Abstract
Solute transport through the bone lacunar-canalicular system (LCS) is essential for osteocyte survival and function, but quantitative data on the diffusivity of various biological molecules in the LCS are scarce. Using our recently developed approach based on fluorescence recovery after photobleaching (FRAP), diffusion coefficients of five exogenous fluorescent tracers (sodium fluorescein, dextran-3k, dextran-10k, parvalbumin, and ovalbumin) were measured in murine tibiae in situ. These tracers were chosen to test the dependency of solute diffusion on molecular weight (376–43,000 Da) and shape (linear vs. globular). Among the five tracers, no fluorescence recovery (and thus mobility) was detected for dextran-10k and the diffusion coefficients (DLCS) of the other four tracers were 295±46, 128±32, 157±88, 65±21 µm2s−1 in the LCS, respectively. Overall, the rate of solute diffusion in the bone LCS showed strong dependency on molecular size and shape. Diffusivity decreased with increasing molecular weight for both linear and globular molecules, with the linear molecules decreasing at a faster rate. Compared with free diffusion (Dfree) in aqueous solutions, the relative diffusivities (DLCS/Dfree) of the four tracers were not significantly different for sodium fluorescein, dextran-3k, parvalbumin, and ovalbumin (55±8.6%, 68.1±17.0%, 79.7±44.7%, 61.0±19.6%, respectively). This result did not agree with the homogenous molecular sieve model proposed for the osteocytic pericellular matrix structure. Instead, a heterogeneous porous model of the pericellular matrix may account for the observed solute transport in the LCS. In summary, the present study provides quantitative data on diffusion of various nutrients and signaling molecules in the LCS that are important for bone metabolism and mechanotrusduction.
Keywords: osteocyte, lacunar-canalicular system, FRAP, pericellular matrix, dextran, proteins
INTRODUCTION
Osteocytes, the most abundant cells in bone, are believed to serve as mechanical sensors and as modulators of other bone cells during bone adaptation and remodeling [1–5]. Recent evidence suggests that osteocytes play more active roles in bone physiology through matrix maintenance and modification and the regulation of systemic phosphate metabolism [1, 3]. These interconnected osteocytes are encased within the lacunar-canalicular pore system (LCS) [6] where they may detect external mechanical loading in terms of substrate deformation, load-induced fluid flow, pressure, as well as secondary signals such as streaming potentials and chemo-transport [1–5, 7, 8] and subsequently transduce biochemical response signals to other osteocytes or effector cells either intracellularly via gap junctions [9, 10] or extracellularly via the pericellular space [7]. Osteocytes express a variety of secretory molecules that could fulfill these regulatory functions such as adenosine-5'-triphosphate (ATP) [11], nitric oxide [12, 13], prostaglandin E2 [14, 15], osteoprotegerin (OPG) and its soluable ligand (sRANKL) [16–18], dentin matrix protein-1 (DMP-1) [19], sclerostin [20], and fibroblasts growth factor (FGF23) [1]. Transport of these signaling molecules, along with nutrients and metabolic wastes, is essential for the survival and proper functioning of osteocytes [21, 22]. However, it remains difficult to quantitatively measure molecular transport in situ and in vivo due to i) the small dimensions of the osteocyte LCS, and ii) the presence of mineralized tissue.
With a newly developed in situ imaging method based on fluorescence recovery after photobleaching [23], measurement of in situ and real-time solute diffusion has become feasible. Utilizing the optical sectioning capability and the high spatial resolution of laser scanning confocal microscopy, solute diffusivity in the tiny canalicular channels (order 0.5 micron diameter) can be quantified by time series imaging of the fluorescence recovery after photobleaching of individual osteocyte lacuna (order 10 micron) [23]. Compared with the few previous studies attempted to measure dynamic transport in bone utilizing the solute desorption method [24, 25], FRAP has the obvious advantages of high speed (i.e., measurement can be completed within minutes vs. hours and days in the desorption methods [24, 25]), high spatial resolution (i.e., at lacunar-canalicular level vs. at mm level for the tissue blocks [24, 25]), and minimal invasiveness (i.e., no sectioning is needed) [21, 26]. Using this FRAP technique, we have previously quantified diffusion of a small molecule (sodium fluorescein, 376 Da) in murine tibia in situ and in real-time by fitting the FRAP data to a two-compartment model [23].
The objective of the present study was to quantify diffusion of molecules with varying molecular weight and shape in the bone LCS using the established FRAP approach. Representative fluorescent tracers, as surrogates of the biological signaling molecules or nutrients found in bone, were selected with a range of molecular weights and of either linear or globular shape. Our hypothesis was that solute diffusion in the bone LCS decreases with increasing molecular weight and this relationship varies with molecular shape. We also explored mechanisms such as steric exclusion to explain retarded tracer diffusion in LCS as compared to free diffusion in aqueous solutions. These systemic studies yielded quantitative data on the mobility of various molecules that are important in bone mechanotransduction and metabolism. The results can also help mapping the distribution of some commonly used drugs such as anti-resorptive bisphosphonates and parathyroid hormone segments in bone. The knowledge of how molecules move in bone provides design guidelines for the formulation of pharmaceutical agents and their carrier systems to ensure effective transport into the bone tissue.
