Abstract
Three circulating human bone alkaline phosphatase (BALP) isoforms (B1, B2, and B/I) can be distinguished in healthy individuals and a fourth isoform (B1x) has been discovered in patients with chronic kidney disease and in bone tissue. The present study was designed to correlate differing glycosylation patterns of each BALP isoform with their catalytic activity towards presumptive physiological substrates and to compare those properties with two recombinant isoforms of the tissue-nonspecific ALP (TNALP) isozyme, i.e., TNALP-flag, used extensively for mutation analysis of hypophosphatasia mutations and sALP-FcD10, a chimeric enzyme recently used as therapeutic drug in a mouse model of infantile hypophosphatasia.
The BALP isoforms were prepared from human osteosarcoma (SaOS-2) cells and the kinetic properties were evaluated using the synthetic substrate p-nitrophenylphosphate (pNPP) at pH 7.4 and 9.8, and the three suggested endogenous physiological substrates, i.e., inorganic pyrophosphate (PPi), pyridoxal 5′-phosphate (PLP), and phosphoethanolamine (PEA) at pH 7.4. Qualitative glycosylation differences were also assessed by lectin binding and precipitation.
The kcat/KM was higher for B2 for all the investigated substrates. The catalytic activity towards PEA was essentially undetectable. The kinetic activity for TNALP-flag and sALP-FcD10 was similar to the activity of the human BALP isoforms. The BALP isoforms differed in their lectin-binding properties and dose-dependent lectin precipitation, which also demonstrated differences between native and denatured BALP isoforms. The observed differences in lectin specificity were attributed to N-linked carbohydrates.
In conclusion, we demonstrate significantly different catalytic properties among the BALP isoforms due to structural differences in posttranslational glycosylation. Our data also suggests that PEA is not an endogenous substrate for the BALP isoforms or for the recombinant TNALP isoforms. The TNALP-flag and the sALP-FcD10 isoforms faithfully mimic the biological properties of the human BALP isoforms in vivo validating the use of these recombinant enzymes in studies aimed at dissecting the pathophysiology and treating hypophosphatasia.
Keywords: bone turnover, glycosylation, hypophosphatasia, kinetics, pyrophosphate
Introduction
Despite the generalized use of alkaline phosphatase (ALP) as a biochemical marker of bone formation, the precise function of bone ALP (BALP) is only now becoming clear. Studies of hypophosphatasia, a rare inherited disorder, caused by missense mutations of the tissue-nonspecific (TNALP) gene, have provided evidence for an important role for ALP in the development and mineralization of the human skeleton [1] and recent studies on TNALP knockout mice, which recapitulate infantile hypophosphatasia [2, 3], suggest a role of BALP as a pyrophosphatase (i.e., cleaving inorganic pyrophosphate (PPi), a potent inhibitor of mineralization), thus promoting mineral deposition in vivo [4].
ALP is a glycoprotein that functions as an ectoenzyme attached to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor [5, 6]. In vitro studies have shown that BALP is released from the surface of osteoblasts in a GPI anchor-intact form attached to membrane vesicles, called matrix vesicles [7–9]. BALP is cleaved from insoluble membrane fragments by GPI-specific phospholipase D (GPI-PLD), before it enters the circulation in an anchor-free (soluble) form [10, 11]. Three endogenous phosphocompounds, PPi, pyridoxal 5′-phosphate (PLP), and phosphoethanolamine (PEA), have been suggested to serve as physiological substrates for BALP since they accumulate in patients with hypophosphatasia, where there is a global deficiency of TNALP [1, 12]. The most commonly used substrate for determination of ALP, however, continues to be the synthetic substrate p-nitrophenylphosphate (pNPP) at pH 9.8 (optimum, in vitro).
In humans, there are four genes that encode for the ALP isoenzymes: tissue-nonspecific, placental, germ cell, and the intestinal locus [13]. TNALP is expressed at high levels in bone and liver tissues, and account for approximately 95% of the total serum ALP activity, with a ratio of approximately 1:1 in healthy adults [14]. Because liver ALP and BALP are encoded by the same gene locus, they are referred to as isoforms of the same TNALP isozyme.
Human bone tissue contains four BALP isoforms (B/I, B1x, B1, and B2), of which the B/I, B1 and B2 isoforms are commonly found in the circulation by high-performance liquid chromatography (HPLC) [15, 16]. The B/I isoform (bone/intestinal) is not a “pure” bone isoform since it co-elutes with circulating intestinal ALP and is composed, on average, of 70% BALP and 30% intestinal ALP. A fourth bone isoform, B1x, has been identified in serum from some patients with chronic kidney disease [17, 18] and in human bone tissue [19]. The circulating levels of these isoforms can vary independently during the pubertal growth spurt [20] and in metabolic bone disease [15, 17, 21], and they differ also with respect to their distribution in human cortical and trabecular bone [22].
Glycosylation is the most common form of posttranslational modification of proteins and the carbohydrate chains of glycoproteins have integral roles in the functional properties of glycoproteins [23]. There are two major types of glycans, N-linked and O-linked. N-linked glycans are linked to asparagine residues in an Asn-X-Ser/Thr motif, and O-linked glycans are linked to serine or threonine. Glycosylation produces numerous structural modifications and increasing evidence indicates that these carbohydrates are important for protein sorting, cell signaling, immune and receptor recognition and cell differentiation among other processes [24]. It has been confirmed that both bone and liver ALP are N-glycosylated but the number of sites is not known [25]. The differences between bone and liver can also be due to differences in O-glycosylation.
