Abstract
The presence of resident Langerhans cells (LCs) in the epidermis makes the skin an attractive target for DNA vaccination. However, reliable animal models for cutaneous vaccination studies are limited. We demonstrate an ex vivo human skin model for cutaneous DNA vaccination which can potentially bridge the gap between pre-clinical in vivo animal models and clinical studies. Cutaneous transgene expression was utilised to demonstrate epidermal tissue viability in culture. LC response to the culture environment was monitored by immunohistochemistry. Full-thickness and split-thickness skin remained genetically viable in culture for at least 72 h in both phosphate-buffered saline (PBS) and full organ culture medium (OCM). The epidermis of explants cultured in OCM remained morphologically intact throughout the culture duration. LCs in full-thickness skin exhibited a delayed response (reduction in cell number and increase in cell size) to the culture conditions compared with split-thickness skin, whose response was immediate. In conclusion, excised human skin can be cultured for a minimum of 72 h for analysis of gene expression and immune cell activation. However, the use of split-thickness skin for vaccine formulation studies may not be appropriate because of the nature of the activation. Full-thickness skin explants are a more suitable model to assess cutaneous vaccination ex vivo.
Keywords: human skin organ culture, viability, Langerhans cell
1. Introduction
The skin is an attractive target for vaccination due to its high accessibility and innate immune responsiveness. Dendritic cells are a heterogeneous group of specialised leucocytes resident mainly in epithelial tissues, including the skin. The epidermis comprises 95% keratinocytes and 2% Langerhans cells (LCs) [1, 2]. LCs are epidermal dendritic cells which function as professional antigen presenting cells (APCs) that, together with dermal dendritic cells (DDCs) in the dermis, constitute the cutaneous immune surveillance apparatus [3-5]. On encountering and subsequently internalising an antigen, a LC matures and migrates across the dermal-epidermal junction to draining lymph nodes, where it presents the antigen to naïve T-lymphocytes to elicit an immune response [6].
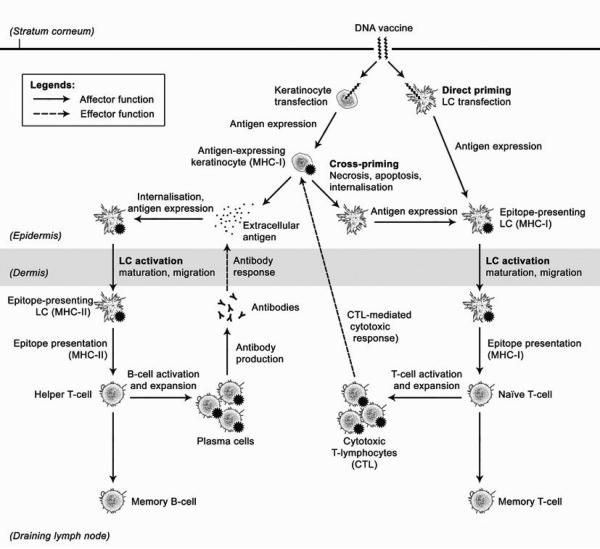
Encoding foreign antigens in the form of a gene, a DNA vaccine can be inserted into host cells for intracellular production of the antigen. Compared to conventional immunisation, which involves the direct inoculation of immunogenic elements, genetic immunisation raises the possibility of poly-immunisation with multiple encoded antigens in a single vaccine [7], co-expression of an adjuvant via the CpG motifs in DNA [8], reduced risks of adverse microbial infection, ease of large-scale production and improved stability [9]. Importantly, whilst conventional peptide-based vaccines activate the humoral arm of adaptive immunity through the major histocompatibility complex class II (MHC II) antigen presentation pathway, DNA vaccines offer the added advantage of activating the cellular arm of adaptive immunity by stimulating both the MHC I (cellular) and MHC II (humoral) pathways of adaptive immunity through direct and cross-priming (Figure 1) [10-12]. In the context of cutaneous DNA vaccination, epidermal LCs are the primary target for direct priming [13]. Keratinocytes, due to their ubiquity and proximity to LCs, represent an appropriate target for cross-priming. There is some evidence that cross-presentation by keratinocytes may be the predominant antigen-presentation pathway for stimulating CD8+ T-lymphocyte responses in genetic immunisation [14]. Berg et al. have shown, using DNA tattooing, that a majority (>98%) of transfected cells consisted of keratinocytes, in contrast with transfected LCs (about 1%) [15]. The accessibility of the skin also makes minimally invasive vaccine administration possible through the use of needle-free approaches, hence improving dose amenability and patient compliance [16].
Figure 1.
A schematic diagram depicting direct priming and cross-priming of CTL responses by epidermal LCs [63].
In order to study local immunological responses to cutaneous vaccination, a representative model of the in vivo environment of human skin is required. Although animal models have been used to study epidermal LC behaviour in vivo and ex vivo [17, 18], the observations arising from their use can be confounded by interspecies variations in morphology, permeability and patterns of cutaneous gene expression [19, 20]. In light of this, human skin explants provide perhaps the closest laboratory model attainable to the in vivo environment in terms of biological complexity and fidelity to human physiology. However, tissue viability deteriorates from the time of excision [21]. The aim of any organ culture system is to minimise the degradation rate and extend explant viability. We have previously reported successful and reproducible ex vivo gene expression in short-term (24 h) skin organ cultures as a result of microneedle-mediated cutaneous delivery of reporter plasmid DNA [22-25]. Similar skin organ culture systems have been used to investigate skin graft preservation [26], cutaneous pharmacokinetics [27], cutaneous immunology [28, 29], toxicology [30] and as a disease model [31]. This report details further work in this area to evaluate the skin organ culture system as an ex vivo model for cutaneous DNA vaccination, focusing on skin viability and LC behaviour, particularly their responses to culture conditions, over a longer term.