MATERIALS AND METHODS
Measurement of Tracer Diffusion in the Bone Lacunar-Canalicular System
Fluorescent Tracers
Five fluorescent tracers including sodium fluorescein, dextrans-3k conjugated with fluorescein, dextran-10k conjugated with fluorescein, parvalbumin conjugated with Alexa Fluor 488, and ovalbumin conjugated with Texas Red were selected, thereby permitting the testing of tracers with various molecular weights (from 376 to 43,000 Da) of either linear (for dextrans) or globular shape (Table 1). Tracers were purchased from Molecular Probes/Invitrogen Corp., (Eugene, OR) except for sodium fluorescein (Sigma-Aldrich, St. Louis, MO). The two dextrans were linear polydisperse molecules with nominal molecular weights of 3,000 or 10,000 Da. The other three tracers were globular molecules. The conjugated fluorophores were chosen based on commercial availability and two competing requirements for FRAP experiments: the fluorophore should be easy to photobleach under high-intensity laser illumination but remains relatively stable (less autofading) during the recovery period under regular scanning. Small molecules typically diffuse faster and thus require shorter recovery imaging time, during which accumulated autofading is not significant. Therefore, we chose fluorescein conjugates for smaller molecules (sodium fluorescein, and dextran-3k). For the dextan-10k, we tested both fluorescein and Texas Red conjugates. Since we did not find different FRAP results for the two conjugates, only the fluorescein-conjugated dextran-10k, which is negatively charged as other tracers, is included here (Table 1). For larger proteins (parvalbumin and ovalbumin), the more stable fluorophores like Alexa Fluor 488 and Texas Red were chosen because a longer recovery imaging period was needed and the stable fluorophores reduced the accumulated autofading during the recovery imaging period. The electric charge of the five tracers was confirmed to be negative using electrophoresis in 1.2% (w/v) agarose gel, where the loading wells were positioned in the center of the electrical field and the molecular charge could be determined by the migration direction.
Table 1.
The fluorescent tracers used for diffusion measurements within the bone LCS in situ
| tracer | MW (Da) |
shape | dye/tracer ratio (mol/mol) |
[C] (mg/ml) |
Vol. (ml) |
dye in blooda (mM) |
circulation time (hr) |
|---|---|---|---|---|---|---|---|
| sodium fluorescein | 376 | spherical | 1 | 10 | 0.2 | 1.662 | 0.5 |
| dextran-3k | 3,000 | Linear | 1 | 10 | 0.5 | 0.477 | 1.5 |
| (fluorescein) | |||||||
| dextran-10k | 10,000 | Linear | 1 | 10 | 0.5 | 0.143 | 3 |
| (fluorescein) | |||||||
| parvalbumin | 12,300 | Globular | 2.2 | 5 | 0.2 | 0.056 | 1.5 |
| (Alexa Fluor 488) | |||||||
| ovalbumin | 43,000 | Globular | 4 | 10 | 0.5 | 0.134 | 7 |
| (Texas Red) | |||||||
Notes: The calculations were based on a blood volume of 3 ml for a mouse of 30g body weight.
Animal Preparation
Skeletally mature C57BL/6J mice (three to five-month old, N = 60) were slowly injected with one of the five tracers via the tail vein under Avertin anesthesia. The tracer’s molecular weight (MW), shape, dye/tracer labeling ratio, solution concentration, injected volume per animal, and the estimated concentrations of fluorophore in blood are summarized in Table 1. The tracer was allowed to circulate until a maximal intensity was reached in the bone tissue. The “circulation time” to achieve the maximal intensity (listed in Table 1) was chosen based on either previous studies (for sodium fluorescein [23]), or our preliminary tests, where the other four tracers were injected into the animals and allowed to circulate for various time periods (1–14 hr), among which the one giving the brightest signal was chosen for the particular tracer in this study. After the pre-defined circulation time, the animals were sacrificed with carbon dioxide. All the animal procedures were approved by the Institutional Animal Use and Care Committee.
FRAP Experiments
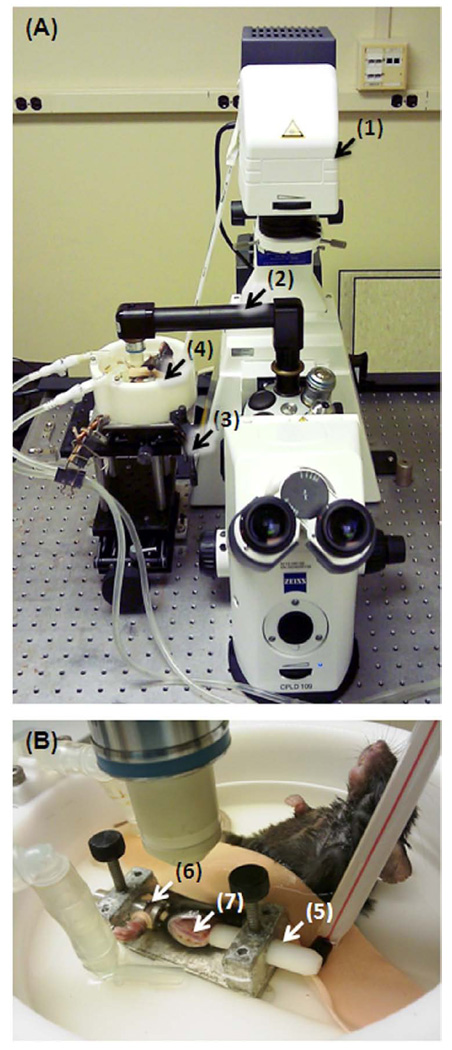
The left tibiae were exposed at the medial-anterior surface and rigidly fixed at the knee and ankle joints in a custom-made holder placed inside a water bath containing phosphate buffered saline (PBS) solution at the room temperature (25°C) (Fig. 1). One to five FRAP experiments were performed on different lacunae per tibia using Zeiss LSM 510 confocal microscope equipped with a lens inverter (LSM Technology, Etters, PA) and a 40× 0.8-numerical aperture water dipping lens (Achroplan, Zeiss) (Fig. 1A). The “L” shaped lens inverter altered the laser path so that the exposed tibia could be imaged from the top (Fig. 1B). The procedure for FRAP experiments was similar to that described in previous studies [23]. Briefly, the microscope was focused on a plane 20–50 µm below the medial-anterior surface of the tibia, and a cluster of fluorescent lacunae identified. The central lacuna was then chosen as the target for FRAP. Using the FRAP tool in the Zeiss LSM software, a time series of images were automatically captured prior to and after photobleaching. The focal plane containing the selected lacuna was first scanned twice (pre-bleach images) with an image size of 512×512 pixels at a speed of ~4sec/frame under normal illumination (488 or 514 nm excitation for fluorescein/Alexa Fluor 488 or Texas Red, 25% power output, 15–25% transmission, one to three Arial unit pinhole). The selected lacuna was then photobleached under 100% transmission for 50–100 iterations (~10–30 sec) till the fluorescence intensity was decreased to approximately 40–60% of the original level. Immediately after photobleaching, a total of 30–125 scans (recovery images) were recorded at ~4 sec/frame until the fluorescent intensity of the photobleached region achieved a plateau. During the FRAP procedure, the fluorescence intensity in the target lacuna was monitored in real-time using the Zeiss LSM ROI tool. The experiment time for imaging one animal varied from 1–4 hrs with larger tracers taking longer times.