Most studies aimed at elucidating the biochemical basis for the pathogenesis of hypophosphatasia make use of recombinant human TNALP expressed in vitro [13]. One such recombinant enzyme, TNALP-flag, expressed in COS-1 or Chinese hamster ovary cells, has been used to characterize the residual kinetic properties of different hypophosphatasia mutations [26]. Another recombinant TNALP, a fusion protein containing the TNALP ectodomain, an Fc domain and a polyaspartate sequence, has recently been expressed in Chinese hamster ovary cells and used to prevent all the manifestations of infantile hypophosphatasia by means of enzyme replacement therapy [27]. The present study was designed with two goals in mind. First, to investigate the catalytic properties of the four known BALP isoforms B/I, B1x, B1, and B2, in relation to three suggested endogenous substrates, PPi, PLP and PEA, at physiological pH 7.4, and the synthetic substrate pNPP at pH 7.4 and pH 9.8. Second, to relate the kinetic behavior of the two most significant human recombinant TNALP isoforms, TNALP-flag and sALP-FcD10 to that of the purified human isoforms. As part of this study, we characterized postulated differences in posttranslational glycosylation patterns among the BALP isoforms and investigated the role of these carbohydrate side-chains in influencing their catalytic properties.
Materials and methods
Human BALP isoforms and recombinant human TNALPs
The BALP isoforms (B/I, B1x, B1, and B2) were prepared from human osteosarcoma SaOS-2 cells (ATCC-LGC Promochem, Rockville, MD, USA) and a stable, high-BALP subpopulation of those cells was used throughout the current studies [28, 29].
Two recombinant forms of TNALP were used for kinetic measurements. The TNALP cDNA (ATCC no. 59635; American Type Culture Collection, Manassas, VA, USA) was used as a template for the construction of the recombinant form TNALP-flag. To facilitate isolation, a FLAG epitope (5′-TTA CTT GTC ATC GTC GTC CTT GTA GTC-3′) and a termination codon to eliminate the GPI-anchoring signal was introduced [26]. TNALP-flag protein was expressed in SV40-transformed African green monkey kidney COS-1 cells as before [26]. The other recombinant form, sALP-FcD10, contains recombinant human TNALP (sALP), the constant region of human IgG1 Fc domain (Fc), and a deca-aspartate (D10) motif for targeting to mineralizing tissue and was expressed in Chinese hamster ovary DG44 cells [27, 30].
Cell culture methods
All reagents were obtained from Sigma (St Louis, MO, USA) if not stated otherwise. The SaOS-2 cells were grown in 100 mm dishes in Dulbecco’s modified Eagle’s medium - high glucose, supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, 40 U/mL nystatin and incubated at 37°C with 95% humidity and 5% CO2. Medium was removed when the cultures were confluent and the dishes were rinsed three times with phosphate-buffered saline and stored at −20°C for extraction of BALP.
Purification of GPI-PLD
GPI-PLD was purified from commercially available human serum (Sigma). Serum was incubated with 9% polyethylene glycol for 1 hour, centrifuged at 2600 × g for 15 minutes to remove insoluble materials. The supernatant was filtered through a 0.22 fm filter, centrifuged through a Vivaspin 20 concentrator, MWCO 300 kDa (Vivascience AG, Hannover, Germany), concentrated with aquacide and dialyzed in “Buffer A” (i.e., 50 mM Tris, 10 mM NaCl, 2 mM CaCl2, pH 7.75). This solution was applied to a DEAE Sepharose column, eluated with 0.010–0.500 M NaCl (total volume 500 mL) in Buffer A. Each fraction was tested for GPI-PLD activity as reported elsewhere [22] and the fractions containing GPI-PLD was pooled, concentrated with aquacide and dialyzed in Buffer A. The solution containing GPI-PLD was applied to a Concanavalin A column and eluated with 0.1 M glucose in Buffer A. The fractions containing GPI-PLD was pooled, concentrated with aquacide, dialyzed with Buffer A and stored at −70°C.
Purification of the BALP isoforms
SaOS-2 cells were homogenized in 100 mM Tris, 1 mM benzamidine and 0.01 mg/mL phenylmethylsulfonyl, pH 8.3, 1 mL per dish and incubated in ice-cool butanol to a final concentration of 30% for 24 hours at 4°C with constant stirring and centrifuged 1500 × g for 15 minutes to separate the two phases. The lower phase was collected and dialyzed with 10 mM Tris, 0.1 mM MgCl2 and 10 μM zinc acetate, pH 8.3 to remove butanol and GPI-PLD was added to a final concentration of 25% together with 0.01% NP-40 and 50 μM zinc acetate and incubated for 16 h at 37°C with constant shaking. To separate the soluble (anchor-depleted) BALP from insoluble (anchor-intact) BALP, the preparation was incubated 1:1 with 20 mM Tris, 0.2 mM MgCl2, 20 μM zinc acetate and 4% Triton X-114, pH 8.3 for 30 minutes in a 37°C water bath and centrifuged at 1500 × g for 10 minutes to separate the two phases.
The upper phase containing soluble BALP was collected, concentrated with aquacide and dialyzed with 12.5 mM Tris, 12.5 mM NaHCO3, 10 μM zinc acetate, pH 8.5 before applied to a Q-Sepharose column and eluted with 0.05–0.20 M sodium acetate (total volume 300 mL) in a buffer of 20 mM Tris and 10 μM zinc acetate, pH 7.6. The isoforms were identified by HPLC.
Measurement of BALP isoforms by HPLC
The BALP isoforms B/I, B1x, B1, and B2 were determined by a previously described HPLC [15, 16]. In brief, the BALP isoforms were separated using a gradient of 0.6 M sodium acetate on a weak anion-exchange column, SynChropak AX300 (250 × 4.6 mm I.D.) (Eprogen, Inc.). The effluent was mixed on-line with the substrate solution (1.8 mM pNPP in a 0.25 M diethanolamine buffer at pH 10.1) and the ensuing reaction took place in a packed-bed post-column reactor at 37°C. The formed product (p-nitrophenol) was then directed on-line through the detector set at 405 nm. The areas under each peak were integrated and the total ALP activity used to calculate the relative activity of each of the detected BALP isoforms.
