Abstract
Background
Hemodialysis patients have a steady increase in blood pressure (BP) over the 44-hour interdialytic interval when ambulatory BP monitoring is used. Home BP recording allows for longer period of monitoring between dialysis and may better define the chronobiology of arterial hypertension. This study sought to determine the optimal time to perform home BP monitoring in hemodialysis patients to improve the strength of prediction of 44-hour interdialytic ambulatory BP.
Study Design
Diagnostic test study
Setting and Participants
This is an ancillary analysis of patients participating in the dry-weight reduction in hypertensive hemodialysis patients (DRIP) trial.
Index test
Home BP measured three times a day for one week using a validated oscillometric monitor on 3 occasions at 4 week intervals after randomization. Home BP measured during the first third, second third and last third of time elapsed after dialysis as well as each third of dialysis was compared to the overall ambulatory BP.
Reference Tests
Interdialytic ambulatory BP measured on 3 occasions at 4 week intervals after randomization.
Results
Over the interdialytic interval, we found an increase in systolic ambulatory BP of 0.30 ± 0.36 mmHg/hr and an increase in systolic home BP of 0.40 ± 0.25 mmHg/hr. This relationship in home BP reached a plateau after approximately 48 hrs. A similar pattern was seen for diastolic home BP. Probing dry-weight steepened the slope of ambulatory BP but did not alter the time-dependent relationship of home BP. Home BP was on average higher (bias) by 14.1 (95% CI 12.0 to 16.2)/5.7 (95% CI 4.6 to 6.9) mmHg. The standard deviation of differences between methods (precision) was 4.6/2.8 mmHg. Measurement of BP during each third of the interdialytic interval gave the best precision as measured by model fit compared to ambulatory BP measurements.
Limitations
Our cohort was overrepresented by African American hemodialysis patients. Whether African American participants have a different pattern of BP response than non-African American participants in the interdialytic period is not known.
Conclusions
Our findings suggest that time elapsed after dialysis must to be considered in interpreting the home BP recordings in hemodialysis patients. Home BP measured in each third of the interdialytic interval is likely to yield the most reliable BP estimate.
Keywords: Home BP, ambulatory BP monitoring, hemodialysis, hypertension, chronobiology
Hypertension is prevalent in hemodi alysis patients and is an important cardiovascular risk factor 1,2. Diagnosis of hypertension in these patients can be facilitated by home blood pressure (BP) monitoring 3-7. In fact, current clinical guidelines call for monitoring home BP for the diagnosis and management of all patients with hypertension 8,9.
The timing of home BP measurement is of increasing interest because of the recognition that a surge in BP in the morning hours may be related to worse cardiovascular outcomes 10. Unlike in patients with intact kidneys, ambulatory BP monitoring in hemodialysis patients demonstrates a linear increase in BP in the interdialytic period. For practical reasons, ambulatory BP monitoring in dialysis patients is often limited to the 44-hour interdialytic period and is cumbersome to perform. Self-recorded home BP can allow measurements over the 3 day dialysis free interval which can potentially disclose novel patterns of BP changes. The existence of time-dependent increase in BP and its pattern of change may have substantial impact on the optimal timing of home BP measurements in these patients. We hypothesized that timing of home BP measurement in relationship to end of dialysis treatment may influence the assessment of overall BP burden in hemodialysis patients.
Accordingly, the purpose of this study was to define the relationship of BP and time elapsed from the end of dialysis. If present, we sought to determine whether timing of home BP measurements can influence agreement with interdialytic ambulatory BP. These relationships were then used to define the optimal timing of home BP measurement in long-term hemodialysis patients.
Methods
Simultaneous home and ambulatory BP monitoring was performed in all patients participating in the dry-weight reduction in hypertensive hemodialysis patients (DRIP) trial 11. We recruited patients 18 years of age or older on long-term hemodialysis for at least 3 months, who had hypertension defined as mean interdialytic ambulatory BP of 135/85 mm Hg or more. After a six hemodialysis run-in phase, during which baseline data were collected, patients were randomized in 1:2 proportion into control group vs. ultrafiltration trial group for 8 weeks. Pre and post BP and weights were averaged over these 6 treatment run-in phase. During this 24 dialysis treatment phase, patients were seen at each dialysis visit and had dry-weight probed as assessed by symptoms and signs related to hypovolemia12,13. Home BP measurements and ambulatory recordings were collected over a two week period. In week 1 home BP was collected and in week 2 ambulatory or vice versa.
