Immunosuppressive drugs have been widely used to control severe cases of ocular inflammation. Azathioprine—a purine nucleoside analog which acts as an antimetabolite by interfering with DNA and RNA synthesis—is one of the immunosuppressive drugs recommended for this purpose.1 Azathioprine is approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis,2 and also has been widely used for organ transplantation3, and various dermatologic4, gastrointestinal5, and rheumatologic diseases including psoriatic arthritis6 and systemic lupus erythematosus.7 For ophthalmic diseases, azathioprine has been used for treatment of corneal graft rejection8 and non-infectious ocular inflammatory conditions,1 such as chronic active iridocyclitis,9 retinal vasculitis,10 Behçet's disease,11, 12 and sympathetic ophthalmia.13 It also has been used in combination with other immunosuppressive agents for serpiginous retinochoroiditis.14 Randomized clinical trials data are limited to use of azathioprine for Behçet's disease11, 12 and a small trial evaluating its use for anterior uveitis.9
The Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study15 includes information regarding the outcomes of a large number of ocular inflammation patients managed at tertiary ocular inflammation centers in the United States using a variety of agents, including azathioprine. In this report, we evaluate the incidence of successful control of inflammation, of corticosteroid-sparing benefits, and of treatment-related complications leading to discontinuation of therapy in patients from the cohort treated with azathioprine as a sole (non-corticosteroid) immunosuppressive agent, followed from the initiation of azathioprine therapy.
METHODS
Data Collection
The methods of the SITE Cohort Study already have been published.15 In brief, all patients with non-infectious ocular inflammatory disease managed at four ocular inflammation subspecialty centers were identified by a comprehensive chart review at four centers, and an approximate 40% random sample was identified at a fifth center. However, data from one center were excluded from this analysis because of its co-management approach–many follow-up observations were missing since they were made by local ophthalmologists and patients were less likely to return if treatment succeeded than if it failed, both of which biased ascertainment of the time-to-occurrence of the primary outcomes of this analysis. The ocular inflammatory diseases included were uveitis, scleritis, mucous membrane pemphigoid (MMP) with ocular involvement, and other less common conditions such as inflammatory orbital disease, peripheral ulcerative keratitis, and autoimmune cicatricial keratitis. Patients with HIV infection and those with infectious ocular inflammation were excluded.
Data for every eye of every patient at every visit were entered into a database by study-trained and -certified expert chart reviewers. Demographic and clinical data recorded included date of birth, sex, race, follow-up time, ocular inflammatory disease diagnosis, associated systemic inflammatory disease diagnoses (when applicable), and previous use of immunosuppressive treatments. Visual acuity, intraocular pressure, and slit-lamp biomicroscopic and ophthalmoscopic findings indicating inflammatory disease status and the presence or absence of complications of inflammation were noted for each eye with an ocular inflammatory diagnosis at each visit. Real-time quality control systems, site visiting, and data quality queries were implemented in order to maximize data quality.
With regards to the retrospective assessment of inflammatory activity at each visit, an overall assessment of activity was used, given that no single clinical finding could establish inflammatory status for all the kinds of ocular inflammation studied. Patients were graded as “inactive” when the chart indicated that there was no inflammatory activity on that visit, as represented by terms such as “quiet”, “quiescent”, “no cells”, and “no active inflammation”. “Slightly active” was chosen when the record indicated minimally active disease, described by words such as, “mild”, “slightly”, “few”, or “trace”. For patients with uveitis, if “occasional” or “rare” cells were recorded without other indications that the examining clinician thought the disease was “active” or “slightly active”, an “inactive” grade was recorded. An “active” grade was applied when statements were recorded indicating signs of active inflammation, including anterior chamber cells of 1+ or higher, vitreous haze of 1+ or more, or the terms “active”, “worsening inflammation”, or “disease progression”. When none of these could be ascertained, data regarding activity at the visit in question were regarded as “missing.” Grades of anterior chamber and vitreous cells and of vitreous haze were recorded in a manner as similar as possible in a retrospective study to the systems adopted by the Standardization of Uveitis Nomenclature Group.16
Patients
Among the 8,562 patients included in the SITE database, all patients starting azathioprine “monotherapy” (no other corticosteroid-sparing immunosuppressive agents1) for non-infectious ocular inflammation during follow-up were identified. Use of topical, periocular, and oral corticosteroids was common in this cohort of patients and the ability to taper oral corticosteroids while maintaining control of inflammation serve as a primary outcome measure of treatment success (see below). Because patients were only included in this analysis if they initiated azathioprine monotherapy during observation, ocular complications prior to initiation of azathioprine therapy and previous use of immunosuppressive drugs were available in the database.
Outcomes Measurements
Each patient's experience was followed at the centers from the initiation of azathioprine until azathioprine was discontinued, an additional immunosuppressive agent was added, the patient stopped attending a SITE clinic, or the study data collection ended, whichever happened first. Treatment success in terms of control of inflammation was evaluated among patients with “active” or “slightly active” disease at the time of initiation of azathioprine monotherapy by the time-to-sustained improvement of global disease activity to the “inactive” level over at least 2 visits spanning at least 28 days. This evaluation was done without regard to use of corticosteroids, which were used according to best medical judgment. Time-to-sustained improvement of global disease activity from “active” to either “slightly active” or “inactive” level also was evaluated as a sensitivity analysis. Treatment success in achieving corticosteroid-sparing benefits was evaluated by the time-to-successful tapering of prednisone to ≤10 mg, ≤5 mg, and 0 mg daily while maintaining control of inflammation over at least 2 visits spanning at least 28 days. Corticosteroid-sparing outcomes were assessed among the patients who did not meet each criterion for corticosteroid-sparing success at the outset of treatment. Prednisone-equivalent doses of alternative systemic corticosteroids were used when needed. The time-to-discontinuation of azathioprine therapy and reasons for discontinuation of azathioprine therapy also were evaluated.