Kinetic measurements
The purified isoforms were diluted to suitable concentrations with buffer containing 12.5 M Tris, 12.5 M NaHCO3 and 10 μM Zn acetate at pH 8.5 [31]. Kinetic properties were evaluated using the substrate pNPP in 1 M diethanolamine buffer containing 1 mM MgCl2 and 20 μM zinc acetate at pH 7.4 and 9.8 and the three substrates PPi, PLP, and PEA in a 50 mM Tris buffer with 1 mM MgCl2 and 20 μM zinc acetate at pH 7.4. For pNPP, at pH 7.4 and 9.8, the absorbance was measured continuously for five and ten minutes, respectively. For the other substrates, the amount of free inorganic phosphate was determined after 60 minutes for PPi, 120 minutes for PLP and 48 hours for PEA [32]. The used concentrations for pNPP pH 9.8 and 7.4 were 0.1–10 mM, PPi and PLP were 2–10 mM, and for PEA 10–50 mM. The kinetic parameters, maximum reaction velocity (Vmax), Michaelis constant (KM) and the catalytic rate constant (kcat) were determined from a Hanes-Wolf plot were the substrate concentration was plotted against the substrate concentration divided with reaction velocity.
To study the effects of terminal sialic acid residues, the isoforms (1 mL each) were treated with 100 μL neuraminidase 20 U/mL in a 20 mM Tris buffer pH 7.6 containing 20 μM zinc acetate, and incubated for 2 hours at 37°C. The kinetic properties were assessed in the same approach as for untreated BALP isoforms.
The kinetic properties for the recombinant TNALP-flag and sALP-FcD10 were determined by the same methods as for the isoforms. The substrates tested were pNPP at pH 7.4 and pH 9.8, PPi, PLP, and PEA at pH 7.4.
Migration of BALP isoforms in a native gel
The four BALP isoforms (with or without neuraminidase treatment) were separated on a Tris/Glycine polyacrylamide gel 4–12%. The isoforms were treated with 5 μL neuraminidase 20 U/mL in a 20 mM Tris buffer at pH 7.6 containing 20 μM zinc acetate to 20 μL of each BALP isoform and incubated for 2 hours in 37°C in a water bath. After electrophoresis, the isoforms were stained in the gel using 1.0 M 2-methyl-2-amino-1,3-propanediol buffer containing 3 mM MgCl2, 4.4 mM naphthyl phosphate and 1 g/L Variamine Blue RT salt (4-aminodiphenylamine diazonium sulphate).
Qualitative determination of glycosylation patterns
The BALP isoforms (B/I, B1x, B1, and B2) were denatured in 70°C for 10 minutes and applied to a SDS polyacrylamide gel and blotted to a nitrocellulose membrane. The glycosylation pattern of the different BALP isoforms was studied with the DIG Glycan Differentiation kit (Roche Applied Science, Basel, Switzerland). This kit contains the following digoxigenin labeled lectins: Datura stramonium agglutinin (DSA), Sambucus nigra agglutinin (SNA), Peanut agglutinin (PNA), Galanthus nivalis agglutinin (GNA), and Maackia amurensis agglutinin (MAA). DSA indicates galactose-β(1–4)-N-acetylglucosamine; SNA, sialic acid terminally linked α(2–6) to galactose or N-acetylgalactosamine; PNA, galactose-β(1–3)-N-acetylgalactosamine; GNA terminal linked mannose; and MAA, sialic acid terminally linked α(2–3) to galactose. Each BALP isoform was immobilized to a nitrocellulose membrane and thereafter incubated with the digoxigenin labeled lectins. The binding of each lectin to a particular carbohydrate structure was visualized with an ALP-labeled anti-digoxigenin antibody. The specificity of the lectin binding was tested with positive controls with known carbohydrate side-chains. Fetuin was a positive control for DSA and MAA, carboxypeptidase Y for GNA, transferrin for SNA, and asialofetuin for PNA.
The N-Glycosidase F deglycosylation kit was used to assess if the linked carbohydrates were N- or O-linked. Each BALP isoform was heat-denatured at 70°C, treated with N-Glycosidase F that cleaves N-linked glycan chains, and thereafter investigated with the DIG Glycan Differentiation kit with the same approach as for the untreated BALP isoforms.
Lectin precipitation
The BALP isoforms were precipitated by incubation with DSA, PNA and SNA. Duplicate aliquots of each BALP isoform at 100–300 U/L were incubated with graded doses of each lectin, in a total volume of 0.1 mL for 30 minutes at 37°C. After precipitation, the samples were centrifuged at 7500 × g for 10 minutes and the remaining BALP isoform activity was measured in the supernatant.