2. Materials and Methods
2.1 Human skin specimens
Human skin specimens were obtained from female subjects (aged 42-78) following mastectomy or breast reduction procedures with full ethical approval and informed patient consent. Skin that was surplus to diagnostic histopathology requirements was excised and transported to the laboratory in organ culture medium (OCM) comprising Dulbecco's modified Eagle medium (DMEM) supplemented with 5% foetal calf serum (FCS), 100 IU mL−1 penicillin and 100 mg mL−1 streptomycin (all from Invitrogen, Paisley, UK) on ice. Subcutaneous adipose tissue was removed by blunt dissection to yield full-thickness skin. Split-thickness skin was prepared by careful lateral dissection to isolate the upper dermis and epidermis from the bulk of the dermal tissue.
2.2 Skin organ culture
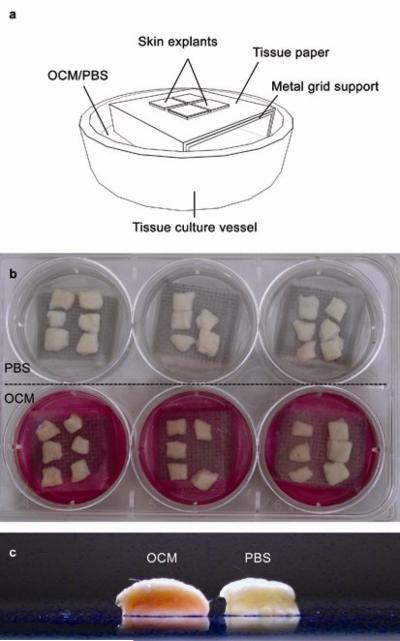
Skin samples measuring approximately 1 cm2 were cultured at the air-liquid interface in a Trowel-type system (Figure 2a-b), in OCM or calcium-free Phosphate buffered saline (PBS; Sigma-Aldrich, Poole, UK) supplemented with 2.5 μg mL−1 amphotericin B (Lonza, Wokingham, UK), at 37 °C and 5% CO2/95% air as described previously [32-34].
Figure 2.
A schematic diagram (a) and photograph (b) of the organ culture setup. The OCM, containing phenol red as a pH indicator, infiltrated full-thickness skin in culture (c) as indicated by the pink colouration of the dermis through to the epidermis. This is contrasted with the relatively pale appearance of the full-thickness skin cultured in PBS. The picture was taken 48 h post-culture, immediately after the skin specimens were removed from the respective culture systems.
2.3 Generation of plasmid DNA
Heat-shock transformation of DH5α™ competent cells (Invitrogen, Paisley, UK) was achieved with pCMVβ (Clontech, Mountain View, CA), the plasmid DNA encoding bacterial β-galactosidase, using a protocol supplied by Invitrogen. The pCMVβ used in the study was amplified from the transformed bacterial stocks and purified using the QIAGEN-tip 2500 Mega Kit (Qiagen, Crawley, UK) according to the manufacturer's protocol [35].
2.4 Microneedle Fabrication
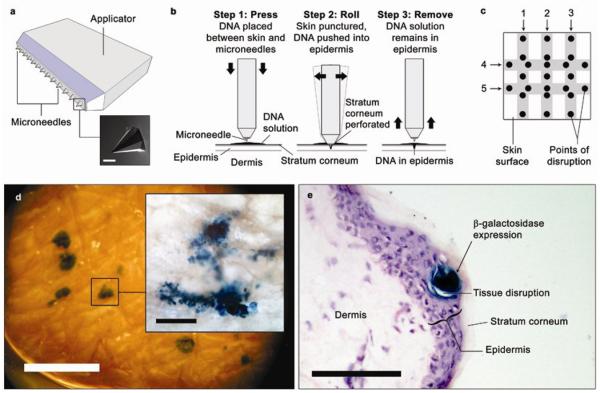
A single-row strip of 13 silicon microneedles with a height-to-base ratio of 3:2 were fabricated using a multi-stage process, as described previously [23, 36]. Briefly, low pressure chemical vapour deposition (LPCD) was used to form a mask on the surface of the wafer and standard photolithography used to create a specific pattern within the mask. The wafer was subsequently immersed in 29% KOH at 79 °C for a defined period of time forming the microneedles. The microneedles utilised in this study had heights of 280 μm, and were mounted on a custom-made applicator with glue (Figure 3a).
Figure 3.
(a) A schematic representation of the microneedle device used to deliver the reporter plasmid DNA into skin explants, and (inset) a scanning electron micrograph (SEM) of a microneedle. (b) The DNA formulation containing pCMVβ was pushed into the epidermis through physical disruptions created in the stratum corneum by the microneedles. (c) The microneedle device was administered 5 times (1-5; shaded areas indicate points of contact between the microneedle device and the skin surface) per 1 cm2 skin specimen, giving a total of 25 potential points of stratum corneum disruption for DNA uptake. (d) Viability of the skin explants at various time points was determined by X-gal staining to detect reporter gene expression in whole skin (scale bar = 1 mm) and (inset) epidermal sheet. (e) 10 μm cryosection of X-gal-stained skin showing localisation of reporter gene expression in the epidermal layer. Scale bars = 100 μm unless otherwise specified.
2.5 Microneedle facilitated delivery of pCMVβ to ex vivo human skin
Both full-thickness and split-thickness skin samples were placed in organ culture (Figure 2a-b). At defined time points (24, 48 and 72 h), the samples were removed from culture in triplicates and a solution of pCMVβ (5 μL at 2.67 μg μL−1) was pipetted on to the epidermal surface. Because the microneedle device was wider than each skin specimen, the microneedle device was aligned with the skin surface such that only the first 5 microneedles came into contact with the skin with each administration. The microneedles were then administered to the pCMVβ-treated region and rocked back and forth in two complete motions (Figure 3b) for a total of 5 times in a criss-cross fashion (Figure 3c). The skin samples were then returned to culture for a further 24 h.