The detailed methods of this trial have been previously published 11. Briefly, the ultrafiltration group underwent an additional weight loss of 0.1kg/10 kg body-weight per dialysis without increasing the time or frequency of dialysis. This additional weight loss was combined with the ultrafiltration volume required to remove interdialytic weight gain to achieve the desired reduction in dry weight. If ultrafiltration was not tolerated based on symptoms and signs such as muscle cramps, need for excessive saline or symptomatic hypotension, the additional prescribed weight loss was reduced by 50%. If ultrafiltration was still not tolerated, the additional weight loss was further reduced by 50% till even 0.2 kg incremental weight loss per dialysis was not tolerated. At this point, the patient was said to be at his or her dry weight. Thus, by this protocol, each patient had to experience symptoms of volume depletion to be at dry-weight. The control group had regular physician visits but no additional reduction in dry-weight. No changes in antihypertensive medication were permitted during the trial.
The results showed a significant difference between randomized groups in change from baseline in weight and ambulatory BP at 4 weeks and 8 weeks. In the intervention group, postdialysis weight was reduced by 0.9 kg at 4 weeks and resulted in −6.9 mm Hg (95% CI: −12.4 to −1.3 mm Hg; P=0.02) greater change in systolic ambulatory BP and −3.1 mm Hg (95% CI: −6.2 to −0.02 mm Hg; P=0.048) greater change in diastolic ambulatory BP. At 8 weeks, dry weight was reduced 1 kg more, systolic ambulatory BP changed −6.6 mm Hg (95% CI: −12.2 to −1.0 mm Hg; P=0.02) more, and diastolic ambulatory BP changed −3.3 mm Hg (95% CI: −6.4 to −0.2 mm Hg; P=0.04) more from baseline in the ultrafiltration group. The Mantel-Hanzel combined odds ratio for systolic BP reduction of > or =10 mm Hg was 2.24 (95% CI: 1.32 to 3.81; P=0.003).
Blood Pressure Monitoring
Ambulatory BP monitoring was performed after the mid-week hemodialysis session for 44 hours at baseline, 4 weeks and 8 weeks. Blood pressures were recorded every 20 minutes during the day (6 AM to 10 PM) and every 30 minutes during the night (10 PM to 6 AM) using a Spacelab 90207 ABP monitor (SpaceLabs Medical Inc, Redmond, WA, USA) in the non-access arm. Recordings began immediately after hemodialysis and terminated immediately before the subsequent dialysis. Accuracy of ambulatory BP recordings was confirmed against auscultated blood pressure at baseline. Hourly means were calculated. These means were then averaged over the entire course of recording to provide systolic and diastolic interdialytic ambulatory blood pressures. The mean interdialytic ambulatory BP of both the ultrafiltration and control groups combined served as the reference test.
Home BP monitoring was performed three times daily for one week at baseline, week 4 and week 8. Patients were asked to record their BP in the morning, afternoon and before going to bed with a validated home BP monitor equipped with a memory device (HEM 705CP, Omron Healthcare, Bannockburn, IL).
The study protocol was approved by the Institutional Review Boards (IRB) and the VA Research and Development Committee and all patients provided written informed consent.
Statistical Analysis
Data were captured an entered into a custom-designed database prospectively. End of dialysis therapy was used as the start time for home BP recording. Time passed since the end of dialysis was during which home BP was measured was divided into thirds. For those home BP readings for which the exact end of dialysis time was not available, we imputed an approximate interval based on the days and shift on which the patient was dialyzed, then computed the interval or hours passed based on that approximate interval. 171 of 3094 readings (5.5%) were imputed in this manner.
We fitted a random coefficient mixed effects model to determine the change in home BP and ambulatory BP over time 14. Specifically, we used the following model to describe changes in home BP: yit = πoi + π1iait + εit, where yit is the home BP for the ith individual (i=1,...,N) at the tth measurement occasion (t=1,...,T), πoi, is the intercept for the ith individual, π1i is the slope for the ith individual, ait represents the value of time for the ith individual at the tth measurement occasion, and εit is the error for the ith individual at the tth measurement occasion. εit was assumed to distribute normally and independently with a mean of zero and const ant variance across time. An unstructured covariance matrix was used to allow for intercepts and slopes to be determined by the data. The unstructured covariance matrix allows the slopes and intercepts to vary independently from each other. Six indicator variables were generated for calendar time (with 3 levels: baseline, week 4, week 8) and treatment allocation (2 levels: UF vs control) and another six by interacting these indicator variables with time elapsed from end of dialysis. These 12 indicator variables were used as fixed effects in a mixed model to determine the influence of calendar time and treatment on slopes and intercepts of home BP recordings. To make head-to-head comparisons, a similar model was fitted for the ambulatory BP data. Night time ambulatory BP measurements were not excluded from these calculations.