Statistics
Frequency distributions of variables for the study population were tabulated. Time-to-event outcomes and incidence rates were calculated using survival analysis methods17 in by-person and by-eye analyses, among patients free of the outcome at the time of initiation of azathioprine therapy. In the primary analyses, outcomes were not accepted until they had been observed for a minimum of 2 visits spanning at least 28 days, to avoid counting transient improvements and brief interruptions of therapy as successes or failures. However, time-to-the first observation of a success or failure outcome (with no requirement that the success continue to be observed over time) was calculated as a sensitivity analysis, given that some patients may have obtained durable benefits of therapy at their last visit. Multiple regression analysis using the Cox proportional hazards model7 was applied to determine adjusted hazard ratios for age, gender, race, type of inflammation, previous treatment, azathioprine dosage, and presence or absence of extraocular immune-mediated disease. By-eye Cox regression analyses incorporated methods to adjust for non-independence of eyes of the same patient.18 All statistical analyses used SAS version 9.1 (SAS Corporation, Cary, North Carolina).
RESULTS
Baseline characteristics of the study subjects
Two hundred and nine patients with ocular inflammatory diseases (2.4% of the total SITE Cohort) initiated azathioprine as a single (non-corticosteroid) immunosuppressive agent during observation. Of the 209 patients, 64 patients from the center employing a co-management approach were excluded so as to avoid underascertainment of outcomes (see above); the remaining 145 patients were included for data analysis in this study (Table 1, see also Supplemental Table, available at www.ajo.com). The median age at initiation of therapy was 50.6 years. Female (68%) and Caucasian (77%) patients formed the majority of the cohort. Ninety-one patients (63%) had uveitis, 16 patients (11%) had scleritis, 33 patients (23%) had MMP, and five patients (3%) had other diagnoses (three with peripheral ulcerative keratitis and two with orbital inflammation). Approximately 76% of patients had bilateral disease. Among the patients with uveitis, 21 (23%) had anterior uveitis, 18 (20%) had intermediate uveitis, and 52 (57%) had posterior uveitis or panuveitis. The median duration of inflammation prior to azathioprine treatment was 1.8 years (interquartile range, 0.51-5.33 years). At the inception of azathioprine therapy, 48% of patients were receiving systemic prednisone > 10 mg daily. Regarding the global activity of inflammation among affected eyes at the time of starting azathioprine, 64% were inactive, 10% were slightly active, and 26% were active.
Table 1.
Characteristics of Patients with Ocular Inflammatory Diseases Upon Initiation of Azathioprine, by Type of Inflammation
| Characteristics | Anterior Uveitis |
Intermediate Uveitis |
Posterior or Panuveitis |
Scleritis | Mucous Membrane Pemphigoid |
Other Ocular Inflammatory Diseases |
Total |
|---|---|---|---|---|---|---|---|
| Person-specific characteristics | |||||||
| Number of patients | 21 | 18 | 52 | 16 | 33 | 5 | 145 |
| Median age, years (range) | 40.5 (9.2 - 70.8) |
44.4 (19.6 - 68.3) |
46.6 (17.5 - 78.8) |
53.9 (24.4 - 65.6) |
70.8 (24.9 - 86.4) |
42.6 (28.8 - 61.1) |
50.6 (9.2 - 86.4) |
| Females | 14 (66.7%) | 13 (72.2%) | 35 (67.3%) | 12 (75.0%) | 20 (60.6%) | 4 (80.0%) | 98 (67.6%) |
| Race: Caucasian | 16 (76.2%) | 16 (88.9%) | 33 (63.5%) | 11 (68.8%) | 31 (93.9%) | 4 (80.0%) | 111 (76.6%) |
| African-American | 4 (19.0%) | 1 (5.6%) | 9 (17.3%) | 4 (25.0%) | 0 (0.0%) | 1 (20.0%) | 19 (13.1%) |
| Other | 1 (4.8%) | 1 (5.6%) | 10 (19.2%) | 1 (6.3%) | 2 (6.1%) | 0 (0.0%) | 15 (10.3%) |
| Bilateral inflammation | 14 (66.7%) | 13 (72.2%) | 38 (73.1%) | 11 (68.8%) | 31 (93.9%) | 3 (60.0%) | 110 (75.9%) |