2.6 Histochemical and semi-quantitative anaylsis of gene expression
Gene expression was determined by X-gal staining of epidermal sheets or cryosections followed by microscopic examination. Epidermal sheets were generated by incubating skin samples in 3.8% ammonium thiocyanate in PBS for 30-40 min. The epidermal sheets were peeled away from the dermis with forceps, fixed in acetone at −20 °C and stained with X-gal for 24 h. Alternatively, the skin specimens were fixed in 2% glutaraldehyde, stained with X-gal for 24 h and snap-frozen in OCT embedding media (RA Lamb, Eastbourne, UK) before cryosectioning at a thickness of 10 μm with a microtome and cryostat (Leica, Milton Keynes, UK). To perform semi-quantitative analysis of gene expression, bright-field micrographs were taken for all X-gal-positive regions of the epidermal sheets. The total area of tissue expressing β-galactosidase was calculated using the NIH ImageJ software and grouped by thickness (split-thickness or full-thickness) and culture medium (OCM or PBS). The relative amount of epidermal transgene expression, X, was determined from the total area of β-galactosidase-positive tissue within each group, A, such that
| Equation 1 |
where Amin and Amax represent the smallest and largest area of all groups, respectively, and grouped into quartiles.
2.7 Structural integrity of skin explants
Full-thickness and split-thickness skin cultured in OCM and PBS were fixed, embedded in OCT embedding medium and sectioned as described above. Structural features of the skin sections were accentuated with H&E stain and examined under bright-field microscopy for gross architectural deterioration.
2.8 Distribution of Langerhans cells in longitudinal skin sections
Skin samples were fixed in formalin for 24 h and subsequently dehydrated in an ethanol gradient before clearing in chloroform. Samples were incubated in two changes of molten paraffin before embedding in solid paraffin. Section of 5 μm were generated using a microtome (Leica, Milton Keynes, UK), rehydrated through an ethanol gradient and then subject to high temperature (95-100 °C) antigen retrieval in Tris-EDTA buffer (pH 9.0) for 40 min, followed by 20 min cooling at room temperature. Immunohistochemistry was performed on the sections using anti-CD207 mouse monoclonal antibody (Abcam, Cambridge, UK) and the VECTASTAIN® Elite ABC Mouse IgG Peroxidase Kit coupled with the DAB Kit (both by Vector Laboratories, Peterborough, UK) and visualised by light microscopy.
2.9 Spatio-temporal changes in Langerhans cell population
Epidermal sheets were prepared and fixed, as per gene expression analysis above, from skin samples cultured in OCM for 24, 48 and 72 h. Immunohistochemical techniques using anti-CD207 mouse monoclonal antibody (Abcam, Cambridge, UK) and the VECTASTAIN® Elite ABC Mouse IgG Peroxidase Kit coupled with the DAB Kit (Vector Laboratories, Peterborough, UK) were used to detect langerin (CD207). Light micrographs were captured (Olympus, Watford, UK) and CD207+ cell areas per 200 μm2 field were calculated using ImageJ (image analysis software developed at the US National Institute of Health). The number of CD207+ cells per field was counted. For a particular field, the mean area occupied by each CD207+ cell was calculated by dividing the total area occupied by CD207+ cells by the number of CD207+ cells.
2.10 Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Differences were calculated using one-way ANOVA, followed by Tukey's post hoc test, and deemed significant at p < 0.05.
3. Results
3.1 Cutaneous gene expression
En face images (Figure 3d) and cryosections (Figure 3e) of cultured skin 48 h following microneedle-assisted DNA delivery highlighted localised transgene expression in the epidermis. This was confined to the immediate vicinity of physical disruptions created by the microneedle device in the epidermal layer. Control skin samples (microneedle-treated without DNA) showed no evidence of gene expression (data not shown).
3.2 Skin viability
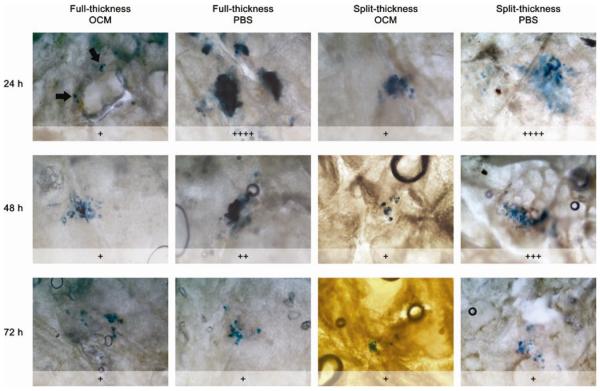
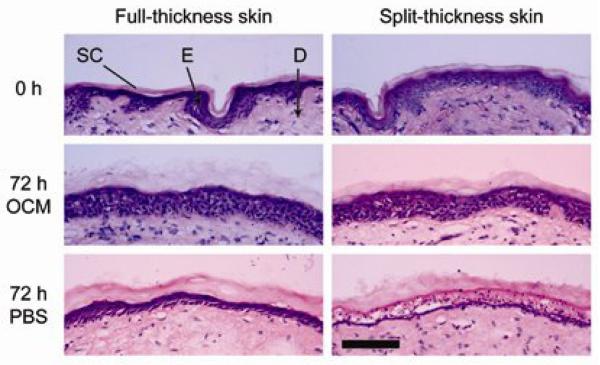
Reporter gene expression was used to determine cellular viability of skin explants with time. β-galactosidase expression was observed in all skin specimens treated with pCMVβ, including those that had been cultured up to 72 h prior to administration of the plasmid. Semi-quantitative analysis of the gene expression data shows that the skin specimens generally exhibited a decline in gene expression with time (Figure 4). Initially, greater gene expression was observed (within the first 48 h) in skin that was cultured in PBS rather than OCM. Skin specimens cultured in OCM showed infiltration of coloured culture medium across the full-thickness skin from the dermis through to the epidermis (Figure 2c). The cultured specimens also appeared swollen from 24 h onwards due to hydration from OCM/PBS (data not shown). Cryosections of cultured skin samples showed no obvious sign of gross degradation or deterioration within skin explants cultured in OCM. However, dissociation of epidermal tissue was observed in those cultured in PBS at 48 h and 72 h (Figure 5).