Fig. 1.
Experimental setup for FRAP measurements of tracer diffusion in intact murine tibiae. (A) The FRAP experiment was performed using a Zeiss LSM 510 confocal module (not shown in the picture) attached to an Axioobserver Z1 invert microscope (1). A lens inverter (2) was used to direct the imaging laser beam to an elevated platform (3) beside the microscope, which was consisted of a height-adjustable jack and a xy table. The animal was placed in a water bath (4) perfused with 25°C PBS solution (heating elements not shown). (B) The left tibia was fastened rigidly at the knee using a rod with a cup-shaped end (5) and at the ankle using a slit and a rubber band (6) under a small compression force. The tibial anterior-medial surface (7) was surgically exposed for FRAP imaging.
Calculation of Tracer Diffusion Coefficients DLCS
Tracer diffusion coefficients were extracted from the FRAP image series using the methods previously described [23]. Briefly, the anatomic parameters such as the perimeter, two-dimensional projection area, total surface area and volume of the photobleached lacuna and the average canalicular length (d) were obtained based on the pre-bleach images using custom-made Matlab codes. These measurements were used to obtain the dimensionless parameter of the fluid volume ratio (Vr) between the contributing canaliculi and the photobleached lacuna [23]. The five tracers was assumed to diffuse mainly through the pericellular fluid space and the intracellular transport, if any, was believed to be negligible in our system, because i) the intracellular cytoskeletons may hinder molecular diffusion more than the pericellular matrix [23] and/or ii) the gap junctions connecting the neighboring cells are limited to small ions and molecules (less than 1000 Da) [9, 10]. The time course of the fluorescence intensity in the photobleached lacunae (I(t’)), including the intensities before photobleaching (I0), immediately after photobleaching (Ib), the new equilibrium after recovery (I∞), were also calculated from the image sequence. Autofading during the recovery imaging was corrected using a reference lacuna that was far away from the photobleached one and assumed to have a constant intensity. The two-compartment model [23] predicted that the experimental data followed this equation:
| (Eq. 1) |
The experimental data were analyzed using linear regression with time and the slope of the fitting line was used to determine the diffusion coefficient (DLCS).
Measurements of Tracer Diffusion Dfree in Aqueous Solution
To obtain the relative diffusivity, diffusion of the linear and globular molecules (dextran-3k, dextran-10k, pavalbumin, and ovalbumin) was measured in PBS solution following the methodology of a previous study [27]. All tested molecules were conjugated with fluorescein or Alexa Fluor 488, because i) these green fluorophores were relatively easy to photobleach compared to the red fluorophores in our system and ii) fast photobleaching ensured an improved accuracy for FRAP measurements [28]. Free diffusion of sodium fluorescein was not measured because its value is well established in literature [29, 30] and its diffusion is too fast in aqueous solution to be accurately measured in our system due to the limited speed of our confocal microscope. Tracers were dissolved in PBS at the concentrations of 0.05, 0.1, 0.05, 0.1 mg/ml for dextran-3k, dextran-10k, parvalbumin, and ovalbumin, respectively. These concentrations were found to be within the linear range of their concentration versus fluorescence intensity curves. The tracer solution was loaded to a testing chamber consisting of a glass slide, a coverslip, and an adhesive spacer (120 µm thickness, Secure-Seal Spacer®, Molecular Probes) containing a central 9 mm diameter well. The spacer was sandwiched in between the glass slide and coverslip to form an imaging chamber filled with tracer solutions. This microscopic chamber was large enough to eliminate interaction between solutes and the chamber wall, but also small enough to eliminate any currents in the solution [27]. For accurate measurements of free diffusion, we tried to capture approximately ten frames during the recovery process. Because tracer recovery in aqueous solution is much faster than in bone, FRAP experiments in aqueous solutions were performed with the pinhole open (to increase the photobleaching efficiency and depth) and by photobleaching a larger circular area (13.6 – 34.7 µm radius), ensuring the recovery process being long enough for data acquisition. In addition, a rectangular image field (512×256 – 512×64) was used to increase the scan speed (~ 200 msec/frame). All the measurements were performed within 1 hour after loading the testing solutions in the chambers to avoid drying and leaking of the solutions. A total number of 15 FRAP experiments were performed for each tracer tested.