| Steroid dose > 10mg daily | 10 (47.6%) | 5 (27.8%) | 29 (55.8%) | 14 (87.5%) | 7 (21.2%) | 5 (100.0%) | 70 (48.3%) |
| Prior azathioprine | 1 (4.8%) | 2 (11.1%) | 9 (17.3%) | 0 (0.0%) | 3 (9.1%) | 0 (0.0%) | 15 (10.3%) |
| Prior antimetabolites (Other than azathioprine) |
3 (14.3%) | 4 (22.2%) | 7 (13.5%) | 3 (18.8%) | 3 (9.1%) | 2 (40.0%) | 22 (15.2%) |
| Prior alkylating agents | 0 (0.0%) | 0 (0.0%) | 4 (7.7%) | 1 (6.3%) | 8 (24.2%) | 2 (40.0%) | 15 (10.3%) |
| Prior T-cell inhibitors | 3 (14.3%) | 8 (44.4%) | 17 (32.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 28 (19.3%) |
| Prior biologics | 0 (0.0%) | 0 (0.0%) | 2 (3.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.4%) |
| Any prior immunosuppressive drug* |
5 (23.8%) | 9 (50.0%) | 25 (48.1%) | 4 (25.0%) | 13 (39.4%) | 2 (40.0%) | 58 (40.0%) |
| Eye-specific characteristics | |||||||
| Number of affected eyes | 35 | 31 | 90 | 27 | 64 | 8 | 255 |
| VA 20/50 or worse | 18 (51.4%) | 15 (48.4%) | 50 (55.6%) | 1 (3.7%) | 34 (53.1%) | 4 (50.0%) | 122 (47.8%) |
| VA 20/200 or worse | 11 (31.4%) | 7 (22.6%) | 22 (24.4%) | 1 (3.7%) | 18 (28.1%) | 1 (12.5%) | 60 (23.5%) |
| Ocular complications, affected eyes, % |
21 (60.0%) | 20 (64.5%) | 49 (54.4%) | 2 (7.4%) | 9 (14.1%) | 1 (12.5%) | 102 (40.0%) |
| Overall activity (affect eye, %) |
|||||||
| - Inactive | 18 (51.4%) | 28 (90.3%) | 67 (74.4%) | 14 (51.9%) | 33 (51.6%) | 3 (37.5%) | 163 (63.9%) |
| - Slightly active | 4 (11.4%) | 1 (3.2%) | 10 (11.1%) | 1 (3.7%) | 8 (12.5%) | 2 (25.0%) | 26 (10.2%) |
| - Active | 12 (34.3%) | 2 (6.5%) | 13 (14.4%) | 12 (44.4%) | 23 (35.9%) | 3 (37.5%) | 65 (25.5%) |
| - Missing | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) |
non-corticosteroid immunosuppressives only
Note: The denominator in each of these was variable depending availability of data collection at outset of azathioprine therapy.
Therapeutic Outcomes
Among patients with active inflammation at the initiation of treatment, the Kaplan-Meier estimate of the proportion with sustained control of inflammation for at least 28 days at or before 6 months was 41% (95% confidence interval (CI), 31-52%), and was 62% (95% CI, 50-74%) at or before 12 months of azathioprine therapy (Table 2). Liberalizing the definition of success to include patients improving to either “slightly active” or “inactive”, an estimated 57% (95% CI, 46-68%) met this success criterion within six months, and 73% (95% CI, 61-83%) within one year. Omitting the requirement that control be sustained, the proportion of initially active patients whose inflammation was “inactive” at any visit within the first 6 months of therapy also was 57% (95% CI, 46-68%), indicating that up to 16% had non-sustained improvement during this period.
Table 2.
Kaplan-Meier Estimates of Time-to-Treatment Success Among Patients with Ocular Inflammatory Diseases Treated with Azathioprine*
| Outcomes | Anterior Uveitis | Intermediate Uveitis |
Posterior or Panuveitis | Scleritis | Mucous Membrane Pemphigoid |
Other Ocular Inflammatory Diseases |
Total |
|---|---|---|---|---|---|---|---|
| Outcomes By Six Months of Therapy | |||||||
| No. of patients treated with azathioprine monotherapy |
21 | 18 | 52 | 16 | 33 | 5 | 145 |
| Controlled inflammation - no activity (%, 95% CI) |
23.7 (9.6 - 51.7) | 69.3 (41.4 - 92.7) | 44.2 (28.2 - 64.2) | 20.0 (3.1 - 79.6) | 43.5 (25.8 - 66.4) | 33.3 (5.5 - 94.6) | 40.8 (31.1 - 52.1) |
| Improved inflammation- to either slightly active or inactive (%, 95% CI) |
28.2 (11.6 - 58.9) | 87.5 (57.7 - 99.3) | 72.0 (51.8 - 89.2) | 29.9 (10.5 - 67.7) | 59.3 (39.7 - 79.8) | 33.3 (5.5 - 94.6) | 56.8 (45.7 - 68.5) |
| Controlled inflammation and prednisone dose - ≤ 10 mg/day (%, 95% CI) |
16.6 (5.6 - 43.2) | 47.0 (22.7 - 79.1) | 36.3 (22.3 - 55.1) | 22.2 (6.1 - 63.5) | 39.5 (22.5 - 62.8) | 20.0 (3.1 - 79.6) | 32.2 (23.8 - 42.5) |