Results
Kinetic properties of the BALP isoforms
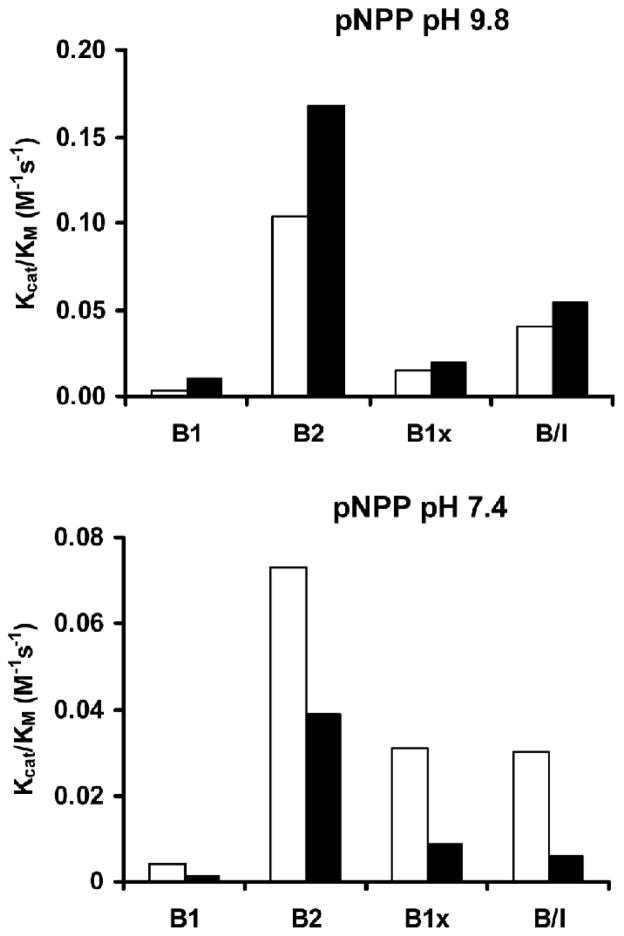
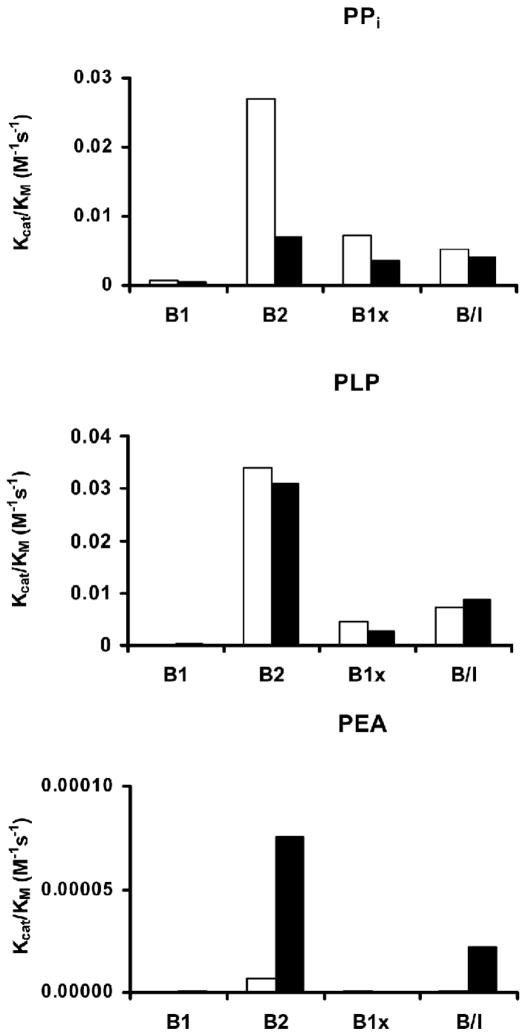
Significantly different catalytic properties were found among the purified human BALP isoforms. The kcat/KM was 35-fold and 18-fold higher for B2 in comparison with B1 for the substrate pNPP at pH 9.8 and pH 7.4, respectively (Fig. 1; Table 1). A similar trend was observed with the other substrates, i.e., kcat/KM was 38-fold higher for B2 in comparison with B1 with PPi, and 415-fold higher with PLP. The catalytic activity, using PEA as substrate, was barely detectable (Fig. 2; Table 1). Treatment with neuraminidase decreased the catalytic activity for all the investigated substrates, except for pNPP at pH 9.8.
Fig. 1.
Kcat/KM for the four BALP isoforms using the synthetic substrate pNPP at pH 9.8 and at 7.4. The BALP B2 isoform had 35-fold higher activity than the B1 isoform for pNPP at pH 9.8. The same trend was observed with pNPP at pH 7.4. The catalytic activities were higher at pH 9.8, but lower at pH 7.4, when the BALP isoforms were treated with neuraminidase. ■ = BALP isoforms treated with neuraminidase.
Table 1.
Catalytic activity for the BALP isoforms using different substrates
| BALP isoform | Vmax (M/min) | Kcat/KM (M−1s−1) | Kcat/KM (M−1s−1) neuraminidase | |
|---|---|---|---|---|
| pNPP pH 9.8 | ||||
| B1 | 0.123·10−6 | 0.003 | 0.010 | |
| B2 | 0.098·10−6 | 0.103 | 0.168 | |
| B1x | 0.079·10−6 | 0.015 | 0.019 | |
| B/I | 0.239·10−6 | 0.040 | 0.054 | |
| pNPP pH 7.4 | ||||
| B1 | 0.114·10−6 | 0.004 | 0.001 | |
| B2 | 0.116·10−6 | 0.073 | 0.039 | |
| B1x | 0.111·10−6 | 0.031 | 0.009 | |
| B/I | 0.208·10−6 | 0.030 | 0.006 | |
| PPi | ||||
| B1 | 4.71·10−6 | 0.001 | 0.0004 | |
| B2 | 1.26·10−6 | 0.027 | 0.0071 | |
| B1x | 6.96·10−6 | 0.007 | 0.0036 | |
| B/I | 4.98·10−6 | 0.005 | 0.0042 | |
| PLP | ||||
| B1 | 0.037·10−6 | 0.00008 | 0.0004 | |
| B2 | 0.713·10−6 | 0.03410 | 0.0310 | |
| B1x | 0.829·10−6 | 0.00450 | 0.0028 | |
| B/I | 1.030·10−6 | 0.00710 | 0.0088 | |
| PEA | ||||
| B1 | 6·10−10 | 0.1·10−6 | 1·10−6 | |
| B2 | 60·10−10 | 7.2·10−6 | 75·10−6 | |
| B1x | 9·10−10 | 0.6·10−6 | — | |
| B/I | 30·10−10 | 1.1·10−6 | 22·10−6 | |
Fig. 2.