Diffusion coefficient Dfree was obtained from the FRAP measurements using the classic Alexrod model [28]:
| (Eq. 2) |
where r is the radius of the photobleached region; rD is a factor accounting for the shape of laser beam (rD = 0.88 for circular beams); t1/2 is the half recovery time when 50% of the initial photobeaching depth is recovered, which was obtained for each FRAP experiment using the kinetic analysis function in the Zeiss LSM software.
Predicted Relative Diffusivity Using Fiber Matrix Theory
The relative diffusivity (DLCS/Dfree) was estimated for each tracer and was usually less than one. This diffusion retardation in the LCS was suggested to be caused by the steric exclusion between the solute and the pericellular matrix [23]. Because the composition and the ultrastructure of the pericellular matrix are largely unknown [31], we tested two possible configurations of the LCS pericellular matrix; as either an ordered array of fibers or a random distribution of fibers. Both models have been applied to modeling transport through the endothelial glycocalyx [32]. The fiber volume fraction was parametrically varied between 0.1–10% with the radius of individual fibers set to be that of glycosaminoglycans (0.6 nm) [32], and the relative diffusivity due to steric exclusion is predicted as:
| (Eq. 3a) |
| (Eq. 3b) |
where vf is the volume fraction of fiber, a is the hydrodynamic radius of the solute, and rf is the radius of the fiber. Because the fiber matrix theory assumes solutes to be spheres, we did not include dextran-10k in our analysis due to its obvious linear configuration. Instead, the dextran-3k data were included, because our preliminary molecular modeling showed that this rod-like molecule has a length (1.8 nm) that is only 20% longer than its cross-sectional diameter (1.5 nm), using Insight II (version 2000, Molecular Simulations Inc., CA).
The hydrodynamic radii (a) of the tracers were estimated from the diffusion coefficients in an aqueous solution (measured in the preceding section) using the Stokes-Einstein equation:
| (Eq. 4) |
where kB is the Boltzmann constant (1.38×10−23 J·K−1), T is room temperature (298 K), μ is the viscosity of PBS assumed to be that of sea water (1.06×10−3 kg·m−1s−1), and Dfree is the tracer diffusion coefficient measured in PBS.
Statistical Analysis
Since no significant variability of the diffusion measurements was found between animals, all FRAP experiments performed in different animals were pooled for each tested tracer. One way ANOVA and post hoc Bonferroni tests were performed to test the difference of diffusivity among the tracer groups, with p < 0.05 indicating a significant difference.
RESULTS
Diffusion in the Bone LCS
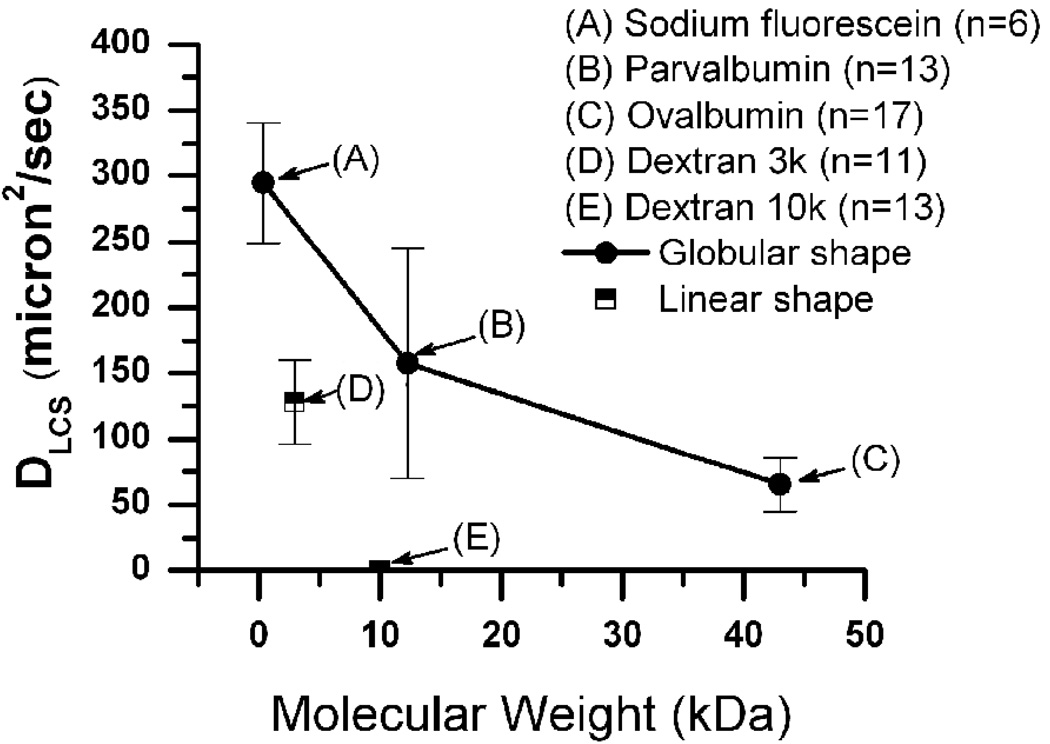
Overall, solute diffusion showed a strong dependence on the molecular weight (size) and the shape of the fluorescent molecule (Fig. 2, Table 2). The diffusion coefficient measured in the bone LCS decreased with increasing molecular weight for both linear and globular molecules, and the linear dextrans moved slower than globular proteins of comparable molecular weights. For instance, diffusion of dextran-10k was barely measurable in the LCS, while the globular parvalbumin of comparable molecular weight (12,300 Da) had a much higher diffusion coefficient (157.4 ± 87.7 µm2s−1), which was even higher than that of a dextran with a four-fold smaller molecular weight (3k Da, 128.0 ± 31.7 µm2s−1, Table 2). The area (A) and volume (Vs) of the photobleached lacuna, contributing canaliculi number (N), canalicular length (d), and the volume ratio between canaliculi and lacuna (Vr) were not significantly different (Table 2).