| Controlled inflammation and prednisone dose - ≤ 5 mg/day (%, 95% CI) |
11.5 (3.0 - 38.6) | 29.9 (12.3 - 61.5) | 16.6 (7.8 - 33.3) | 18.2 (4.9 - 55.3) | 31.5 (16.2 - 55.5) | 0.0 | 20.6 (13.9 - 29.9) |
| Controlled inflammation and prednisone dose - 0 mg/day (%, 95% CI) |
0.0 | 0.0 | 7.1 (2.4 - 20.5) | 0.0 | 11.8 (3.9 - 32.6) | 0.0 | 5.2 (2.4 - 11.3) |
| Outcomes By 12 Months of Therapy | |||||||
| Controlled inflammation - no activity (%, 95% CI) |
34.6 (15.2 - 66.7) | 89.8 (63.6 - 99.4) | 59.7 (40.9 - 79.3) | 73.3 (31.4 - 99.0) | 66.7 (45.6 - 86.3) | 33.3 (5.5 - 94.6) | 62.2 (50.5 – 74.0) |
| Improved inflammation- to either slightly active or inactive (%, 95% CI) |
42.6 (18.7 - 77.4) | 100.0 | 83.2 (63.8 - 95.7) | 76.6 (37.3 - 98.9) | 71.0 (50.5 - 88.6) | 33.3 (5.5 - 94.6) | 72.7 (60.9 - 83.3) |
| Controlled inflammation and prednisone dose - ≤ 10 mg/day (%, 95% CI) |
24.9 (9.8 - 54.8) | 68.2 (40.2 - 92.2) | 44.0 (28.6 - 63.2) | 35.2 (12.8 - 74.7) | 62.0 (41.3 - 82.7) | 46.7 (13.7 - 93.2) | 46.9 (36.9 - 58.0) |
| Controlled inflammation and prednisone dose - ≤ 5 mg/day (%, 95% CI) |
19.5 (6.6 – 50.0) | 64.9 (34.2 - 92.7) | 42.9 (27.1 - 62.9) | 29.9 (10.5 - 67.7) | 48.3 (29.1 - 71.8) | 0.0 | 40.6 (30.8 - 52.2) |
| Controlled inflammation and prednisone dose - 0 mg/day (%, 95% CI) |
8.3 (1.2 - 46.1) | 0.0 | 9.9 (3.8 - 24.3) | 11.1 (1.6 - 56.7) | 17.0 (6.6 - 39.8) | 0.0 | 9.5 (5.2 - 17.1) |
95% confidence intervals are not available for estimates of 0% or 100%. Control of inflammation had to be observed across at least 2 visits spanning 28 days in order to be counted.
Success in achieving complete inactivity of inflammation sustained for at least 28 days varied by the site of ocular inflammation. Approximately 69% (95% CI, 41-93%) of patients with active intermediate uveitis had control of their ocular inflammation within 6 months of treatment, versus 44 % (95% CI, 28-64%) of posterior or panuveitis patients, 43% (95% CI, 26-66%) of MMP patients, 24% (95% CI, 10-52%) of anterior uveitis patients, and 20% (95% CI, 3-80%) of scleritis patients.
Regarding the corticosteroid-sparing benefits of azathioprine, among patients initially receiving >10 mg of prednisone daily, an estimated 32% (95% CI, 24-43%), 21% (95% CI, 14-30%), and 5% (95% CI, 2-11%) succeeded in tapering their prednisone dose to ≤ 10 mg, ≤ 5 mg and 0 mg respectively within the first six months of therapy while maintaining control of inflammation across successive visits spanning at least 28 days. These results continued to improve after six months of therapy, with 46.9 (95% CI, 36.9 - 58.0), 40.6 (95% CI, 30.8 - 52.2), and 9.5 (95% CI, 5.2 - 17.1) respectively achieving these outcomes with 12 months of therapy. Corticosteroid-sparing success by six months was highest for intermediate uveitis (47%; 95% CI, 23-79%), followed by MMP (39%; 95% CI, 23-63%), posterior or panuveitis (36%; 95% CI, 22-55%), scleritis (22%; 95% CI, 6-64%), other ocular inflammatory diseases (20%; 95% CI, 3-80%) and anterior uveitis (17%; 95% CI, 6-43%). Among those who had required oral corticosteroids at the beginning of azathioprine therapy, only a small number of patients with MMP (12%; 95% CI, 4-33%) and posterior or panuveitis (7%; 95% CI, 2-21%) succeeded at completely discontinuing prednisone while maintaining sustained control of inflammation for at least 28 days. No patients in the other groups were observed to successfully discontinue prednisone while maintaining sustained control of inflammation.
Table 3 gives the crude and adjusted hazard ratios for demographic and clinical characteristics evaluated as potential risk or protective factors. Males tended to gain treatment success less often than females, after adjusting for other factors. No consistent patterns of variation in treatment success across age and racial groups were observed.
Table 3.