Figure 4.
Bright-field micrographs showing relative amount of β-galactosidase expression, X, in epidermal sheets derived from full-thickness and split-thickness skin pre-cultured in OCM and PBS, calculated using Equation 1. Reporter gene was expressed in the epidermal layer of explants cultured for up to 72 h prior to gene delivery. A graded decline in the area of β-galactosidase (blue) expression was apparent in explants cultured in PBS, but not those cultured in OCM. Each plus sign represents a quartile, i.e. +: 0% ≤ X ≤ 25%; ++: 25% < X ≤ 50%; +++: 50% < X ≤ 75%; ++++: 75% < X ≤ 100%.
Figure 5.
Histological examination of H&E-stained skin cryosections showing the morphology of the stratum corneum (SC), epidermis (E) and dermis (D) in full-thickness and split-thickness skin at the start of the experiment (0 h) and 72 h post-culture. All images were taken at the same magnification. Scale bar = 100 μm.
3.3 Distribution of LCs in normal skin epidermis
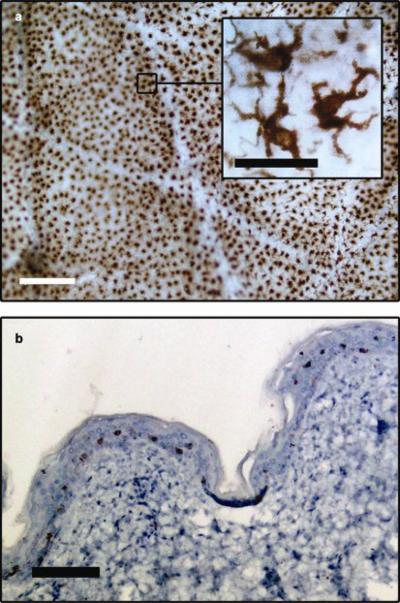
Staining of epidermal sheets for the CD207-specific marker highlighted a network of LCs in the epidermal layer of human skin (Figure 6a). The LCs exhibit numerous protrusions from their cell surfaces (Figure 6a, inset) which are characteristic of dendritic cell morphology and consistent with the literature [37, 38]. The transverse section in Figure 6b illustrates the major architectural features apparent in normal human skin. CD207+ LCs were observed exclusively in the epidermis amidst nucleated cells, and distributed at a relatively consistent depth from the skin surface.
Figure 6.
(a) An en face image of an epidermal sheet from normal human skin showing the extensive network of LCs (brown), which have a dendritic appearance (inset, scale bar = 10 μm). (b) A cross section of the skin shows that the LCs are distributed evenly in the epidermis at a certain distance from the skin surface (scale bar = 100 μm).
3.4 Spatio-temporal changes in LC population
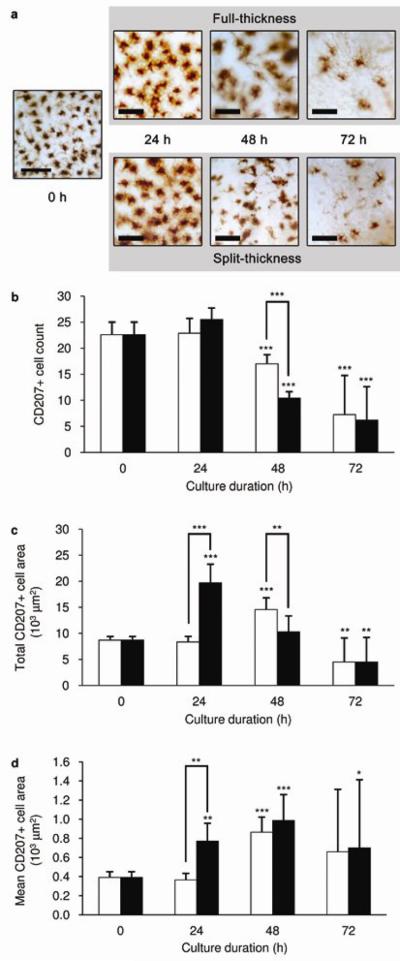
Excised human skin was further assessed for changes in cell composition with culture time. Figure 7a shows representative images of CD207+ LC distribution within organ culture conditions at 0, 24, 48 and 72 h. These images illustrate the observed changes in both LC morphology and number in full-thickness and split-thickness skin. LC number diminished over 72 h in both full-thickness and split-thickness skin (Figure 7b). However, LC depletion was significantly more pronounced within the first 48 h in split-thickness skin than full-thickness skin. In the latter, LC depletion did not become apparent microscopically until 72 h post-culture. Figure 7c shows a strong increase in total CD207-positive area within a defined perimeter in split-thickness skin within the first 24 h. The same pattern of increase was noted in full-thickness skin 24 h later, i.e. at 48 h. The mean area occupied per LC, which is indicative of individual cell size, remained constant for the first 24 h in full-thickness skin in culture (Figure 7d). This is in contrast with split-thickness skin, where the mean area occupied by each LC was significantly increased over the same time period. These results are representative of two independent experiments utilising skin samples from two different donors.
Figure 7.
(a) Representative images of immunostained LCs (brown) in epidermal sheets at the various time points. Scale bars represent 100 μm. (b-c) The decline in epidermal LC number and increase in the total area covered by epidermal LCs in full-thickness (white bars) and split-thickness (black bars) skin over time in tissue culture. (d) Changes in the size of individual LCs was estimated from the calculated area per LC. Skin samples were cultured in OCM. Data are mean ± SD (n = 4). Significance levels are relative to 0 h, unless otherwise indicated, whereby ***p < 0.001, **p < 0.01, *p < 0.05.