Non-linear relationship of time and BP were tested using restricted cubic splines 15. Specifically, we place 5 knots at 0.05, 0.275, 0.5, 0.725 and 0.95 quantiles of the time variable and then tested the covariates for linearity on BP, the response variable. Probing dry-weight did not alter the time-dependent relationship of home BP. Thus the ultrafiltration and control groups were combined to describe the chronobiology of home BP changes.
To determine the optimal time to measure home BP we created averages for each individual for 3 categories of home BP measurements: home BP taken during the first 1/3 of time elapsed since end of dialysis (0 to 14.67 h), home BP obtained during the second 1/3 of time elapsed since end of dialysis (14.67 to 29.33 h) and home BP obtained during the last 1/3 of time elapsed since end of dialysis (29.33 to 44 h) and we compared these to overall home BP (0 to 44h). Home BP measurements after 44 hours after the end of dialysis were not used in this analysis. We compared agreement between home BP recordings made during each 1/3 of dialysis and overall home BP with 44-hour interdialytic ambulatory BP using the likelihood ratio test in nested models. When models were not nested, we tested model fits using the Bayesian information criterion (BIC). Lower the BIC better is the model fit. The absolute difference between models can be used to grade the evidence according to the following criteria: absolute difference 0−2 (weak), 2−6 (positive), 7−10 (strong) and >10 (very strong) 16.
The nominal level of significance was set at two sided p of <0.05 and all statistical analyses were performed with Stata version 10.1 (StataCorp LP, College Station, TX).
Results
The baseline characteristics of 142 patients who had simultaneous home and ambulatory BP recordings are shown in Table 1. The remaining 8 patients had missing home BP recordings. The patients were mostly African American given the demographics of our dialysis units. Most patients were on antihypertensive medications.
Table 1.
Clinical characteristics of the study population
| Clinical Characteristic | n=142 |
|---|---|
| Age (years) | 54.2 ± 12.7 |
| Men | 98 (69%) |
| Race | |
| White | 14 (10%) |
| African American | 124 (87%) |
| Other | 4 (3%) |
| Pre-dialysis BP (mmHg) | 159.7 ± 16.1/86.6 ± 11.2 |
| Post-dialysis BP | 143.0 ± 18.2/77.9 ± 11.2 |
| Pre-dialysis weight (kg) | 84.5 ± 20.0 |
| Post-dialysis weight (kg) | 81.5 ± 19.4 |
| Body Mass Index (kg/m2) | 27.3 ± 6.1 |
| Years of dialysis | 4.1 ± 5.2 |
| Etiology of end-stage renal disease | |
| Diabetes Mellitus | 55 (39%) |
| Hypertension | 68 (48%) |
| Glomerulonephritis | 5 (4%) |
| Polycystic Kidney Disease | 3 (2%) |
| Other | 11 (8%) |
| Current Smoker | 47 (33%) |
| History of | |
| Congestive Heart Failure | 20 (14%) |
| Myocardial Infarction | 19 (13%) |
| Stroke | 13 (9%) |
| Urea reduction ratio | 73.9 ± 6.7 |
| Albumin (g/dl) | 3.7 ± 0.4 |
| Hemoglobin (g/dl) | 12.1 ± 1.2 |
| Antihypertensive medications | 119 (84%) |
| Number of BP medications in users | 2.6 ± 1.4 |
± indicates standard deviation. Parentheses have percent of patients.
Note: Conversion factors for units: urea nitrogen in mg/dL to mmol/L, ×0.357; albumin and hemoglobin in g/dL to g/L, ×10
Table 2 shows the mean intercept and slopes of ambulatory BP in the randomized groups. Overall, the intercept ambulatory systolic BP was 134.8 ± 14.8 mmHg with a slope of 0.30 ± 0.36 mmHg/hr and ambulatory diastolic BP was 78.0 ± 11.2 mmHg with a slope of 0.11 ± 0.18 mmHg/hr. Significant changes from baseline in intercept BP and slopes of BP in the ultrafiltration group at 4 and 8 weeks compared to control group were noted. Thus, probing dry-weight steepened the increase in systolic and diastolic ambulatory BP over time.
Table 2.
