that enable gating of different actions in response to predicted body state differences. It is proposed that drug users and dependent individuals show an altered sensitivity to interoceptive stimuli and fail to engage the appropriate modulatory areas, e.g. the anterior cingulate and ventromedial prefrontal cortex, to initiate adaptive behaviors.
This neuroanatomical processing model is similar to what has been proposed by Gray and Critchley, who suggested that reduced urge to smoke after insula lesion in human smokers is “attributable to disruption of the capacity to represent (and hence respond to) internal interoceptive signals that are perceived subjectively as anxiety and tension…[and] are generated in response to psychological processing of smoking-related cues generated by conditioned reinforcers” (Gray and Critchley 2007). A well-established role for the insula in the formation of conditioned associations in fear conditioning paradigms (Bermudez-Rattoni et al. 1997) is consistent with this view that emphasizes conditioned aversive emotional states. However, we (Paulus 2007), Craig (Craig 2007), and Naqvi and Bechara (Naqvi and Bechara 2008) argue that both approach behaviors to positive hedonic stimuli and avoidance of negative hedonic stimuli are regulated by interoceptive input provided by the insula, and our model allows for both positive and negative valuation processes to influence drug-seeking and taking behavior.
6. Conclusions and Future Directions
It is well established that interoception undergoes both Pavlovian and instrumental learning. Therefore, future investigation may develop new interventions targeted at interoceptive stimulation to alter the internal state so as to decrease the incentive motivational properties of drug-related cues. However, insular injury in humans (Naqvi et al. 2007) and rodents (Contreras et al. 2007) may also result in disruption of interoceptive regulation of direct, not just conditioned, positive or negative hedonic processes. Further studies are needed to assess directly whether insular integrity is critical to the experience of the direct rewarding effects of abused drugs, and/or the negative affective consequences of withdrawal, independent of or in addition to a role in conditioned drug associations. Thus, both human and animal insula lesion studies, while compelling in their overall implications, leave unresolved which addiction-related behaviors in fact critically involve interoceptive processing and the insula. Moreover, it is also unclear whether anatomical differences between rodent and human insula contribute to differential effects on modulating drug effects. Lastly, it remains to be determined exactly which connections of the neural substrates underlying interoceptive processing (e.g., insular and anterior cingulate cortex) are critical in driving drug-related urges, in conjunction with the well-established neural substrates underlying reward-related phenomena (e.g., ventral striatum, amygdala, orbitofrontal cortex, and mid-brain dopamine systems). Taken together, the integration of the interoceptive system into the existing approaches to drug addiction will provide a useful opportunity to deepen our understanding of addictive disorders, and point toward behavioral and pharmacologic targets for intervention.
Acknowledgments
During the preparation of this article, the authors were supported by NIH grants DA13186, DA016663 (M.P.P.); AA13419, DA021182, and DA024194 (S.F.T.); DA010475 (G.S.), and Dept. of Veterans Affairs Clinical (M.P.P.) and Biomedical Laboratory (G.S.) Research Merit Awards.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Introini-Collison I, Coleman-Mesches K, McGaugh JL. Insular cortex and amygdala lesions induced after aversive training impair retention: effects of degree of training. Neurobiology of learning and memory. 1997;67:57–63. doi: 10.1006/nlme.1996.3747. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Breiner MJ, Stritzke WG, Lang AR. Approaching avoidance. A step essential to the understanding of craving. Alcohol Res Health. 1999;23:197–206. [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Guillaume J, Balasko M, Fleury A. Pleasure in decision-making situations. BMC Psychiatry. 2002;2:7. doi: 10.1186/1471-244X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling Near-Misses Enhance Motivation to Gamble and Recruit Win-Related Brain Circuitry. Neuron. 2009;61:1–10. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and Emotion: a Neuroanatomical Perspective. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of Emotions. New York: Guilford Press; 2007. pp. 272–290. [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186 (Suppl 1):S107–S111. doi: 10.1007/s00408-007-9045-7. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117:467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99 (Suppl 1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Misiurek J, Jurkowski AJ, McCarthy G. Dynamic and strategic aspects of executive processing. Brain Res. 2004;1000:78–84. doi: 10.1016/j.brainres.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Loewenstein G. Out of control: Visceral influences on behavior. Organizational Behavior & Human Decision Processes. 1996;65:272–292. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Neural basis of reward and craving--a homeostatic point of view. Dialogues. Clin Neurosci. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An Insular View of Anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. Addiction as vulnerabilities in the decision process. Behav Brain Sci. 2008;31:461–487. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up [news] Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Rose AK, Grunsell L. The subjective, rather than the disinhibiting, effects of alcohol are related to binge drinking. Alcohol Clin Exp Res. 2008;32:1096–1104. doi: 10.1111/j.1530-0277.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton EG, Gorelick DA. Mechanisms of alcohol craving and their clinical implications. Recent Dev Alcohol. 1998;14:177–195. doi: 10.1007/0-306-47148-5_8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Hansen S. Alcohol alliesthesia: food restriction increases the palatability of alcohol through a corticosterone-dependent mechanism. Physiol Behav. 1999;67:409–415. doi: 10.1016/s0031-9384(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Cox JE, Cook EW, III, Weller RE. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48:139–144. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- Thewissen R, van den HM, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100:387–396. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23:215–224. [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RG. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–162. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99:1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. Neuropharmacology. 2008. A somatic marker theory of addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Exp Clin Psychopharmacol. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Zhao C, Cabanac M. Experimental study of the internal signal of alliesthesia induced by sweet molecules in rats. Physiol Behav. 1994;55:169–173. doi: 10.1016/0031-9384(94)90026-4. [DOI] [PubMed] [Google Scholar]