Factors Associated with Successful Azathioprine Therapy Among Patients with Ocular Inflammatory Diseases*
| Characteristics | Conditions | Crude HR Controlled Inflammation, Inactive (95% CI) |
Adjusted HR Controlled Inflammation, Inactive (95% CI) |
Crude HR Improved Inflammation, Inactive or Slightly Active (95% CI) |
Adjusted HR Improved Inflammation, Inactive or Slightly Active (95% CI) |
Crude HR Corticosteroid-Sparing Success, ≤ 10 mg Prednisone/day (95% CI) |
Adjusted HR Corticosteroid-Sparing Success, ≤ 10 mg Prednisone/day (95% CI) |
|---|---|---|---|---|---|---|---|
| Sex | Male (vs Female) | 0.71 (0.42 - 1.20) | 0.47 (0.24 - 0.93) | 0.66 (0.39 - 1.11) | 0.55 (0.31 - 0.98) | 0.72 (0.42 - 1.24) | 0.42 (0.22 - 0.80) |
| Race | Caucasian | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| African-American | 0.99 (0.48 - 2.04) | 0.70 (0.24 - 1.99) | 1.05 (0.54 - 2.06) | 0.81 (0.25 - 2.65) | 0.93 (0.51 - 1.68) | 0.56 (0.20 - 1.62) | |
| Other | 0.98 (0.55 - 1.75) | 0.81 (0.34 - 1.95) | 0.96 (0.52 - 1.78) | 0.72 (0.31 - 1.67) | 1.57 (0.89 - 2.77) | 1.62 (0.68 - 3.85) | |
| Age | <18 years | 0.26 (0.02 - 2.80) | 0.69 (0.04 - 11.80) | 0.37 (0.06 - 2.18) | 1.17 (0.21 - 6.59) | 0.43 (0.03 - 5.69) | 1.32 (0.04 - 47.33) |
| 18-39 years | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 40-54 years | 1.40 (0.71 - 2.78) | 1.41 (0.61 - 3.25) | 1.33 (0.65 - 2.69) | 1.63 (0.75 - 3.52) | 2.88 (1.39 - 5.96) | 2.42 (0.93 - 6.31) | |
| 55-64 years | 0.98 (0.43 - 2.26) | 0.97 (0.32 - 2.93) | 0.83 (0.39 - 1.77) | 0.95 (0.32 - 2.79) | 2.59 (1.06 - 6.30) | 2.35 (0.79 - 6.94) | |
| 65 years or more | 1.40 (0.73 - 2.68) | 1.28 (0.50 - 3.30) | 1.04 (0.57 - 1.89) | 1.41 (0.56 - 3.57) | 3.61 (1.67 - 7.80) | 3.13 (1.09 - 8.98) | |
| Type of inflammation |
Anterior uveitis | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Intermediate uveitis | 3.73 (1.04 - 13.36) | 3.44 (0.87 - 13.71) | 6.19 (1.86 - 20.61) | 4.03 (0.85 - 19.00) | 4.09 (1.23 - 13.58) | 4.75 (1.01 - 22.29) | |
| Posterior/panuveitis | 2.08 (0.71 - 6.06) | 1.92 (0.53 - 6.94) | 2.48 (0.85 - 7.25) | 1.54 (0.43 - 5.56) | 2.25 (0.81 - 6.23) | 2.52 (0.64 - 9.86) | |
| Scleritis | 0.80 (0.23 - 2.86) | 0.51 (0.12 - 2.06) | 1.16 (0.29 - 4.71) | 0.71 (0.15 - 3.29) | 0.90 (0.25 - 3.26) | 0.62 (0.16 - 2.40) | |
| Mucous membrane pemphigoid |
2.33 (0.75 - 7.23) | 1.91 (0.56 - 6.48) | 1.90 (0.63 - 5.75) | 1.83 (0.58 - 5.75) | 3.35 (1.14 - 9.79) | 2.61 (0.72 - 9.46) | |
| Other ocular inflammatory diseases |
0.95 (0.15 - 6.23) | 1.08 (0.15 - 7.90) | 0.58 (0.12 - 2.89) | 0.36 (0.04 - 3.33) | 1.29 (0.17 - 9.71) | 1.54 (0.16 - 15.22) | |
| Previous Immunosuppressive therapy |
Azathioprine | 0.60 (0.20 - 1.75) | 0.95 (0.27 - 3.36) | 0.74 (0.36 - 1.54) | 0.71 (0.30 - 1.67) | 0.51 (0.26 - 1.00) | 0.84 (0.22 - 3.10) |
| Other antimetabolite(s) | 0.42 (0.19 - 0.94) | 0.32 (0.09 - 1.13) | 0.42 (0.18 - 0.99) | 0.33 (0.12 - 0.91) | 0.56 (0.25 - 1.25) | 0.29 (0.05 - 1.62) | |
| T-cell inhibitor(s) | 0.57 (0.30 - 1.10) | 0.34 (0.12 - 0.94) | 1.11 (0.63 - 1.98) | 0.91 (0.35 - 2.35) | 0.45 (0.22 - 0.92) | 0.37 (0.12 - 1.17) | |
| Alkylating agent(s) | 1.15 (0.67 - 1.97) | 1.14 (0.54 - 2.38) | 0.83 (0.46 - 1.51) | 0.87 (0.39 - 1.91) | 1.27 (0.75 - 2.16) | 1.02 (0.50 - 2.06) | |
| Dosage | 125 mg/day or more (vs. lower doses) |
1.26 (0.73 - 2.19) | 1.10 (0.62 - 1.95) | 1.19 (0.66 - 2.12) | 1.21 (0.69 - 2.14) | 1.19 (0.68 - 2.08) | 1.00 (0.55 - 1.81) |
| Systemic (extraocular) autoimmune disease |
Yes | 0.85 (0.50 - 1.44) | 0.70 (0.32 - 1.52) | 0.57 (0.34 - 0.95) | 0.47 (0.19 - 1.14) | 0.81 (0.48 - 1.37) | 0.63 (0.27 - 1.45) |
95% CI = 95% confident interval; N/A= not applicable. Control of inflammation had to be observed across at least 2 visits spanning 28 days in order to be counted.