Modeled Slopes and Intercepts of Ambulatory BP over the 48-hour interdialytic interval
| Systolic BP | Diastolic BP | |||||
|---|---|---|---|---|---|---|
| BP Component | Baseline | 4 week time point | 8 week time point | Baseline | 4 week time point | 8 week time point |
| Intercept | ||||||
| Number of readings control group (±SD) | 90 ± 23 | 87 ± 24 | 81 ± 22 | 90 ± 23 | 87 ± 24 | 81 ± 22 |
| Control group BP (mmHg; baseline and change from baseline) | 142.4 | −2.9‡ | −3.0‡ | 80.5 | −1.6† | −1.3* |
| 95% CI | 138.1 to 146.6 | −4.4 to −1.4 | −4.5 to −1.4 | 78.2 to 84.8 | −2.7 to −0.6 | −2.3 to −0.3 |
| Number of readings ultrafiltration group (±SD) | 90 ± 22 | 89 ± 21 | 80 ± 26 | 90 ± 22 | 89 ± 21 | 80 ± 26 |
| Ultrafiltration group BP (mmHg; baseline and change from baseline) | 140.6 | −14.1‡ | −14.2‡ | 81.5 | −7.7‡ | −8.1‡ |
| 95% CI | 137.7 to 143.5 | −15.1 to −13.0 | −15.3 to −13.2 | 79.3 to 83.8 | −8.4 to −7.0 | −8.9 to −7.4 |
| Difference in BP between groups (UF-Control) (mmHg) | −1.8 | −11.2 | −11.3 | 0.03 | −6.0 | −6.8 |
| 95% CI | −6.9 to 0.5 | −13.0 to −9.4 | −13.2 to −9.4 | −3.9 to 4.0 | −7.3 to −4.8 | −8.1 to −5.6 |
| p value of difference between groups | 0.5 | <0.001 | <0.001 | 1.0 | <0.001 | <0.001 |
| Slopes | ||||||
| Control group (mmHg/hr; baseline and change from baseline) | 0.26 | 0.03 | −0.08* | 0.10 | .00 | −0.06† |
| 95% CI | 0.15 to 0.38 | −0.03 to 0.09 | −0.14 to −0.01 | 0.04 to 0.16 | −0.05 to 0.04 | −0.11 to −0.02 |
| Ultrafiltration group (mmHg/hr; baseline and change from baseline) | 0.22 | 0.19‡ | 0.12‡ | 0.06 | 0.10‡ | 0.09‡ |
| 95% CI | 0.14 to 0.30 | 0.15 to 0.24 | 0.07 to 0.16 | 0.02 to 0.10 | 0.07 to 0.13 | 0.06 to 0.12 |
| Difference between groups (UF-Control) (mmHg/hr) | −0.04 | 0.16 | 0.20 | −0.04 | 0.10 | 0.15 |
| 95% CI | −0.18 to 0.09 | 0.08 to 0.24 | 0.12 to 0.28 | −0.11 to 0.03 | 0.05 to 0.15 | 0.09 to 0.20 |
| p value of difference between groups | 0.5 | <0.001 | <0.001 | 0.3 | <0.001 | <0.001 |
0.01<p<0.05
0.001<p<0.01
p<0.001. Night time BP recordings were not excluded.
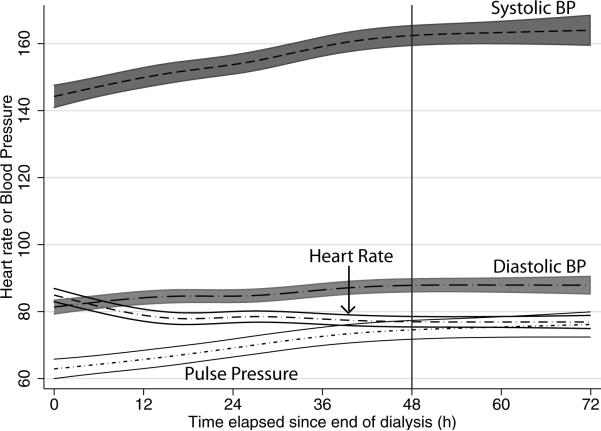
Figure 1 shows that the relationship of BP over time was nonlinear. There were 861 recordings between 44−72 hours in 140 patients. After approximately 2 days, the growth curve in systolic BP plateaued. Since systolic BP increased at a more rapid rate than diastolic BP, there was amplification of pulse pressure over time. Consistent with increase in BP, there was slowing noted in the heart rate.
Figure 1.
Non-linear relationships of home blood pressure and heart rate over an interdialytic interval modeled using restricted cubic splines. After about 48 hours (vertical line) the changes in home BP and heart rate plateau. Pulse pressure is amplified over time between dialysis treatments.