Fig. 2.
Diffusion coefficients decreased with increasing molecular weight for both linear and globular molecules; and the linear molecules had further reduced diffusivity than globular molecules with comparable molecular weights.
Table 2.
FRAP Measurements of diffusion coefficients for tracers in the bone LCS
| Tracer | Number of measurements | A (µm2) | Vs (µm3) | N | d (µm) | Vr | D (µm2s−1) |
|---|---|---|---|---|---|---|---|
| fluorescein | 6 | 82±23 | 395±182 | 11±3 | 20±3 | 0.03±0.01 | 294.8±45.8 |
| dextran-3k | 11 | 65±23 | 271±133 | 9±3 | 21±3 | 0.03±0.01 | 128.0±31.7a |
| parvalbumin | 13 | 97±52 | 528±394 | 13±7 | 25±4 | 0.03±0.01 | 157.4±87.7a |
| ovalbumin | 17 | 94±28 | 450±193 | 13±4 | 26±5 | 0.04±0.01 | 65.3±20.7 |
Notes: Dextran-10k was not reported here because no recovery was found. Data were reported as mean ± standard deviation. No significant difference was found for A, Vs, N, d, and Vr.
No significant difference was found for D between Dextran-3k and parvalbumin (p>0.05), while the other groups differed significantly among each other (p< 0.05).
Definitions of Keys: A = cross-sectional area of the photobleached lacuna; Vs = total surface area of the photobleached lacuna; N = contributing canaliculi number (22% of the total canaliculi emanating from the lacuna at a number density of 0.18/µm2)[23]; d = average canalicular length; Vr = fluid volume ratio between contributing canaliculi and lacuna; D = diffusion coefficient of tracer in the bone LCS.
Relative Diffusivity in the LCS
The free diffusion coefficients of the four tested tracers were 187.7±18.2, 107.7±5.9, 197.0±11.0, and 106.5±14.9 (µm2 s−1) for dextran-3k, dextran-10k, parvalbumin and ovalbumin in aqueous solutions, respectively (Table 3). The free diffusion of sodium fluorescein reported in literature [30] was also included. Using these free diffusion coefficients as references, the relative diffusivities of these tracers in the LCS over the free diffusion were found to vary from 55% – 80% (Table 3) with no significant difference among the four tracer groups.
Table 3.
Free diffusion in PBS and relative diffusivity of the five tracers in the bone LCS
| Tracer | Dfree (µm2/s) | Dlcs/Dfreeb |
|---|---|---|
| sodium fluorescein | 540a | 55±9% |
| dextran-3k | 187.7±18.2 | 66±20% |
| dextran-10k | 107.7±5.9 | 0% |
| parvalbumin | 197.0±11.0 | 80±45% |
| ovalbumin | 106.5±14.9 | 61±20% |
Notes: The value of free diffusion reported in literature was used to calculate the relative diffusivity for sodium fluorescein [29, 30].
Mean values of the free diffusions were used in the calculations of relative diffusivities for the tracers, which were reported as mean ± standard deviation. No significant difference of the relative diffusivity (DLCS/Dfree) was detected among the tracer groups (p > 0.05).
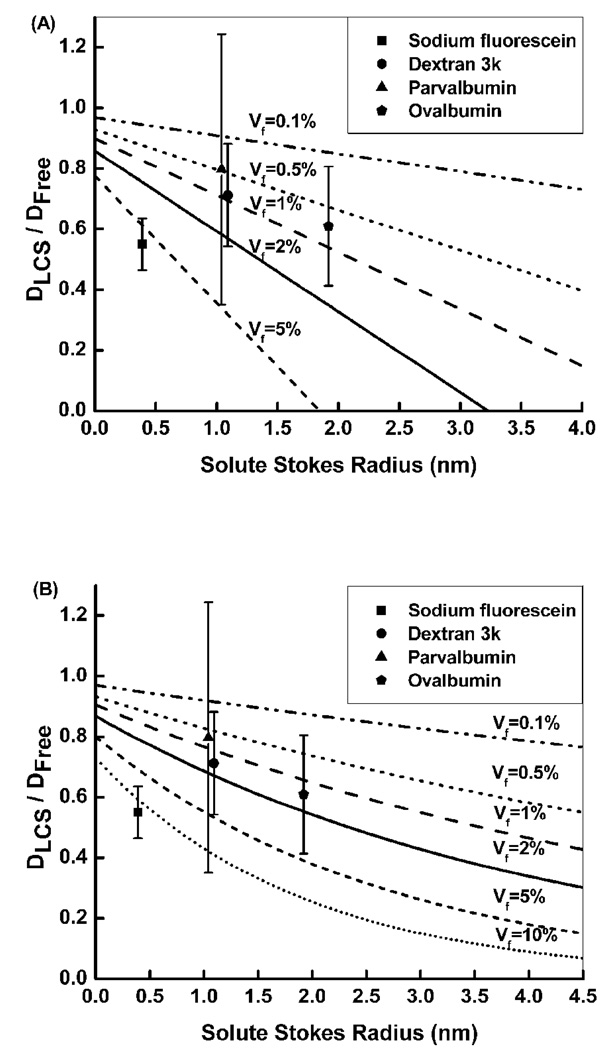
The relative diffusivity for the four tracers with varying molecular size did not fit with either the ordered fiber array or the random fiber matrix model (Fig. 3). The examined data points did not fall on the same predicted line for any fiber volume fraction (0.1–10%), and the pericellular matrix appeared to be denser for sodium fluorescein (5 or 10%) and sparser for larger molecules (0.5–2% for dextran-3k, parvalbumin, and ovalbumin).