4. Discussion
The aim of cutaneous DNA vaccination is to facilitate transgenic expression of a specific antigen by the host skin tissue to generate a whole-body immune response. In order to exploit and study this chain of events ex vivo, it is imperative to ensure that the skin model remains viable long enough for the uptake and expression of the transgene, as well as any local-level immune responses downstream, to occur. The viability of the skin model in this context hinges on fully functional cellular machineries required for both nucleic acid and protein synthesis. In the past, ex vivo skin viability has been largely defined and measured by various surrogate parameters, such as oxygen consumption and enzymatic activity [39], which do not address the genetic aspect of tissue viability. Using cell culture methods such as quantification of cell growth, Hautier et al. [26] showed that keratinocytes remain viable in similar culture conditions >7 days, but the study offers no insight into the susceptibility of the tissue to transfection. In the present study, successful reporter gene expression in situ served as the end point which defined the viability of ex vivo skin, hence addressing all the biological requirements for DNA vaccine expression. Whilst the viability data did not distinguish between the viability of LCs and keratinocytes, some preceding studies have shown modulated changes in gene expression patterns of LCs in culture during this time [40, 41], thus suggesting that the LCs could be expected to still be viable.
Importantly, this study demonstrated that both split-thickness and full-thickness skin cultures retained their capability to express transgenes up to 72 h post-culture (Figure 4). This was not restricted by the minimal nutritional specifications of PBS compared with OCM. Conventionally, for short-term (within 24 h) cutaneous gene expression studies, split-thickness skin has been used to facilitate OCM diffusion to the epidermal tissue, thus in principle maximising perfusion and explant viability [24, 32, 33]. In this study, it was observed that fluid uptake from the culture medium through the basal surface of the dermis took place in culture (Figure 2c). This uptake was not precluded by the thicker dermis in full-thickness skin compared to split-thickness skin. As a result, the skin specimens became swollen due to hydration within 24 h in culture, after which no noticeable change in tissue volume was observed (data not shown). In the process of gene delivery, the skin specimens were compressed by the microneedle device and returned to culture in the compressed state. These became swollen again 24 h after being returned to the organ culture. That the skin explants retained their biological functionality for 3 days (Figure 5), even when cultured in PBS, highlighted the resilience of the human skin in culture. The supply of key nutrients to the explants during this time, with the use of enriched culture medium, was not an absolute requirement. It has been suggested that excised tissue may be susceptible to multiple impairments of vital intracellular systems including membrane integrity, aerobic respiration, protein and DNA repair and synthesis which, if prolonged, ultimately lead to necrosis due to hypoxia, undernourishment and accumulation of toxic waste products [42]. In this study, it was observed that any such damage was minimal in the organ culture model employed in this study within the time frame investigated. However, there was some evidence of delamination within epidermal tissues cultured in PBS, hence suggesting that PBS may not be a suitable medium to use with this model system. It is not certain at this time what caused the structural changes, but it is likely due to the relatively higher NaCl concentrations in PBS than OCM, as NaCl has been used successfully for epidermal sheet separation, albeit at much higher concentrations than PBS (1 M) [43].
Interestingly, the semi-quantitative analysis suggests that more gene expression was observed in skin cultured in Ca2+-free PBS than in OCM containing 5% FCS and 1.8 mM Ca2+ (Figure 4). Where PBS was used, the onset of epidermal delamination, which became apparent after 48 h, could have facilitated lateral diffusion of the DNA solution within the epidermis such that gene expression was observed over a larger area at the earlier time points. Also, the disruption in the three-dimensional structure of the tissue perhaps made individual cells more accessible to the DNA solution, leading to enhance DNA uptake. Alternatively, there is evidence that FCS contains both stimulatory and inhibitory factors which orchestrate the proliferation and differentiation of keratinocytes in an integrated manner [44]. Haberland et al. [45] has reported that bovine serum albumin (BSA) present in FCS is toxic to keratinocytes. FCS and high Ca2+ levels also both inhibit the growth of mouse keratinocytes [46, 47]. However, because the composition of FCS is undefined and varies from batch to batch, it is not possible to attribute this discrepancy to any particular component of OCM with great certainty. Meanwhile, ex vivo organ cultures have been reported to be better preserved morphologically and exhibit enhanced levels of DNA synthesis in serum-free, minimal nutrient media [48]. Hence, the idea that such media may be able to prolong skin viability compared to FCS-supplemented media is not entirely inconceivable. In addition, while it is important to recognise that gene expression may not truly reflect skin viability in absolute terms, a consistent trend of decline in gene expression over time in the skin explants was observed (Figure 4). It also has to be acknowledged that inter-individual biological variability may play a role. However, the study was conducted with experimental parameters standardised wherever possible. The data shown were derived from a single donor to remove inter-individual variability but reflect our past experience with work of this nature.
In terms of immunology, because both direct and cross-priming are feasible mechanisms for genetic immunisation [49-51], the targets of DNA vaccination should include both keratinocytes and LCs. From the histochemical data (Figure 3d-e), coupled with the knowledge that the epidermis is made up predominantly of keratinocytes, it is highly likely that the majority (if not all) of the transfected cells were keratinocytes. Even though not verified in this particular study, direct transfection of LCs is possible [52, 53]. Since cutaneous gene expression is usually observed to take place within 24 h of gene delivery, although maturation and migration of LCs have been observed as early as 16 h post-stimulation in human skin [54], a 48 h window is sufficient to investigate an early-phase immune response in LCs. The organ culture system allows for this.