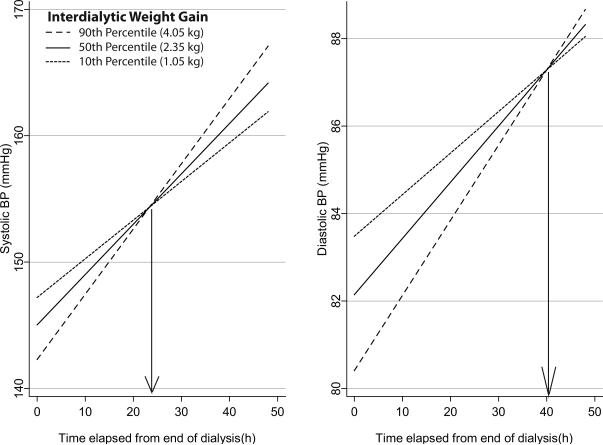
Since we were interested in evaluating the linear changes in hemodynamics we excluded recordings obtained after 48 hours from end of dialysis. We found that the intercept (SD) of systolic BP was 145.0 (18.3) and the linear slope was 0.40 (0.25) mmHg/hr. Over the two day interdialytic period, median weight gain was 2.35 kg, 10th percentile weight gain was 1.05 kg and 90th percentile weight gain was 4.03 kg. Each kg weight gain caused the intercept BP to change by −1.6 mmHg (95% CI −2.9 mmHg to −0.4 mmHg) and the linear slope by 0.07 mmHg/hr (95% CI 0.23 to 0.12). Figure 2 shows the effect of interdialytic weight gain on the interdialytic BP. Those who gained more weight had a lower intercept but steeper slopes. At approximately 24 hours after the end of dialysis the impact of interdialytic weight gain on home BP was seen to be minimal. For diastolic BP, however, the effect of weight gain was seen to be minimized approximately 40 hours after the end of dialysis.
Figure 2.
Effect of interdialytic weight gain on home BP changes. The weight gain category was interacted with the fixed effect of time. Home BP rises 0.4 mmHg/hour systolic and 0.13 mmHg/hour diastolic in the interdialytic period in those who gain median weight (2.35 kg over 2 days). In those who gain more weight the intercept systolic BP is lower (by 1.6 mmHg/kg systolic and 1.0 mmHg/kg diastolic) but the rate of rise is steeper (by 0.07 mmHg/hr/kg systolic and 0.025 mmHg/hr/kg diastolic). In those who gain less weight have a slower rate of rise in interdialytic BP. The point where BP is least influenced by weight gain is about 24 hours for systolic and 40 hours of diastolic BP. Thus, sampling home BP over each third of time elapsed after dialysis will give the most reliable estimates of interdialytic ambulatory BP.
Table 3 shows the mean intercept and slopes of systolic home BP in the randomized groups. Over the interdialytic interval, we found an increase in systolic home BP of 0.40 ± 0.25 mmHg/hr. Although the change from baseline in intercept home BP in the ultrafiltration group at 8 weeks was significant compared to the change from baseline in the control group at 8 weeks, no changes in slopes was noted. The interaction term noted as difference between groups on slopes was no different from zero. Thus, we found that probing dry-weight did not perturb the extent of increase in systolic home BP over time.
Table 3.
Slopes and Intercepts of Home BP over the 48-hour interdialytic interval
| Systolic BP | Diastolic BP | |||||
|---|---|---|---|---|---|---|
| BP Component | Baseline | 4 week time point | 8 week time point | Baseline | 4 week time point | 8 week time point |
| Intercept | ||||||
| Number of readings control group (±SD) | 11 ± 4 | 13 ± 4 | 13 ± 4 | 11 ± 4 | 13 ± 4 | 13 ± 4 |
| Control group (mmHg; baseline and change from baseline) | 153.1 | −5.0 | −1.4 | 84.4 | −0.7 | 0.2 |
| 95% CI | 147.3 to 158.9 | −9.5 to −0.4 | −6.0 to 3.3 | 80.7 to 88.1 | −3.1 to 1.8 | −2.4 to 2.7 |
| Number of readings ultrafiltration group (±SD) | 11 ± 4 | 13 ± 4 | 12 ± 4 | 11 ± 4 | 13 ± 4 | 12 ± 4 |
| Ultrafiltration group (mmHg; baseline and change from baseline) | 149.4 | −8.2† | −13.3‡ | 84.6 | −4.2† | −6.5† |
| 95% CI | 145.4 to 153.4 | −11.4 to −4.9 | −16.6 to −10.1 | 82.1 to 87.1 | −5.9 to −2.4 | −8.3 to −4.8 |
| Difference between groups (UF-Control) (mmHg) | −3.7 | −3.2 | −12.0* | 0.2 | −3.5* | −6.7‡ |
| 95% CI | −10.7 to 3.4 | −8.7 to 2.4 | −17.7 to −6.3 | −4.2 to 4.7 | −6.5 to −0.5 | −9.8 to −3.6 |
| p value of difference between groups | 0.3 | 0.3 | <0.001 | 0.9 | 0.02 | <0.001 |
| Slopes | ||||||
| Control group (mmHg/hr; baseline and change from baseline) | 0.27 | 0.09 | 0.05 | 0.12 | 0.00 | 0.02 |
| 95% CI | 0.12 to 0.41 | −0.08 to 0.26 | −0.12 to 0.23 | 0.04 to 0.19 | −0.10 to 0.08 | −0.08 to 0.11 |
| Ultrafiltration group (mmHg/hr; baseline and change from baseline) | 0.37 | 0.07 | 0.08 | 0.10 | 0.05 | 0.04 |