Fig. 3.
Relative diffusivity (DLCS/Dfree) predicted from the fiber matrix theory assuming (A) an ordered fiber array and (B) a randomly distributed fiber matrix. Our data did not fit well with either model. The pericellular matrix appeared denser for small solutes (sodium fluorescein) and sparser for larger solutes.
DISCUSSION
The present study aimed to quantify diffusion of molecules of various size and shape in the bone lacunar-canalicular system within an intact long bone. Transport of functional molecules is critical for bone’s mechanosensation and metabolism, because these molecules are involved in cell-to-cell signaling and tissue nutrition (some example molecules are shown in Table 4). Using our newly developed FRAP approach [23], we have injected five exogenous fluorescent tracers to mimic the functional molecules (Table 4) into live animals and measured their mobility in the pericellular space inside the lacunar-canalicular system of intact murine tibiae immediately post mortem.
Table 4.
Examples of biologically relevant molecules found in bone
| Molecule | MW (Da) | Functions | Reference |
|---|---|---|---|
| nitric oxide (NO) | 30 | promoting bone formation and inhibiting bone resportion | [12, 13] |
| glucose | 180 | nutrition | |
| prostaglandin E2 (PGE2) | 352 | promoting bone formation | [14, 15] |
| adenosine-5'-triphosphate | 507 | modulating intracellular Ca2+ | [11] |
| (ATP) | |||
| sclerostin | 24,000 | inhibiting osteoblastic bone formation | [20] |
| soluable receptor activator of NFkappaB ligand (sRANKL) | 24,000 | modulating osteoclastgenesis and bone loss | [18] |
| fibroblasts growth factor (FGF23) | 25,000 | modulating cell proliferation and differentiation | [1] |
| dentin matrix protein-1 (DMP-1) | 100,000 | inhibiting mineralization of bone | [19] |
| osteoprotegerin (OPG) | 60,000 or 120,000 | inhibiting osteoclast formation | [17] |
Note: the molecules listed here are of spherical or globular shape. Liner diffusible molecules such as collagen and proteoglycan fragments (the byproducts of tissue turnover process) have no well-defined molecular weights, and thus are not included here.
Our results demonstrated that diffusivity of both linear and globular solutes in the bone LCS was quite sensitive to the molecular size and shape (Fig. 2 and Table 2). Transport of larger-sized molecules in bone, as expected, was slower than smaller molecules with similar shapes (Fig. 2). Furthermore, linear molecules moved slower in the LCS than globular molecules of similar molecular weight (Fig. 2). For example, parvalbumin (12 kDa) diffused at 157 µm2 s−1 in LCS, while the linear dextran-10k moved so slow in the LCS that its recovery was barely measurable up to 20 min post photobleaching (i.e., twice the typical scanning time for the largest ovalbumin tracer) using the current setup. We also note an approximate twofold decrease in diffusivity for dextran-10k compared with parvalbumin in aqueous solutions (Table 3). These results were consistent with the increased drag coefficient (and thus slow diffusion) experienced by rod-like objects than spheres in aqueous solution [33]. The drag force experienced by the tracers in the bone LCS can be attributed to multiple molecular interactions between the solutes and the extracellular solid and fluid phases, including (but not limited to) electrostatic interactions, hydrophobic/hydrophilic compartmental partitioning, and physical tangling. In this study, we did observe various immobilized fractions (10–25%) for the tested tracers as shown in the reduced recovered fluorescence intensities compared with pre-bleach intensities (data not shown). Further investigations need to quantify the molecular interactions in bone in situ.
The size- and shape-dependency of the local diffusion in the LCS was consistent with the overall rate of tracer perfusion from the blood stream to the bone interstitium as shown in the “circulation” times, the lag between the time of tracer injection and the time when the maximal fluorescence intensity was reached within the LCS (Table 1). The “circulation” time reflected the overall perfusion speed that the tracer leaked out of the blood vessel walls and subsequently penetrated into the LCS system. The “circulation” time for heavier molecules was usually longer than lighter molecules of the same molecular shape, e.g., sodium fluorescein (0.5 hr) < parvalbumin (1.5 hr) < ovalbumin (7 hr) for globular molecules; dextran-3k (1.5 hr) < dextran-10k (3 hr) for linear molecules (Table 1). For molecules of similar molecular weight, linear molecules appeared to penetrate the bone LCS slower than globular ones (dextran-10k: 3 hr vs. parvalbumin 12kDa: 1.5 hr, Table 1).
We tried to infer the ultrastructure of the pericellular matrix from our relative diffusivity data as in our previous studies [23]. Our present results do not support the molecular sieve model with either a regular array or a random distribution of glycosaminoglycan (GAG) side chains. A regular array of GAG fiber matrix was originally proposed to fill the fluid space within the LCS [7]. With new electron microscopic images of the endothelial glycocalyx, an alternative structure of spherical clusters attached to core proteins was proposed where the spherical clusters are of 10–12 nm in diameter and distributed every 20 nm in all three directions (schematics of the proposed beads-on-string structure can be found in [34, 35]). In our previous investigations [23], the steric exclusion from the fibers (12.6 % volume fraction) of this beads-on-string structure was found to be able to explain the diffusion reduction for sodium fluorescein. The present study using sodium fluorescein found a consistent result with a denser fiber volume fraction at similar level (~10%). In contrast, the results from other larger molecules can only be explained by assuming a much sparser fiber volume fraction (0.5–2%).