Understanding the behaviour of LCs residing within ex vivo human skin maintained in organ culture is prerequisite to the use of such systems as models for early immune initiation with regards to DNA vaccination. Importantly, within organ culture systems, LC activation, maturation and migration may be useful determinants of vaccine efficacy and therefore can be used in the development of novel vaccine formulations and delivery methods. The turnover time of LCs in vivo is approximately 4 months [55]. LC activation manifests itself as a reduction in epidermal LC density and an increase in cell size [56]. By merely subjecting human skin explants to ex vivo culture conditions, spontaneous LC depletion can be induced [57, 58]. In preparing skin explants for culture, it is possible that sufficient tissue damage stimulates release of cytokines and chemokines, such as TNF-α and IL-1β from keratinocytes and dermal DCs. Such cell-signalling molecules serve as potent migration signals to LCs [59, 60]. In preparing split-thickness skin, it is inevitable that further physical damage is brought about in the dermis and this is likely to induce chemotactic LC maturation and migration. In relation to this, the ability to maintain the viability of full-thickness human skin under the same culture conditions will minimise physical damage to the dermis and, in the process, prevent premature activation of LCs. Thus, by using full-thickness skin instead of split-thickness skin in the organ culture model, the LCs are able to remain unperturbed for longer in the epidermis, making it practical for subsequent immunological investigations. This window of opportunity could possibly be extended with the use of serum-free culture medium, as FCS containing xenogenic serum products is immunogenic to human tissue and thus may accelerate the depletion of LCs from the cultured epidermis [61, 62].
Admittedly, the ex vivo skin model lacks an influx of other immune cells and biochemicals such as neutrophils, natural killer cells and complement proteins from the circulatory system. This is recognised as a limitation of the ex vivo skin model. Animal models, on the other hand, are perfused by a systemic circulation but lacks fidelity to true human biology. The two models therefore complement each other. The ex vivo skin model is intended to be used not exclusively but as a complement to animal models.
5. Conclusion
The study has addressed a number of fundamental issues pertaining to the use of human organ culture systems for DNA vaccination. Firstly, it has demonstrated that both full-thickness and split-thickness skin will remain viable in the organ culture for at least 72 h, during which time a limited supply of nutrients in the culture medium is unlikely to be a limiting factor to their viability. Secondly, targeted DNA delivery to the epidermis can be achieved using microneedles to attain in situ transgene expression. Thirdly, spontaneous LC activation from the epidermis in culture can be delayed by using full-thickness instead of split-thickness skin. Thus, the organ culture system provides a robust platform on which the immunological aspects of DNA vaccination can be investigated ex vivo. We aim to exploit this model to investigate early markers of immune response following cutaneous DNA vaccine delivery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bauer J, Bahmer FA, Worl J, Neuhuber W, Schuler G, Fartasch M. A strikingly constant ratio exists between Langerhans cells and other epidermal cells in human skin. A stereologic study using the optical disector method and the confocal laser scanning microscope. J Invest Dermatol. 2001;116(2):313–8. doi: 10.1046/j.1523-1747.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- 2.McGrath JA, Eady RAJ, Pope FM. Anatomy and Organization of Human Skin. Rook's Textbook of Dermatology: Blackwell Publishing. 2004 3.1-3.84-3.1-3. [Google Scholar]
- 3.Klareskog L, Tjernlund U, Forsum U, Peterson PA. Epidermal Langerhans cells express Ia antigens. Nature. 1977;268(5617):248–50. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- 4.Rowden G, Lewis MG, Sullivan AK. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977;268(5617):247–8. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- 5.Stingl G, Wolff-Schreiner EC, Pichler WJ, Gschnait F, Knapp W, Wolff K. Epidermal Langerhans cells bear Fc and C3 receptors. Nature. 1977;268(5617):245–6. doi: 10.1038/268245a0. [DOI] [PubMed] [Google Scholar]
- 6.Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I. Langerhans cell migration. Clin Exp Dermatol. 2000;25(5):413–8. doi: 10.1046/j.1365-2230.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 7.Garmory H, Brown K, Titball R. DNA vaccines: improving expression of antigens. Genet Vaccines Ther. 2003;1(1):2. doi: 10.1186/1479-0556-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ban E, Dupre L, Hermann E, Rohn W, Vendeville C, Quatannens B, et al. CpG motifs induce Langerhans cell migration in vivo. Int Immunol. 2000;12(6):737–45. doi: 10.1093/intimm/12.6.737. [DOI] [PubMed] [Google Scholar]
- 9.WHO DNA vaccines. undated [cited 20 November 2008]; Available from: http://www.who.int/biologicals/areas/vaccines/dna/en/index.html.