| 95% CI | 0.27 to 0.47 | −0.05 to 0.19 | −0.04 to 0.20 | 0.05 to 0.16 | −0.01 to 0.11 | −0.03 to 0.10 |
| Difference between groups (UF-Control) (mmHg/hr) | 0.10 | −0.02 | 0.03 | −0.01 | 0.06 | 0.02 |
| 95% CI | −0.07 to 0.27 | −0.23 to 0.19 | −0.19 to 0.24 | −0.10 to 0.08 | −0.06 to 0.17 | −0.09 to 0.14 |
| p value of difference between groups | 0.2 | 0.8 | 0.8 | 0.8 | 0.3 | 0.7 |
0.01<p<0.05
0.001<p<0.01
p<0.001
To test the notion, whether timing of home BP measurement would influence the accuracy and precision of ambulatory BP measurements, we calculated average home BP recorded in the first third, middle third and last third of dialysis. Table 4 shows that the average systolic and diastolic BP increased over time from a low of 147.3/82.4 mmHg in the approximately the first 15 hours after dialysis to a high of 161.3/87.5 mmHg in the 15 hours preceding the next dialysis. The overall home BP was calculated as the mean of the mean for each third of the inter-dialytic period. The middle third most closely approximated the overall home BP and ambulatory BP for systolic BP. The model fits as assessed by Bayesian Information Criterion were best for the overall home BP followed by BP recordings made in the middle of the interdialytic interval for systolic BP. A similar pattern was seen for diastolic BP. In the case of diastolic BP, as for systolic BP, Bayesian Information Criterion indicated the best model for when home BP was sampled in each third of the interdialytic interval followed by BP recordings made in the last third of the interdialytic interval.
Table 4.
Timing of Home BP Measurement and Prediction of Ambulatory BP
| Home BP Timing in relation to end of dialysis | |||||
|---|---|---|---|---|---|
| Parameter | 44-h Ambulatory BP | First 1/3 | Second 1/3 | Last 1/3 | Overall |
| Systolic BP | |||||
| BP (mmHg) | 139.7 | 148.2 | 152.8 | 161.2 | 153.8 |
| 95% CI | 137.7 to 141.7 | 145.1 to 151.3 | 150.0 to 155.6 | 158.2 to 164.1 | 151.0 to 156.6 |
| Mean (ambulatory-home) | −7.9 | −13.2 | −21.6 | −14.1 | |
| 95% CI of diff (ABP-home) | −10.5 to −5.3 | −15.4 to −11.0 | −23.9 to −19.2 | −16.2 to −12.0 | |
| SD between patients | 12.3 | 11.6 | 11.7 | 11.8 | |
| SD between methods (ABP, home) | 5.2 | 4.8 | 5.7 | 4.6 | |
| SD residual | 13.8 | 13.0 | 13.0 | 12.2 | |
| - Log likelihood | −3101.0 | −3057.2 | −3063.5 | −3019.1 | |
| BIC | 6235.0 | 6147.3 | 6159.9 | 6071.1 | |
| Order of model fit compared to ambulatory | 4 | 2 | 3 | 1 | |
| Diastolic BP | |||||
| BP (mmHg) | 79.3 | 82.9 | 84.7 | 87.4 | 84.9 |
| 95% CI | 77.5 to 81.1 | 80.9 to 84.8 | 82.8 to 86.6 | 85.5 to 89.4 | 83.0 to 86.8 |
| Mean (ambulatory-home) | −3.7 | −5.5 | −8.3 | −5.7 | |
| 95% CI of diff (ABP-home) | −5.1 to −2.3 | −6.7 to −4.4 | −9.5 to −7.1 | −6.9 to −4.6 | |
| SD between patients | 9.9 | 10.1 | 10.2 | 10.1 | |
| SD between methods (ABP, home) | 3.5 | 2.5 | 2.7 | 2.8 | |
| SD residual | 7.6 | 6.9 | 6.8 | 6.3 | |
| - Log likelihood | −2716.0 | −2650.9 | −2646.3 | −2606.8 | |
| BIC | 5465.0 | 5334.8 | 5325.6 | 5246.7 | |
| Order of model fit compared to ambulatory | 4 | 3 | 2 | 1 | |
| BIC = Bayesian information criterion. The strength of evidence for differences between model fits was very strong | |||||
Discussion
The main findings of the present study are: (1) home BP increases over time after hemodialysis treatment; (2) this relationship is non-linear and plateaus after approximately 2 days; (3) probing dry-weight does not perturb the time-dependent relationship of home BP but steepens the slope for ambulatory BP; (4) increase in interdialytic weight gain reduces the intercept BP but steepens the slope of BP changes; (5); the timing of home BP monitoring influences the accuracy and precision of the measurements and that (6) time elapsed after dialysis must to be considered in interpreting the home BP recordings in hemodialysis patients.