Kcat/KM for the four BALP isoforms using the three suggested endogenous substrates: inorganic pyrophosphate (PPi), pyridoxal 5′-phosphate (PLP) and phosphoethanolamine (PEA) at pH 7.4. B2 had the highest activity for all the natural substrates. The catalytic activity for PEA was barely detectable for all four BALP isoforms. ■ = BALP isoforms treated with neuraminidase.
Kinetic properties of recombinant TNALP-flag and sALP-FcD10
TNALP-flag had higher catalytic activities than sALP-FcD10 for the substrates PPi (3.5-fold) and PLP (1.7-fold), while sALP-FcD10 had higher catalytic activity than TNALP-flag for pNPP at pH 9.8 (2.5-fold) and at pH 7.4 (6.9-fold). The catalytic activity for both TNALP-flag and sALP-FcD10 is comparable with the activity of the human BALP isoforms using pNPP at pH 9.8, as well as PPi and PLP at pH 7.4. The activity, using PEA as a substrate, was next to nothing for both TNALP-flag and sALP-FcD10 (Table 2).
Table 2.
Catalytic activity for the recombinant human isoforms TNALP-flag and sALP-FcD10 using different substrates
| Recombinant ALP forms | Vmax (M/min) | Kcat/KM (M−1s−1) | |
|---|---|---|---|
| pNPP pH 9.8 | TNALP-flag | 0.508·10−6 | 0.056 |
| sALP-FcD10 | 0.568·10−6 | 0.138 | |
| pNPP pH 7.4 | TNALP-flag | 0.043·10−6 | 0.0016 |
| sALP-FcD10 | 0.081·10−6 | 0.0111 | |
| PPi | TNALP-flag | 3.220·10−6 | 0.026 |
| sALP-FcD10 | 0.817·10−6 | 0.0074 | |
| PLP | TNALP-flag | 0.940·10−6 | 0.012 |
| sALP-FcD10 | 0.320·10−6 | 0.0070 | |
| PEA | TNALP-flag | - | 0.006·10−6 |
| sALP-FcD10 | 0.021·10−6 | 20·10−6 |
Glycosylation studies
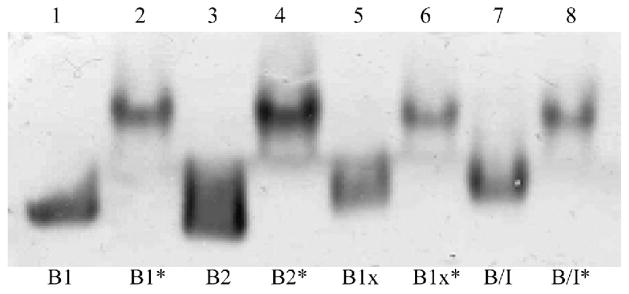
The BALP isoforms, B/I, B1x, B1 and B2, showed different migration patterns through native gels, which indicates that they differ with respect to charge and shape. However, all four BALP isoforms migrated more similar after treatment with neuraminidase, which indicates that the gel-migrating differences were due to terminal sialic acid residues (Fig. 3).
Fig. 3.
Migration of BALP isoforms in a native gel. B1, B2, B1x and B/I are untreated BALP isoforms and B1*, B2*, B1x* and B/I* are isoforms treated with neuraminidase prior analysis. The untreated isoforms had different migration patterns through the gel, but more similar migration patterns after treatment with neuraminidase.
Qualitative glycosylation patterns of the denatured BALP isoforms were investigated with the DIG Glycan Differentiation kit, which utilize five different lectins (i.e., GNA, SNA, DSA, PNA, and MAA). GNA, that bind terminal linked mannose, showed reactivity with B/I, and SNA that indicates sialic acid, terminally linked α(2–6) to galactose or N-acetylgalactosamine, and DSA that indicates galactose-β(1–4)-N-acetylglucosamine, had reactivity with B1 and B1x, but not with B2 and B/I. PNA that bind galactose-β(1–3)-N-acetylgalactosamine, showed reactivity only with B2, and MAA that indicates sialic acid terminally linked α(2–3) to galactose did not bind to any of the BALP isoforms (Table 3).
Table 3.
Specific binding of different lectins to denatured BALP isoforms in order to characterize different patterns of glycosylation
| BALP isoforms |
||||
|---|---|---|---|---|
| Lectins | B1 | B2 | B1x | B/I |
| DSA | +++ | — | +++ | — |
| SNA | +++ | — | +++ | — |
| PNA | — | (+) | — | — |
| GNA | — | — | — | + |
| MAA | — | — | — | — |
— no reaction, (+) weak reaction, + reaction, ++ strong reaction, +++ very strong reaction
None of the applied lectins had any reactivity towards the BALP isoforms after treatment with N-Glycosidase F, which indicates that all of the demonstrated lectin binding capacities of denatured BALP isoforms were due to N-linked oligosaccharides.