- 10.Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur J Immunol. 2003;33(10):2645–54. doi: 10.1002/eji.200324341. [DOI] [PubMed] [Google Scholar]
- 11.Dickgreber N, Stoitzner P, Bai Y, Price KM, Farrand KJ, Manning K, et al. Targeting antigen to MHC class II molecules promotes efficient cross-presentation and enhances immunotherapy. J Immunol. 2009 Feb 1;182(3):1260–9. doi: 10.4049/jimmunol.182.3.1260. [DOI] [PubMed] [Google Scholar]
- 12.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004 Jun;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 13.Timares L, Safer KM, Qu B, Takashima A, Johnston SA. Drug-inducible, dendritic cell-based genetic immunization. J Immunol. 2003;170(11):5483–90. doi: 10.4049/jimmunol.170.11.5483. [DOI] [PubMed] [Google Scholar]
- 14.Cho JH, Youn JW, Sung YC. Cross-priming as a predominant mechanism for inducing CD8(+) T cell responses in gene gun DNA immunization. J Immunol. 2001 Nov 15;167(10):5549–57. doi: 10.4049/jimmunol.167.10.5549. [DOI] [PubMed] [Google Scholar]
- 15.van den Berg JH, Nujien B, Beijnen JH, Vincent A, van Tinteren H, Kluge J, et al. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum Gene Ther. 2009 Mar;20(3):181–9. doi: 10.1089/hum.2008.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58(1):68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126(4):787–96. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 18.Stutte S, Jux B, Esser C, Forster I. CD24a expression levels discriminate Langerhans cells from dermal dendritic cells in murine skin and lymph nodes. J Invest Dermatol. 2008;128(6):1470–5. doi: 10.1038/sj.jid.5701228. [DOI] [PubMed] [Google Scholar]
- 19.Hengge UR, Walker PS, Vogel JC. Expression of naked DNA in human, pig, and mouse skin. J Clin Invest. 1996;97(12):2911–6. doi: 10.1172/JCI118750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panchagnula R, Stemmer K, Ritschel WA. Animal models for transdermal drug delivery. Methods Find Exp Clin Pharmacol. 1997;19(5):335–41. [PubMed] [Google Scholar]
- 21.Tomita Y, Nihira M, Ohno Y, Sato S. Ultrastructural changes during in situ early postmortem autolysis in kidney, pancreas, liver, heart and skeletal muscle of rats. Leg Med. 2004;6(1):25–31. doi: 10.1016/j.legalmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Birchall J, Coulman S, Anstey A, Gateley C, Sweetland H, Gershonowitz A, et al. Cutaneous gene expression of plasmid DNA in excised human skin following delivery via microchannels created by radio frequency ablation. Int J Pharm. 2006;312(12)::15–23. doi: 10.1016/j.ijpharm.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Birchall J, Coulman S, Pearton M, Allender C, Brain K, Anstey A, et al. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch micro-fabricated micro-needles. J Drug Target. 2005;13(7):415–21. doi: 10.1080/10611860500383705. [DOI] [PubMed] [Google Scholar]
- 24.Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N, et al. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3(1):65–75. doi: 10.2174/156720106775197510. [DOI] [PubMed] [Google Scholar]
- 25.Pearton M, Allender C, Brain K, Anstey A, Gateley C, Wilke N, et al. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm Res. 2008;25(2):407–16. doi: 10.1007/s11095-007-9360-y. [DOI] [PubMed] [Google Scholar]
- 26.Hautier A, Sabatier F, Stellmann P, Andrac L, Nouaille De Gorce Y, Dignat-George F, et al. Assessment of organ culture for the conservation of human skin allografts. Cell Tissue Bank. 2008;9(1):19–29. doi: 10.1007/s10561-007-9042-3. [DOI] [PubMed] [Google Scholar]
- 27.Roychowdhury S, Cram AE, Aly A, Svensson CK. Detection of haptenated proteins in organotypic human skin explant cultures exposed to dapsone. Drug Metab Dispos. 2007;35(9):1463–5. doi: 10.1124/dmd.107.015560. [DOI] [PubMed] [Google Scholar]
- 28.de Gruijl TD, Ophorst OJ, Goudsmit J, Verhaagh S, Lougheed SM, Radosevic K, et al. Intradermal delivery of adenoviral type-35 vectors leads to high efficiency transduction of mature, CD8+ T cell-stimulating skin-emigrated dendritic cells. J Immunol. 2006;177(4):2208–15. doi: 10.4049/jimmunol.177.4.2208. [DOI] [PubMed] [Google Scholar]
- 29.Lehé CL, Jacobs JJ, Hua CM, Courtellemont P, Elliott GR, Das PK. Subtoxic concentrations of allergenic haptens induce LC migration and maturation in a human organotypic skin explant culture model: a novel method for identifying potential contact allergens. Exp Dermatol. 2006;15(6):421–31. doi: 10.1111/j.0906-6705.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs JJ, Lehe C, Cammans KD, Das PK, Elliott GR. An in vitro model for detecting skin irritants: methyl green-pyronine staining of human skin explant cultures. Toxicol In Vitro. 2002;16(5):581–8. doi: 10.1016/s0887-2333(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 31.Yasuoka H, Larregina AT, Yamaguchi Y, Feghali-Bostwick CA. Human skin culture as an ex vivo model for assessing the fibrotic effects of insulin-like growth factor binding proteins. Open Rheumatol J. 2008;2:17–22. doi: 10.2174/1874312900802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birchall J, Coulman S, Pearton M, Allender C, Brain K, Anstey A, et al. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch microfabricated microneedles. J Drug Target. 2005;13(7):415–21. doi: 10.1080/10611860500383705. [DOI] [PubMed] [Google Scholar]
- 33.Pearton M, Allender C, Brain K, Anstey A, Gateley C, Wilke N, et al. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm Res. 2008;25(2):407–16. doi: 10.1007/s11095-007-9360-y. [DOI] [PubMed] [Google Scholar]
- 34.Trowell OA. A modified technique for organ culture in vitro. Exp Cell Res. 1954;6(1):246–8. doi: 10.1016/0014-4827(54)90169-x. [DOI] [PubMed] [Google Scholar]
- 35.Birchall JC, Kellaway IW, Gumbleton M. Physical stability and in-vitro gene expression efficiency of nebulised lipid-peptide-DNA complexes. Int J Pharm. 2000;197(12)::221–31. doi: 10.1016/s0378-5173(00)00339-2. [DOI] [PubMed] [Google Scholar]
- 36.Wilke N, Mulcahy A, Ye SR, Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectronics J. 2005;36:650–6. [Google Scholar]