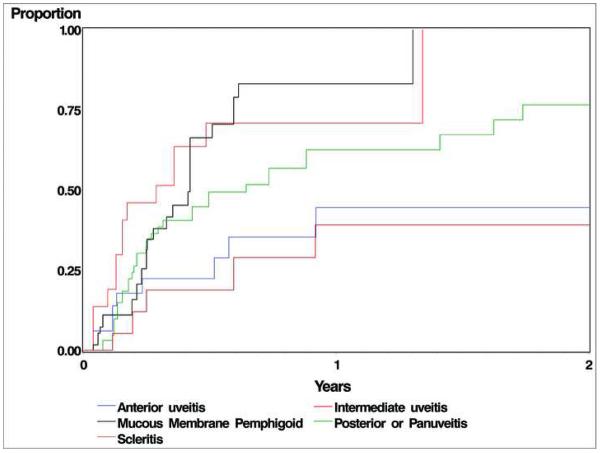
Compared to those with other sites of ocular inflammation, patients with intermediate uveitis and mucous membrane pemphigoid generally were more likely to achieve both control of inflammation and corticosteroid-tapering success than the other groups (see Figure). Patients with posterior or panuveitis also tended to gain success more often than those with anterior uveitis, although results were not statistically significant. For corticosteroid-sparing success outcomes, intermediate uveitis patients were several-fold more likely to achieve corticosteroid-sparing success compared to anterior uveitis patients at the ≤ 10 mg (crude HR=4.08, p=0.021; adjusted HR=6.42, p=0.010) and ≤ 5 mg prednisone levels (crude HR=3.50, p=0.048; adjusted HR=6.68, p=0.005) respectively. The MMP patients also achieved corticosteroid-sparing success more often than anterior uveitis patients at the ≤10 mg (crude HR=3.34, p=0.028; adjusted HR=2.11, p=0.219), ≤5 mg (crude HR=4.55, p=0.012; adjusted HR=4.64, p=0.017) and complete discontinuation of prednisone levels (crude HR=4.94, p=0.017; adjusted HR=6.93, p=0.060).
Figure.
Time-to- control of inflammation sustained for at least 28 days while taking a prednisone dose of 10 mg/day or less among patients with ocular inflammation, by type of inflammation.
Prior use of antimetabolites other than azathioprine was associated with an approximate 60% lower likelihood of control of inflammation (see Table 3). Prior use of T-cell inhibitors also tended to be associated with a lesser likelihood of success, although results were less consistent. Prior use of alkylating agents was not associated with a significantly different likelihood of favorable response. Patients with an associated systemic inflammatory disease tended to fare less well, but in most analyses the difference observed was not statistically significant (see Table 3). Use of higher dosages of azathioprine (125 mg or more per day) did not appear to affect the chance of achieving treatment success to an important degree.
Discontinuation of azathioprine
During a median follow-up of 230 days (interquartile range, 62-679 days), 99 patients (68%) were observed to discontinue azathioprine (Table 4). The overall rate of discontinuation was 0.45/person-year (95% CI, 0.37-0.55), and the Kaplan-Meier estimate of the proportion discontinuing therapy for any reason within one year was 47% (95% CI, 39-57%). Within the first year of therapy, an estimated 17% discontinued therapy because of ineffectiveness, and an additional 9% were placed on a second immunosuppressive agent. Thirty-five patients (24%) stopped therapy because of adverse drug effects (24.1% by one year). The rate of discontinuation of azathioprine due to systemic adverse effects was 0.16/person-year (PY) (95% CI, 0.11-0.22/PY). The most common side effect leading to discontinuation was gastrointestinal upset (9%, 0.06/PY), followed by bone marrow suppression (5%, 0.03/PY), elevated liver enzymes (4%, 0.03/PY), infection (2%, 0.01/PY), and allergic reaction (1%, 0.01/PY). Other developments leading to discontinuation of azathioprine in single patients were paresthesia, arthralgia, unspecified bladder disease, emotional disturbance, and severe fatigue. The reasons for discontinuation were not specified for 22 patients (15%). If these patients had the same distribution of reasons for discontinuing azathioprine, the proportion discontinuing treatment because of toxicity by one year would be 28.4%.
Table 4.
Reasons for Discontinuation of Azathioprine Among Patients with Ocular Inflammatory Diseases*
| Reasons | No. of Affected Patients (%) |
Rate (Events per Person-Year) |
95% Confidence Interval (CI) on Rate |
Kaplan-Meier Estimate at 1 Year (%, 95% CI) |
Kaplan-Meier Estimate at 2 Years (%, 95% CI) |
|---|---|---|---|---|---|
| Due to side effects | 35 (24%) | 0.16 | (0.11 , 0.22) | 24.1 (17.2 - 33.2) | 31.1 (22.5 - 42.1) |
| - Gastrointestinal upset | 13 (9%) | 0.06 | (0.03 , 0.10) | 10.8 (6.3 - 18.0) | 10.8 (6.3 - 18.0) |
| - Bone marrow suppression | 7 (5%) | 0.03 | (0.01 , 0.07) | 3.5 (1.3 - 9.1) | 8.3 (3.4 - 19.5) |
| - Elevated liver enzymes | 6 (4%) | 0.03 | (0.01 , 0.06) | 4.9 (2.0 - 11.8) | 6.7 (2.9 - 15.1) |
| - Infection | 3 (2%) | 0.01 | (0.002 , 0.04) | 2.4 (0.6 - 10.3) | 2.4 (0.6 - 10.3) |
| - Allergy | 2 (1%) | 0.01 | (0.001 , 0.03) | 1.6 (0.4 - 6.3) | 1.6 (0.4 - 6.3) |
| - Other side effects | 5 (3%) | 0.02 | (0.01 , 0.05) | 4.2 (1.5 - 11.2) | 6.7 (2.6 - 16.9) |
| Due to ineffectiveness | 22 (15%) | 0.10 | (0.06 , 0.15) | 17.4 (11.2 - 26.4) | 20.7 (13.6 - 30.8) |
| Due to remission of ocular inflammatory disease |
20 (14%) | 0.09 | (0.06 , 0.14) | 5.9 (2.4 - 14.0) | 14.2 (7.4 - 26.5) |
| Unspecified | 22 (15%) | 0.10 | (0.06 , 0.15) | 10.7 (5.8 - 19.2) | 22.2 (13.7 - 34.9) |
| Total (stopping azathioprine for any reason) |
99 (68%) | 0.45 | (0.37 , 0.55) | 47.4 (39.1 - 56.5) | 63.6 (54.7 - 72.5) |
More than one cause could have been scored as contributing to discontinuation of drug.