The increase in BP in the interdialytic period has been well recognized 17,18. However, these studies are limited by evaluating BP only before and after dialysis. Given that there are measurements at only two points in any given patient, the pattern of BP change is not possible to describe. We have, using ambulatory BP monitoring, demonstrated that the linear increase in BP is superimposed on a circadian variation in BP 19. This study extends the findings of the previous study through repeated measurements over longer interdialytic intervals that span the weekend. Ambulatory BP monitoring is typically not utilized over 3 days and our study makes the novel observation that the BP rise plateaus after the first two days. This may be due to accumulation of salt and water and uremic toxins that may stretch the limits of increase in BP. Our data suggests that the increase in interdialytic BP may not simply reflect salt and water accumulation during the interdialytic period. We found that the average interdialytic slope of 0.40 mmHg/hr is changed by 0.07 mmHg/hr with each kg increase in interdialytic weight gain. Since the average weight gain we noted was 2.35 kg over the interdialytic period, adjusting for the weight gain such than a zero weight gain occurs in the interdialytic interval does not reduce the rate of rise in BP to zero. This suggests accumulation of uremic factors (such as inhibitors of nitric oxide synthase, asymmetric dimethyl arginine) may play a role in non-volume mediated increase in BP 20. Furthermore, as shown in Figure 1, the increase in diastolic BP did not match the increase in systolic pressure with may lead to a detrimental amplification of pulse pressure over the interdialytic interval—a phenomenon we have also observed with ambulatory BP monitoring 21.
Since increase in interdialytic weight also evokes greater removal of intradialytic weight, it is little surprise that those who gain more weight also have a lower postdialysis BP. Our model suggests that approximately 24 hours of dialysis the BP reaches a point of fulcrum (Figure 2) which may be the most opportune time to measure systolic home recordings. At this time, the magnitude of interdialytic weight gain would have the least influence on BP recordings. Indeed when we divided the home BP recordings in thirds in relationship to the end of dialysis, we found that the middle third of the interdialytic period was associated with the greatest agreement in BP with ambulatory BP monitoring for systolic BP. The point of fulcrum for diastolic BP was achieved in the last third of the interdialytic interval (Figure 2). Accordingly, the last third of the interdialytic interval had the best agreement with the ambulatory BP. However, when BP was recorded in each third of the interdialytic interval, it gave the best precision in predicting ambulatory BP. Accordingly, in patients on hemodialysis, the timing of measurement in relation to hemodialysis may have substantial implications for interpreting the results.
Probing dry-weight did not alter the time-dependent relationship of home BP. Systolic BP is the major problem in hemodialysis patients 22,23. Thus, if limited home BP monitoring was to be performed, timing the measurements between 18−30 hours after completion of dialysis would still give reliable results over time. The changes in home BP suggest that reduction in arterial pressure evoked by probing dry-weight was caused by reduction in intercept pressure which is in contrast to change in slope of arterial pressure associated with increasing number of antihypertensive drugs. In contrast to home BP, probing dry-weight steepened the slope of ambulatory BP. The greater number of recordings including those obtained at night may have facilitated the detection of steepened slope of BP change during the interdialytic period with ambulatory BP monitoring.
Ambulatory BP is a cumbersome technique and not well suited for the day-to-day management of hypertension. In particular, ambulatory BP is particularly difficult to use over the 3 day period spanning the weekend in patients undergoing conventional three times a week hemodialysis. Our data provides evidence that home BP agrees well with ambulatory BP provided we can sample BP over the entire interdialytic interval.
A limitation of our study is that it was overrepresented by African American hemodialysis patients. Whether African American participants have a different pattern of BP response than non-African American participants in the interdialytic period is not known. However, greater representation by non-African American participants would have made the study more generalizable. Whether the improvement in precision in our study was simply due to averaging a greater number of BP recordings was not tested, but in a prior study we have shown that simply increasing the number of BP measurements does not increase the precision of these recordings 24. Home BP measurements and ambulatory BP recordings were made in different weeks for practical reasons. Perhaps simultaneous home and ambulatory BP recordings (if this were technically possible) may have further increased the precision of home BP recordings. A strength of our study was the use of validated home BP monitor which was equipped with a memory device and all participants were instructed in its use.