- 37.Cavanagh LL, Weninger W. Dendritic cell behaviour in vivo: lessons learned from intravital two-photon microscopy. Immunol Cell Biol. 2008 Jul;86(5):428–38. doi: 10.1038/icb.2008.25. [DOI] [PubMed] [Google Scholar]
- 38.Figueroa CD, Caorsi I. Ultrastructural and morphometric study of the Langerhans cell in the normal human exocervix. J Anat. 1980 Dec;131(Pt 4):669–82. [PMC free article] [PubMed] [Google Scholar]
- 39.Messager S, Hann AC, Goddard PA, Dettmar PW, Maillard JY. Assessment of skin viability: is it necessary to use different methodologies? Skin Res Technol. 2003;9(4):321–30. doi: 10.1034/j.1600-0846.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 40.de Gruijl TD, Sombroek CC, Lougheed SM, Oosterhoff D, Buter J, van den Eertwegh AJ, et al. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J Immunol. 2006;176(12):7232–42. doi: 10.4049/jimmunol.176.12.7232. [DOI] [PubMed] [Google Scholar]
- 41.Rambukkana A, Bos JD, Irik D, Menko WJ, Kapsenberg ML, Das PK. In situ behavior of human Langerhans cells in skin. Lab Invest. 1995;73(4):521–31. [PubMed] [Google Scholar]
- 42.Sterne GD, Titley OG, Christie JL. A qualitative histological assessment of various storage conditions on short term preservation of human split skin grafts. Br J Plast Surg. 2000 Jun;53(4):331–6. doi: 10.1054/bjps.1999.3279. [DOI] [PubMed] [Google Scholar]
- 43.Willsteed EM, Bhogal BS, Das A, Bekir SS, Wojnarowska F, Black MM, et al. An ultrastructural comparison of dermo-epidermal separation techniques. Journal of Cutaneous Pathology. 1991;18(1):8–12. doi: 10.1111/j.1600-0560.1991.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 44.Wille JJ, Pittelkow MR, Shipley GD, Scott RE. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol. 1984 Oct;121(1):31–44. doi: 10.1002/jcp.1041210106. [DOI] [PubMed] [Google Scholar]
- 45.Haberland A, Schreiber S, Maia CS, Rubbelke MK, Schaller M, Korting HC, et al. The impact of skin viability on drug metabolism and permeation -- BSA toxicity on primary keratinocytes. Toxicol In Vitro. 2006;20(3):347–54. doi: 10.1016/j.tiv.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Bertolero F, Kaighn ME, Camalier RF, Saffiotti U. Effects of serum and serum-derived factors on growth and differentiation of mouse keratinocytes. In Vitro Cell Dev Biol. 1986;22(7):423–8. doi: 10.1007/BF02623533. [DOI] [PubMed] [Google Scholar]
- 47.Varani J. Preservation of human skin structure and function in organ culture. Histol Histopathol. 1998;13(3):775–83. doi: 10.14670/HH-13.775. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen-Le XK, Corcos J, Briere N. Rat prostate explants in serum-free organ culture: a comparison of two media and gas mixtures. Prostate. 1997;32(1):43–8. doi: 10.1002/(sici)1097-0045(19970615)32:1<43::aid-pros6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 49.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103(20):7783–8. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan M, Peng J, Jabbar IA, Liu X, Filgueira L, Frazer IH, et al. Despite differences between dendritic cells and Langerhans cells in the mechanism of papillomavirus-like particle antigen uptake, both cells cross-prime T cells. Virology. 2004;324(2):297–310. doi: 10.1016/j.virol.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 51.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174(4):2220–7. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 52.Larregina AT, Watkins SC, Erdos G, Spencer LA, Storkus WJ, Beer Stolz D, et al. Direct transfection and activation of human cutaneous dendritic cells. Gene Ther. 2001;8(8):608–17. doi: 10.1038/sj.gt.3301404. [DOI] [PubMed] [Google Scholar]
- 53.Timares L, Safer KM, Qu B, Takashima A, Johnston SA. Drug-inducible, dendritic cell-based genetic immunization. J Immunol. 2003;170(11):5483–90. doi: 10.4049/jimmunol.170.11.5483. [DOI] [PubMed] [Google Scholar]
- 54.Ouwehand K, Santegoets SJ, Bruynzeel DP, Scheper RJ, de Gruijl TD, Gibbs S. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur J Immunol. 2008;38(11):3050–9. doi: 10.1002/eji.200838384. [DOI] [PubMed] [Google Scholar]
- 55.Krueger GG, Daynes RA, Emam M. Biology of Langerhans cells: Selective migration of Langerhans cells into allogeneic and xenogeneic grafts on nude mice. Proc Natl Acad Sci U S A. 1983;80(6):1650–4. doi: 10.1073/pnas.80.6.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araki M, Imafuku S, Furue M, Shimada S, Tamaki K. Activation pattern of Langerhans cells in the afferent and efferent phases of contact hypersensitivity. J Investig Dermatol Symp Proc. 1999 Sep;4(2):164–8. doi: 10.1038/sj.jidsp.5640202. [DOI] [PubMed] [Google Scholar]
- 57.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172(5):1483–93. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rambukkana A, Bos JD, Irik D, Menko WJ, Kapsenberg ML, Das PK. In situ behavior of human Langerhans cells in skin organ culture. Lab Invest. 1995;73(4):521–31. [PubMed] [Google Scholar]
- 59.Griffiths CE, Dearman RJ, Cumberbatch M, Kimber I. Cytokines and Langerhans cell mobilisation in mouse and man. Cytokine. 2005;32(2):67–70. doi: 10.1016/j.cyto.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Nishibu A, Ward BR, Boes M, Takashima A. Roles for IL-1 and TNFalpha in dynamic behavioral responses of Langerhans cells to topical hapten application. J Dermatol Sci. 2007;45(1):23–30. doi: 10.1016/j.jdermsci.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson LF, deSerres S, Herzog SR, Peterson HD, Meyer AA. Antigenic cross-reactivity between media supplements for cultured keratinocyte grafts. J Burn Care Rehabil. 1991;12(4):306–12. doi: 10.1097/00004630-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Meyer AA, Manktelow A, Johnson M, deSerres S, Herzog S, Peterson HD. Antibody response to xenogeneic proteins in burned patients receiving cultured keratinocyte grafts. J Trauma. 1988;28(7):1054–9. doi: 10.1097/00005373-198807000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006 Jan;126(1):32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]