Forty-six patients (32%) remained on azathioprine at the end of observation. The majority of these cases (35 patients) continued on azathioprine monotherapy, although 11 patients had been placed on additional immunosuppressive agents. Of these 11, only 2 had been placed on a second agent because of ongoing active inflammation, while the others received additional immunosuppression to facilitate corticosteroid tapering as part of a multiple immunosuppressive drug regimen. Twenty patients (14%) stopped therapy because of disease remission. The Kaplan-Meier estimates of the proportion discontinuing therapy for disease remission within one and two years respectively were 6% (95% CI, 2-14%) and 14% (95% CI, 7%-27%).
DISCUSSION
These results indicate that azathioprine is moderately effective for patients with active ocular inflammation or in need of a corticosteroid-sparing agent, particularly if several months can be allowed for the drug to have its effect. However, some patients will not respond to therapy, and so clinicians must be prepared to change to alternative immunosuppressive agents in the event of failure to achieve treatment goals within 6-12 months, particularly if patients' clinical status is unstable while awaiting the therapeutic effect of azathioprine. Our results suggest that azathioprine may be especially favorable for patients with intermediate uveitis.
A previous, moderate-sized study of the outcomes of azathioprine conducted at one of the centers participating in the present study found that 58% (95% CI: 42%-82%) of a similar spectrum of ocular inflammation cases were controlled using 10 mg or less or prednisone within six months of therapy.19 Using the same approach (omitting the requirement that controlled inflammation be sustained and a 10 mg or less prednisone threshold for corticosteroid-sparing), our six month results (46.8% success) were within this confidence interval. However, because up to 16% of patients were observed to have transient success, our lower estimates of sustained corticosteroid-sparing success with azathioprine (approximately one-third by six months and one-half by 12 months) may provide a more realistic assessment of treatment success. Smaller prior studies in uveitis patients also observed moderate benefits of azathioprine therapy.9, 20
A 2-year double-masked, placebo-controlled trial of azathioprine therapy in 73 Turkish male patients with Behçet's disease found that azathioprine reduced the incidence of ocular involvement in patients initially free of ocular inflammation by more than 50%.11 Follow-up of the subset of these patients with ocular involvement treated with azathioprine found that 17 patients (68%) were in remission after discontinuation of treatment during the median follow-up time of 90 months.12 In our cohort, results for patients with Behçet's Disease were similar to those for patients with other forms of ocular inflammation in this study (data not shown). Extrapolating the rate of discontinuation for remission observed in our study to 90 months yields similar remission results.
Our results were consistent across the various outcomes in finding that cases of intermediate uveitis responded significantly better to azathioprine than anterior uveitis, with 89.8% achieving complete control of inflammation sustained for at least 28 days and 68.2% meeting corticosteroid-sparing objectives before 12 months of therapy. This pattern of response was not observed in our study of patients treated with methotrexate,21 suggesting that azathioprine might be especially effective for intermediate uveitis. Patients with MMP and posterior or panuveitis also tended to respond better to azathioprine than patients with anterior uveitis in our hands. However, because this is an observational study, indications-for-treatment bias cannot be excluded as a potential explanation of these associations, for instance if clinicians tended to use azathioprine after alkylating agent therapy for MMP, which might have lingering benefits after its discontinuation. Our observation that patients who previously had used other antimetabolites or T-cell inhibitors tended to respond to azathioprine less often may reflect that such patients on average may have more severe disease.
Our results indicated that azathioprine was discontinued due to side effects at a rate of 0.16 /PY (95% CI, 0.11-0.22), and provided an estimate that 24% (95% CI, 17.2% -33.2%) of patients would discontinue azathioprine due to side effects within one year, versus 0.13/PY and 17.5% within one year in our similar study evaluating methotrexate for ocular inflammatory diseases.21 The true incidence rate of drug-induced side effects would be higher than this, because only adverse events resulting in discontinuation of treatment are captured in this rate, and because 15% (0.10 /PY; 95% CI, 0.06-0.15) discontinued treatment for reasons not specified in the medical records, some of whom may have discontinued for toxicity. Among 38 patients treated with azathioprine therapy in the previous report from one of our centers, the rate of side effects was 0.29 /PY and the rate of discontinuation of azathioprine due to side effects was 0.24 /PY19 –slightly higher than our overall results. Gastrointestinal complications, which are well-known,22 were the most common side effects leading to discontinuation in our study. Other side effects we observed also have been previously described; nearly all would be detectable by following guidelines for the administration of the drug,1 and reversible with dose reduction or discontinuation of therapy. Our previous review of the available literature regarding cancer risk with azathioprine suggested there is likely no or little increase in the risk of cancer with use of this agent for ocular inflammation.23 Absence of increased risk of mortality with azathioprine therapy was confirmed in ocular inflammation patients from this cohort, among whom the risk of mortality from any cause for patients treated with azathioprine with respect to those not treated with immunosuppressive drugs was 0.99 (95% CI: 0.72 - 1.38), and the relative risk of mortality due to cancer was 1.13 (95% CI: 0.60 - 2.14), after adjusting for confounding variables.24
Potential methodological limitations of this retrospective, observational study include indications-for-treatment bias, missing data, and incomplete follow-up. While the patients studied appear to have had severe ocular inflammation, based on the presence of a large number of ocular complications of ocular inflammation at the onset of therapy (see Supplemental Table, available at www.ajo.com), such patients are commonly encountered in the tertiary ocular inflammation practices where immunosuppressive therapy often is administered. The percentage of missing data (0.4% for overall inflammatory activity) was low in this study. The survival analysis approach assumes that patients lost to follow-up fared similarly to those continuing at the center. Censoring of patients who were started on a second agent may in fact have slightly inflated the proportion of patients counted as having treatment success, because such patients likely were placed on a second agent because of inadequacy of response to azathioprine. We were unable to directly compare success with other agents because the reasons for selection of azathioprine as opposed to other agents were unknown and may have varied substantially. It was necessary to group cases by site/type of inflammation (anterior uveitis, intermediate uveitis, etc) in order to achieve group sizes sufficiently large for meaningful statistical comparison; there may be heterogeneity in the responsiveness of specific disease subtypes to azathioprine that is not captured by this approach.