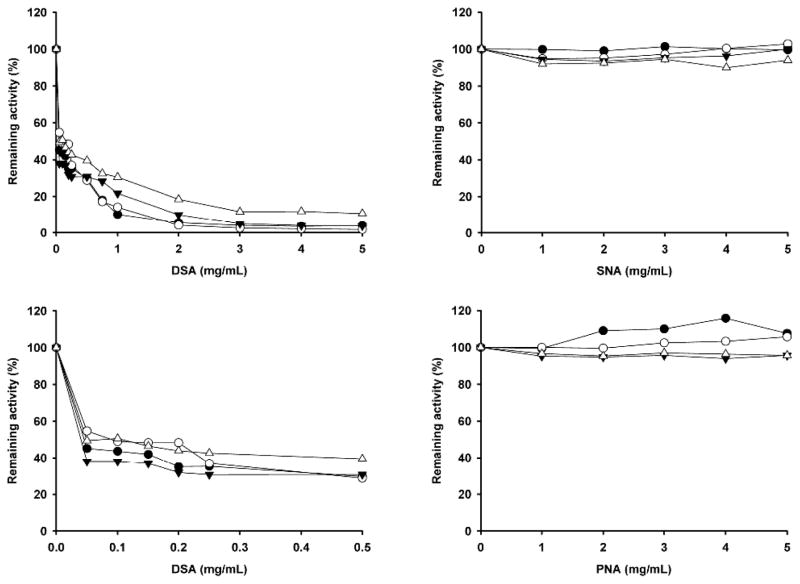
The effects of the dose-dependent lectin precipitation with DSA, SNA and PNA on native BALP isoforms are shown in Figure 4. DSA, which binds galactose-β(1–4)-N-acetylglucosamine, precipitated all four isoforms. SNA, that binds sialic acid, terminally linked α(2–6) to galactose or N-acetylgalactosamine and PNA, that binds galactose-β(1–3)-N-acetylgalactosamine, did not precipitate any of the four BALP isoforms at lectin concentrations of 0–5 mg/mL.
Fig. 4.
Dose-dependent lectin precipitation on native BALP isoforms: B1 (●), B2 (○), B1x (▼;), and B/I (△). Each data point is expressed as a mean of samples in duplicate. (A) DSA, 0–5 mg/mL; (B) DSA, 0–0.5 mg/mL; (C) SNA, 0–5 mg/mL; and (D) PNA, 0–5 mg/mL. DSA caused significant precipitation, even at low concentrations, for all four BALP isoforms, whereas no major precipitation was observed for SNA and PNA.
Discussion
Circulating ALP has been used for many years as a biochemical marker of bone turnover, specifically bone formation, and for monitoring the treatment of patients with metabolic bone disease. At least six different ALP isoform peaks can be separated and quantified by weak anion-exchange HPLC in serum from healthy individuals: three BALP isoforms (B/I, B1 and B2) and three liver ALP isoforms (L1, L2 and L3) [15, 16]. In healthy adults, the BALP isoforms, B/I, B1 and B2, account on average for 4, 16 and 37%, respectively, of the total serum ALP activity [19]. In serum, the minor fraction B/I is not a pure bone isoform as it co-elutes with the intestinal ALP isozyme and is composed, on average, of 70% bone and 30% intestinal ALP. The BALP isoforms differ with respect to sensitivity to precipitation with wheat germ lectin, i.e., B1 and B2 have more (or more reactive) sialic acid residues compared with B/I [31]. The reported differences in the content sialic acid differences result also in differences in molecular weights and the sialic acid residues present in BALP affect the immunoreactivity of monoclonal antibodies (MAbs) against bone ALP [33].
In this paper, we have extended the characterization of the glycosylation differences between these BALP isoforms. The isoforms did not show the same migration pattern in native gels, suggesting that they differ in charge and shape; however, they had the same migration pattern in native gels when the terminal sialic acid residues were removed with neuraminidase. In addition, the differences in lectin binding properties disappeared after treatment with N-Glycosidase F, which removes N-linked glycans. These observations confirm indeed that the core structure of the BALP isoforms is the same, in agreement with the fact that they result from the expression of a single ALPL gene locus, and that the isoforms differ only in posttranslational modifications [13]. The dose-dependent precipitation with the lectins DSA, SNA and PNA showed different results than the binding of the same lectins to the isoforms immobilized to the nitrocellulose membrane. The isoforms were native in the precipitation study and denatured when bound to the nitrocellulose membrane. The N-linked glycans are situated close to the active site of the BALP molecule [33]. In its native form, the protein structure prevents the lectin from binding, but when the protein is denatured the lectin can bind to the protein. The N-linked glycans plays a specific role in the catalytic activity and can be the reason to the different kinetic of the isoforms.
It is of interest, and of methodological importance, that we have previously evaluated the specificities and target epitopes of 19 monoclonal antibodies raised against BALP and found that some were capable of distinguishing to some extent between the BALP isoforms [33]. The epitope specificities of both of the monoclonal antibodies used in available commercial assays [34, 35] were grouped with those antibodies with specificity toward an antigenic domain close to the active site of BALP. Four of the 5 putative N-glycosylation sites are located within or in close proximity to this domain. Considering the results from the present study that the observed differences in lectin specificity were attributed to N-linked carbohydrates, it is not surprising that these MAbs work sufficiently well in the routine clinical laboratory setting for measurement of BALP in patients with metabolic bone disease.
Three endogenous compounds, i.e., PPi, PLP, and PEA, have been suggested as physiological substrates for BALP [1, 12]. Importantly, we found largely different kinetic properties of each of the BALP isoforms at physiological pH for the physiological substrates PPi and PLP. The B2 isoform had significantly higher kcat/KM values for all the investigated substrates. In human serum the activity of B2 is much higher than for B1. This difference in activity of the isoforms in the circulation can thus be explained by the isoforms different catalytic activity. The amount of B2 is less than the amount of B1 in serum; however, a decrease of B2 will influence the total ALP activity and show a bigger loss of activity than a decrease of B1. Different reaction kinetics for the BALP isoforms could result in differences in their ability to regulate the inhibitory levels of the substrate PPi. All isoforms showed extremely low activity with PEA. The kcat/KM with PEA was about 10,000 times lower than for PPi and about 800–5000 times lower than for PLP. This finding indicates that PEA is most likely not a physiological substrate of BALP [1, 12]. However, PEA could still be an endogenous substrate for other isoforms, e.g., liver ALP, also expressed from the TNALP locus. A study of patients with adult hypophosphatasia reported an inverse correlation of urinary levels of PEA and phosphoserine with residual liver ALP but not BALP [36]. Thus, it remains to be investigated if glycosylation differences in liver ALP may enhance the ability of those TNALP isoforms to catalyze PEA.