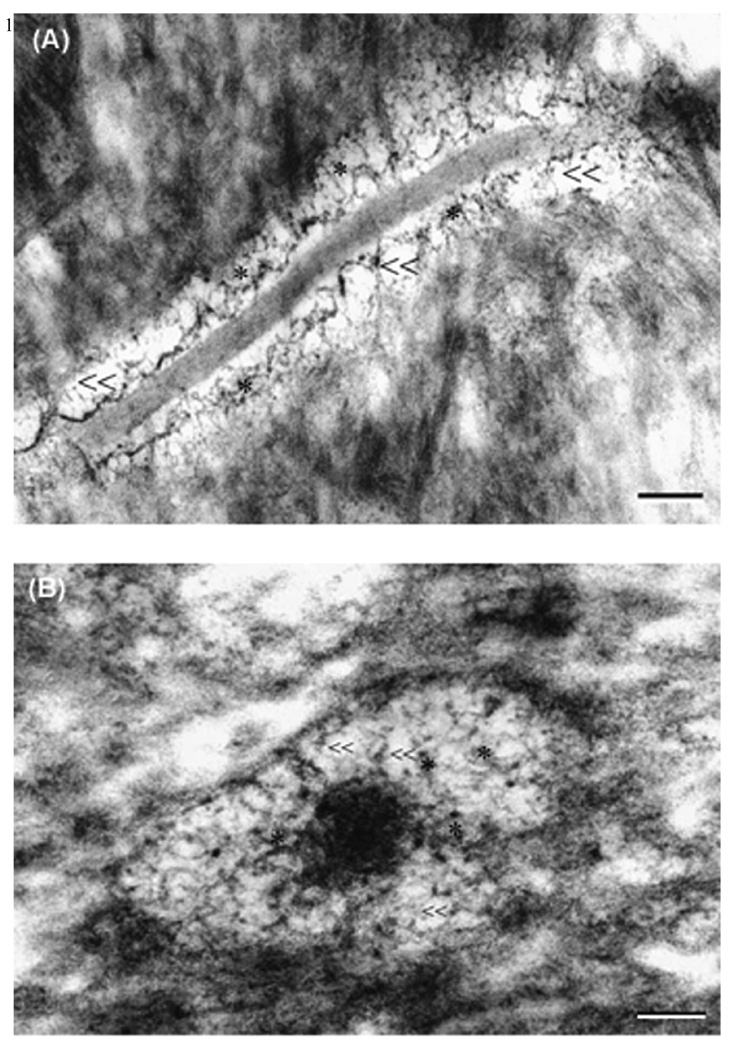
This apparent contradiction of fiber volume fractions for small and large molecules could be explained if a hierarchical porous model were adopted. If the pericellular matrix in the bone LCS were assumed to be consisted of the clusters on strings as proposed in endothelial glycocalyx [34, 35], there are two fundamentally distinct domains in the fluid annulus: the more packed clusters consisted of closely spaced GAG fibers with a characteristic pore size of 2–3 nm [36]; and the more porous domain between these clusters with a characteristic pore size of 8–10 nm. Because the hydrodynamic Stoke diameters for dextran 3-k (~2 nm), parvalbumin (~2 nm) and ovalbumin (~4 nm) are close or exceed the GAG spacing (2–3 nm), these large tracers would be excluded from the clusters and preferentially diffuse through the larger pores among the clusters. The diffusion would be much hindered for smaller tracers such as sodium fluorescein, because they could penetrate into the clusters and become trapped there, while the effective steric exclusion effect would be greatly reduced for these larger molecules and the fiber matrix appeared less dense for them. Preferential flow paths have been found in heterogeneously packed chromatographic columns for protein separation [37, 38]. We speculate that the differential routes taken by the small and large solutes in heterogeneous porous media would increase tortuosity and decrease diffusivity for small molecules at a greater degree, as shown in our data in the bone LCS. Furthermore, our preliminary electron microscopic photographs of the osteocyte pericellular matrix appeared to support the existence of heterogeneous pores (Fig. 4). We cannot completely rule out the possibility that the bigger pores in the electron microscopic photographs were caused by pericellular matrix collapsing during the histological processing. However, the application of perfusion-fixation in live animals and the use of oxygen-carrying blood substitute and ruthenium III hexamine trichloride, as shown in stabilizing filamentous endothelial glycocalyx and osteocyte pericellular matrix in previous electron microscopic studies [31, 39], was expected to greatly reduce the possibility of matrix collapsing and shrinkage artifacts. Another possible explanation for the poor agreement of the measured diffusivity and the fiber matrix prediction is that the pericellular matrix may have degraded during the experiments. Because it took longer time to perform FRAP studies for larger molecules, the degree of the pericellular matrix degradation may be more pronounced than that occurred for small tracers. We are currently addressing this issue by performing paired FRAP experiments on the same lacunae in vivo and post mortem.
Fig. 4.
Electron micrographic images showed a heterogeneous porous pericellular matrix in the osteocyte canaliculi. Both large pores (“≪”) and small pores (“*”) were clearly identified in a (A) longitudinal and (B) a cross-sectional canalicular images. Bar = 100 nm.