The chronobiology of arterial pressure changes clarified for the first time by careful home BP monitoring over the entire interdialytic interval has substantial clinical implications. The time-dependent amplification of pulse pressure suggests that hemodialysis patients may accumulate a potent additional cardiovascular risk factor over the interdialytic interval which would be most pronounced over the period spanning the weekend 25. The lack of impact of dry-weight reduction on slope of interdialytic BP changes suggests that antihypertensive drugs which are associated with a blunted interdialytic slope 19 may have an important and synergistic role in the treatment of hypertension in these patients. These data suggest that home BP monitoring should include each third of the interdialytic interval; however if this is not possible, the period surrounding 24 hours after the end of dialysis may provide the most stable BP recordings. Home BP monitoring can thus be valuable for the diagnosis and management of hypertension in hemodialysis patients 6. Implementation of home BP monitoring for the diagnosis and management of hypertension can begin to address the control of this important cardiovascular risk factor in hemodialysis patients 2.
Acknowledgements
We are indebted to the participating hemodialysis patients who volunteered their time; staff of the dialysis units at Dialysis Clinics, Inc, Clarian Health, and the Roudebush VA Medical Center; the faculty of the Division of Nephrology for allowing us to study their patients; the research technicians, research fellows and to the members of the Data and Safety Monitoring Board.
Support: This work was supported by grant 5RO1-NIDDK062030-05 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None.
REFERENCES
- 1.Agarwal R, Nissenson AR, Batlle D, et al. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R. Hypertension and survival in chronic hemodialysis patients-Past lessons and future opportunities. Kidney Int. 2005;67:1–13. doi: 10.1111/j.1523-1755.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Andersen MJ, Bishu K, et al. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69:900–906. doi: 10.1038/sj.ki.5000145. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Brim NJ, Mahenthiran J, et al. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 5.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R. How should hypertension be assessed and managed in hemodialysis patients? Home BP, not dialysis unit BP, should be used for managing hypertension. Semin Dial. 2007;20:402–405. doi: 10.1111/j.1525-139X.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R. Hypertension diagnosis and prognosis in chronic kidney disease with out-of-office blood pressure monitoring. Curr Opin Nephrol Hypertens. 2006;15:309–313. doi: 10.1097/01.mnh.0000222700.14960.18. [DOI] [PubMed] [Google Scholar]
- 8.Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 10.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Alborzi P, Satyan S, et al. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charra B, Laurent G, Chazot C, et al. Clinical assessment of dry weight. Nephrol Dial Transplant 11 Suppl. 1996;2:16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10:392–403. doi: 10.1681/ASN.V102392. [DOI] [PubMed] [Google Scholar]
- 14.Holden JE, Kelley K, Agarwal R. Analyzing Change: A Primer on Multilevel Models with Applications to Nephrology. Am J Nephrol. 2008;28:792–801. doi: 10.1159/000131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr., Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 16.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press; Oxford: 2003. [Google Scholar]
- 17.Inrig JK, Patel UD, Gillespie BS, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;50:108–18. 118. doi: 10.1053/j.ajkd.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leypoldt JK, Cheung AK, Delmez JA, et al. Relationship between volume status and blood pressure during chronic hemodialysis. Kidney Int. 2002;61:266–275. doi: 10.1046/j.1523-1755.2002.00099.x. [DOI] [PubMed] [Google Scholar]
- 19.Kelley K, Light RP, Agarwal R. Trended cosinor change model for analyzing hemodynamic rhythm patterns in hemodialysis patients. Hypertension. 2007;50:143–150. doi: 10.1161/HYPERTENSIONAHA.107.091579. [DOI] [PubMed] [Google Scholar]
- 20.Zoccali C, Mallamaci F, Maas R, et al. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int. 2002;62:339–345. doi: 10.1046/j.1523-1755.2002.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Light RP. Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol. 2007;294:F303–F308. doi: 10.1152/ajprenal.00575.2007. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R. Systolic hypertension in hemodialysis patients. Semin Dial. 2003;16:208–213. doi: 10.1046/j.1525-139x.2003.16041.x. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Lewis RR. Prediction of hypertension in chronic hemodialysis patients. Kidney Int. 2001;60:1982–1989. doi: 10.1046/j.1523-1755.2001.00997.x. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Andersen MJ, Light RP. Location Not Quantity of Blood Pressure Measurements Predicts Mortality in Hemodialysis Patients. Am J Nephrol. 2007;28:210–217. doi: 10.1159/000110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klassen PS, Lowrie EG, Reddan DN, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]