Some anatomical differences in the skeletal content of the BALP isoforms have been described in human femora. Cortical bone had about 2-fold higher activities of B1 compared with B2, and trabecular bone had about 2-fold higher activities of B2 compared with B1 [19, 22]. Ten-fold higher activities in serum have been reported for the BALP isoforms B1 and B2 during childhood and adolescence in comparison with adults [20]. The circulating levels of the BALP isoforms vary also independently during the pubertal growth spurt. Girls, 15–16 years, have higher levels than boys for the calculated ratio B2/B1 due to a more rapid decline of B2 compared with the BALP B1 isoform after puberty [20].
We deemed it important to compare the behavior of two commonly used recombinant TNALP isoforms with those of the BALP isoforms, B1, B2 and B1x, and B/I, to better understand how they relate to physiological in vivo circumstances. The two recombinant isoforms TNALP-flag and sALP-FcD10 showed similar catalytic activities as the human BALP isoforms for all the tested substrates. Therefore, the TNALP-flag and the sALP-FcD10 isoforms faithfully mimics the biological properties of the human BALP isoforms in vivo and further validates the use of these recombinant enzymes in studies aimed at dissecting the pathophysiology of hypophosphatasia. This sALP-FcD10 isoform has recently been used successfully for enzyme replacement therapy to prevent infantile hypophosphatasia in a murine model of the disease [27], and its use to treat advanced infantile hypophosphatasia and adult hypophosphatasia is under investigation. It is reassuring to see that the sALP-FcD10 properties resemble those of the endogenous human BALP isoforms.
In conclusion, the BALP isoforms have different catalytic properties due to structural differences in their posttranslational N-linked glycosylation. The B2 isoform had significantly higher kcat/KM values in comparison with the other BALP isoforms for all the investigated substrates. In addition, our results suggest also that PEA is not an endogenous substrate for the human BALP isoforms or for the recombinant isoforms. The kinetic activity for the recombinant isoform TNALP-flag, used extensively for mutation analysis of hypophosphatasia; and sALP-FcD10, a chimeric enzyme recently used as therapeutic drug in a mouse model of hypophosphatasia, is comparable with the human BALP isoforms. Future investigations will focus on the clinical significance and functional mechanisms of the revealed molecular differences between the BALP isoforms, which ultimately could advance our understanding of bone cell biology and skeletal mineralization.
Acknowledgments
This study was supported by grants from the Swedish Research Council, the Swedish Society of Medicine, and the County Council of Östergötland and grant DE012889 from the National Institutes of Health, USA. We thank Philippe Crine at Enobia Pharma for the generous contribution of the recombinant BALP isoform sALP-FcD10.
This work was supported by grants from the Swedish Research Council, the Swedish Society of Medicine, and the County Council of Östergötland, and grant DE012889 from the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439–61. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 2.Narisawa S, Fröhlander N, Millán JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–46. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millán JL, MacGregor GR, Whyte MP. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–26. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millán JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper NM. Glycosyl-phosphatidylinositol anchored membrane enzymes. Clin Chim Acta. 1997;266:3–12. doi: 10.1016/s0009-8981(97)00161-7. [DOI] [PubMed] [Google Scholar]
- 6.Fedde KN, Lane CC, Whyte MP. Alkaline phosphatase is an ectoenzyme that acts on micromolar concentrations of natural substrates at physiologic pH in human osteosarcoma (SAOS-2) cells. Arch Biochem Biophys. 1988;264:400–9. doi: 10.1016/0003-9861(88)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HC, Reynolds JJ. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vesicles and their role in calcification. Dev Biol. 1973;34:211–27. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]
- 8.Fedde KN. Human osteosarcoma cells spontaneously release matrix-vesicle-like structures with the capacity to mineralize. Bone Miner. 1992;17:145–51. doi: 10.1016/0169-6009(92)90726-t. [DOI] [PubMed] [Google Scholar]
- 9.Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millan JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–7. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anh DJ, Dimai HP, Hall SL, Farley JR. Skeletal alkaline phosphatase activity is primarily released from human osteoblasts in an insoluble form, and the net release is inhibited by calcium and skeletal growth factors. Calcif Tissue Int. 1998;62:332–40. doi: 10.1007/s002239900441. [DOI] [PubMed] [Google Scholar]
- 11.Anh DJ, Eden A, Farley JR. Quantitation of soluble and skeletal alkaline phosphatase, and insoluble alkaline phosphatase anchor-hydrolase activities in human serum. Clin Chim Acta. 2001;311:137–48. doi: 10.1016/s0009-8981(01)00584-8. [DOI] [PubMed] [Google Scholar]
- 12.Whyte MP, Landt M, Ryan LM, Mulivor RA, Henthorn PS, Fedde KN, Mahuren JD, Coburn SP. Alkaline phosphatase: placental and tissue-nonspecific isoenzymes hydrolyze phosphoethanolamine, inorganic pyrophosphate, and pyridoxal 5′-phosphate. Substrate accumulation in carriers of hypophosphatasia corrects during pregnancy. J Clin Invest. 1995;95:1440–5. doi: 10.1172/JCI117814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millán JL. From biology to applications in medicine and biotechnology. Weinheim, Germany: WILEY-VCH Verlag GmgH & Co. KGaA; 2006. Mammalian alkaline phosphatase. [Google Scholar]