In summary, the present studies quantified diffusivities of five tracers representing a spectrum of biologically relevant molecules with molecular weights varying from 376 Da up to 43,000 Da and of either linear or globular shape. In the bone LCS larger molecules moved slower than smaller ones; and linear molecules moved slower than globular ones of similar size. The mobility of all tracer molecules in bone was reduced compared with that in free aqueous solution, but the reduction was not significantly different among the examined tracers. Our data suggest that the pericellular matrix may have a hierarchical pore system where the large pores form a preferential pathway for fast diffusion of large molecules. These in situ measurements will help our understanding of the mobility of various nutrients and signaling molecules in bone. These data can also help quantitative understanding of the distribution of some commonly used treatments in bone such as anti-resorptive bisphosphonates and parathyroid hormone segments. The knowledge of how molecules move in bone provides design guidelines for the formulation of pharmaceutical agents and their carrier systems to ensure effective transport into the bone tissue.
ACKNOWLEGEMENT
This study was supported by grants from NIH (AR054385 and P20RR016458). The authors thank the valuable comments and technical help from Drs. Chris Price, Randy Duncan, Mary C. Farach-Carson, Xinqiao Jia, and Ms. Sue Seta of University of Delaware. We also thank Dr. Junmin Zhu from the Western Case Reserve University for running molecular simulations on dextrans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116:281–290. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 2.Fritton SP, Weinbaum S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annual Review of Fluid Mechanics. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol. 2000;159:7–13. doi: 10.1016/s0303-7207(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 4.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddle RC, Donahue HJ. From Streaming Potentials to Shear Stress: 25 Years of Bone Cell Mechanotransduction. Journal of Orthopaedic Research. 2009;27:143–149. doi: 10.1002/jor.20723. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RR, Milgram JW, Robinson RA. Morphology of the osteon. An electron microscopic study. J Bone Joint Surg Am. 1966;48:1239–1271. [PubMed] [Google Scholar]
- 7.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 8.Burger EH, Klein-Nulend J, van der Plas A, Nijweide PJ. Function of osteocytes in bone--their role in mechanotransduction. J Nutr. 1995;125:2020S–2023S. doi: 10.1093/jn/125.suppl_7.2020S. [DOI] [PubMed] [Google Scholar]
- 9.Guo XE, Takai E, Jiang X, Xu Q, Whitesides GM, Yardley JT, Hung CT, Chow EM, Hantschel T, Costa KD. Intracellular calcium waves in bone cell networks under single cell nanoindentation. Mol Cell Biomech. 2006;3:95–107. [PubMed] [Google Scholar]
- 10.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–1462. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol. 1996;271:E205–E208. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- 13.McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–936. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- 14.Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol. 1991;261:C428–C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- 15.Reich KM, McAllister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology. 1997;138:1014–1018. doi: 10.1210/endo.138.3.4999. [DOI] [PubMed] [Google Scholar]
- 16.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 18.Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, Nakamura K, Seiki M, Tanaka S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J Biol Chem. 2006;281:36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- 19.Hassan AH, Evans CA, Zaki AM, George A. Use of bone morphogenetic protein-2 and dentin matrix protein-1 to enhance the osteointegration of the Onplant system. Connect Tissue Res. 2003;44:30–41. [PubMed] [Google Scholar]
- 20.Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354. [PubMed] [Google Scholar]
- 21.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 22.Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Wang Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, Schaffler MB. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005;102:11911–11916. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Seara MA, Wehrli SL, Wehrli FW. Diffusion of exchangeable water in cortical bone studied by nuclear magnetic resonance. Biophys J. 2002;82:522–529. doi: 10.1016/S0006-3495(02)75417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang SB, Stipanich N, Soremi EA. Diffusion of glucose in stressed and unstressed canine femur in vitro. Ann N Y Acad Sci. 1974;238:139–148. doi: 10.1111/j.1749-6632.1974.tb26784.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone's interstitial fluid pathway in vivo. Bone. 2004;34:499–509. doi: 10.1016/j.bone.2003.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braeckmans K, Remaut K, Vandenbroucke RE, Lucas B, De Smedt SC, Demeester J. Line FRAP with the confocal laser scanning microscope for diffusion measurements in small regions of 3-D samples. Biophys J. 2007;92:2172–2183. doi: 10.1529/biophysj.106.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu BM, Adamson RH, Curry FR. Determination of microvessel permeability and tissue diffusion coefficient of solutes by laser scanning confocal microscopy. J Biomech Eng. 2005;127:270–278. doi: 10.1115/1.1865186. [DOI] [PubMed] [Google Scholar]
- 30.Fu BM, Curry FE, Weinbaum S. A diffusion wake model for tracer ultrastructure-permeability studies in microvessels. Am J Physiol. 1995;269:H2124–H2140. doi: 10.1152/ajpheart.1995.269.6.H2124. [DOI] [PubMed] [Google Scholar]
- 31.You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:505–513. doi: 10.1002/ar.a.20050. [DOI] [PubMed] [Google Scholar]
- 32.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 33.Truskey GA, Yuan F, Katz DF. Transport Phenomena in Biological Systems. Upper Saddle River, NJ: Pearson Education, Inc.; 2009. [Google Scholar]
- 34.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136:239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 35.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg L, Hellmann W, Kleinschmidt AK. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975;250:1877–1883. [PubMed] [Google Scholar]
- 37.Billen J, Desmet G. Understanding and design of existing and future chromatographic support formats. J Chromatogr A. 2007;1168:73–99. doi: 10.1016/j.chroma.2007.07.069. discussion 71-2. [DOI] [PubMed] [Google Scholar]
- 38.Billen J, Gzil P, Vervoort N, Baron GV, Desmet G. Influence of the packing heterogeneity on the performance of liquid chromatography supports. J Chromatogr A. 2005;1073:53–61. doi: 10.1016/j.chroma.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 39.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]