- 14.Magnusson P, Degerblad M, Sääf M, Larsson L, Thorén M. Different responses of bone alkaline phosphatase isoforms during recombinant insulin-like growth factor-I (IGF-I) and during growth hormone therapy in adults with growth hormone deficiency. J Bone Miner Res. 1997;12:210–20. doi: 10.1359/jbmr.1997.12.2.210. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson P, Löfman O, Larsson L. Determination of alkaline phosphatase isoenzymes in serum by high-performance liquid chromatography with post-column reaction detection. J Chromatogr. 1992;576:79–86. doi: 10.1016/0378-4347(92)80177-r. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson P, Löfman O, Larsson L. Methodological aspects on separation and reaction conditions of bone and liver alkaline phosphatase isoform analysis by high-performance liquid chromatography. Anal Biochem. 1993;211:156–63. doi: 10.1006/abio.1993.1247. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson P, Sharp CA, Magnusson M, Risteli J, Davie MW, Larsson L. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001;60:257–65. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 18.Swolin-Eide D, Hansson S, Larsson L, Magnusson P. The novel bone alkaline phosphatase B1x isoform in children with kidney disease. Pediatr Nephrol. 2006;21:1723–9. doi: 10.1007/s00467-006-0231-2. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson P, Larsson L, Magnusson M, Davie MW, Sharp CA. Isoforms of bone alkaline phosphatase: characterization and origin in human trabecular and cortical bone. J Bone Miner Res. 1999;14:1926–33. doi: 10.1359/jbmr.1999.14.11.1926. [DOI] [PubMed] [Google Scholar]
- 20.Magnusson P, Häger A, Larsson L. Serum osteocalcin and bone and liver alkaline phosphatase isoforms in healthy children and adolescents. Pediatr Res. 1995;38:955–61. doi: 10.1203/00006450-199512000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson P, Larsson L, Englund G, Larsson B, Strang P, Selin-Sjögren L. Differences of bone alkaline phosphatase isoforms in metastatic bone disease and discrepant effects of clodronate on different skeletal sites indicated by the location of pain. Clin Chem. 1998;44:1621–8. [PubMed] [Google Scholar]
- 22.Magnusson P, Sharp CA, Farley JR. Different distributions of human bone alkaline phosphatase isoforms in serum and bone tissue extracts. Clin Chim Acta. 2002;325:59–70. doi: 10.1016/s0009-8981(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 23.Carr SA, Huddleston MJ, Bean MF. Selective identification and differentiation of N- and O-linked oligosaccharides in glycoproteins by liquid chromatography-mass spectrometry. Protein Sci. 1993;2:183–96. doi: 10.1002/pro.5560020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Nosjean O, Koyama I, Goseki M, Roux B, Komoda T. Human tissue non-specific alkaline phosphatases: sugar-moiety-induced enzymic and antigenic modulations and genetic aspects. Biochem J. 1997;321 ( Pt 2):297–303. doi: 10.1042/bj3210297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Mauro S, Manes T, Hessle L, Kozlenkov A, Pizauro JM, Hoylaerts MF, Millan JL. Kinetic characterization of hypophosphatasia mutations with physiological substrates. J Bone Miner Res. 2002;17:1383–91. doi: 10.1359/jbmr.2002.17.8.1383. [DOI] [PubMed] [Google Scholar]
- 27.Millán JL, Narisawa S, Lemire I, Loisel TP, Boileau G, Leonard P, Gramatikova S, Terkeltaub R, Pleshko Camacho N, McKee MD, Crine P, Whyte MP. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farley JR, Kyeyune-Nyombi E, Tarbaux NM, Hall SL, Strong DD. Alkaline phosphatase activity from human osteosarcoma cell line SaOS-2: an isoenzyme standard for quantifying skeletal alkaline phosphatase activity in serum. Clin Chem. 1989;35:223–9. [PubMed] [Google Scholar]
- 29.Farley JR, Hall SL, Herring S, Tarbaux NM, Matsuyama T, Wergedal JE. Skeletal alkaline phosphatase specific activity is an index of the osteoblastic phenotype in subpopulations of the human osteosarcoma cell line SaOS-2. Metabolism. 1991;40:664–71. doi: 10.1016/0026-0495(91)90081-7. [DOI] [PubMed] [Google Scholar]
- 30.Rea DW, Ultee ME, Chen SX, Loisel TP. Solutions for purification of Fc-fusion proteins. BioPharm Int. 2008 March;(Suppl):20–25. [Google Scholar]
- 31.Magnusson P, Farley JR. Differences in sialic acid residues among bone alkaline phosphatase isoforms: a physical, biochemical, and immunological characterization. Calcif Tissue Int. 2002;71:508–18. doi: 10.1007/s00223-001-1137-4. [DOI] [PubMed] [Google Scholar]
- 32.Heinonen JK, Lahti RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981;113:313–7. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- 33.Magnusson P, Ärlestig L, Paus E, Di Mauro S, Testa MP, Stigbrand T, Farley JR, Nustad K, Millán JL. Monoclonal antibodies against tissue-nonspecific alkaline phosphatase. Report of the ISOBM TD9 workshop. Tumour Biol. 2002;23:228–48. doi: 10.1159/000067254. [DOI] [PubMed] [Google Scholar]
- 34.Gomez B, Jr, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, Kung VT. Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem. 1995;41:1560–6. [PubMed] [Google Scholar]
- 35.Broyles DL, Nielsen RG, Bussett EM, Lu WD, Mizrahi IA, Nunnelly PA, Ngo TA, Noell J, Christenson RH, Kress BC. Analytical and clinical performance characteristics of Tandem-MP Ostase, a new immunoassay for serum bone alkaline phosphatase. Clin Chem. 1998;44:2139–47. [PubMed] [Google Scholar]
- 36.Millán JL, Whyte MP, Avioli LV, Fishman WH. Hypophosphatasia (adult form): quantitation of serum alkaline phosphatase isoenzyme activity in a large kindred. Clin Chem. 1980;26:840–5. [PubMed] [Google Scholar]