The major strength of this study is a larger sample size than that of previous reports, improving the study's ability to estimate treatment success or failure, adverse effects and safety profiles. Standardization of data entry by expert chart reviewers, including site visiting to ensure correct application of pre-specified data entry protocols across the different sites, and appropriate statistical analyses16 also were strengths of our study methodology. The outcome definitions we used (requiring sustained control of inflammation for at least 28 days before scoring a success) are likely more realistic than other approaches, as indicated by the large proportion of patients who achieved “success” in controlling inflammation for just a single visit, followed by a relapse of inflammation.
In conclusion, our data suggest that azathioprine can be tolerated by the majority of ocular inflammation patients, and that the side effects which do occur are likely to be reversible with discontinuation of therapy or dose-adjustment. The drug appears moderately effective for contributing to control of inflammation and allowing tapering of systemic corticosteroids to 5 or 10 mg/day of prednisone when given as a single non-corticosteroid immunosuppressive therapy in addition to management with corticosteroids according to best medical judgment. Patients with intermediate uveitis appear to fare especially well with azathioprine. We would benefit from additional studies to evaluate whether combination therapy would increase the likelihood of treatment success and/or adverse effects, and whether genetic or other factors might be useful to identify patients especially likely or unlikely to respond to therapy, approaches which could potentially increase the success rate with azathioprine therapy. Although the success rate with azathioprine is moderate, and treatment-limiting (reversible) toxicity is not uncommon, its advantages include relatively lower cost especially compared to most alternative agents, some evidence for safety during pregnancy,25, 26 and decades of clinical experience making it less likely that unrecognized long-term toxicities of therapy exist than with newer treatments.
Supplementary Material
Acknowledgement
A. Funding/support
Supported primarily by the National Eye Institute, Bethesda, Maryland (grant EY-014943 [Dr. Kempen]), the Paul and Evanina Mackall Foundation (New York, New York), and Research to Prevent Blindness (New York, New York). Dr. Kempen is a Research to Prevent Blindness James S. Adams Special Scholar Award recipient. Drs. Jabs and Rosenbaum are recipients of Research to Prevent Blindness Senior Scientific Investigator Awards. Dr. Levy-Clarke was previously supported by and Dr. Nussenblatt is supported by intramural funds of the National Eye Institute (Bethesda, Maryland). Dr. Suhler also received support from the Veterans' Administration.
B. Financial disclosures
Dr. Foster has served as a consultant to Allergan, Bausch & Lomb, and Sirion; owns equity or stock options in Eyegate; and has received speaking fees from Alcon, Allergan, Inspire, Ista, Bausch & Lomb, and Centocor. Dr. Jabs has served a consultant to Abbott, Genzyme Corporation, Novartis, Allergan, the Emmes Corporation (DSMC member), and the Johns Hopkins University Dana Center for Preventive Ophthalmology. Dr. Kempen has served as a consultant to Lux Biosciences and has participated in clinical trials funded by Allergan and Eyegate. Dr. Rosenbaum has served as a consultant to Abbott, Amgen, Allergan, Lux Biosciences, and Novartis; holds equity ownership/stock options in Amgen; and has received research support from Genentech, Lux Biosciences, and Abbott. Dr. Suhler has received research support from Eyegate, Genentech, Lux Biosciences, and Abbott.
C. Contributions of authors
Design of the study (SP, JHK, CN), conduct of the study (SP, JHK, EBS); collection (SP, JHK, CN, TL, SSP, JTR, JET, CSF, DAJ, GAL, RBN, EBS), management (SP, JHK, JTR, JET, CSF, DAJ, GAL, RBN, EBS), analysis (SP, JHK, CN, EBS), interpretation of the data (SP, JHK, CN, EBS); and preparation (SP, JHK, EBS), review (SP, JHK, CN, TL, SSP, JTR, JET, CSF, DAJ, GAL, RBN, EBS), approval of the manuscript (SP, JHK, CN, TL, SSP, JTR, JET, CSF, DAJ, GAL, RBN, EBS)
D. Conformity of author information
The protocol was approved by institutional review boards of all participating institutes, and conducted in accordance with the principles of the Declaration of Helsinki and the HIPAA compliance.
E. Other acknowledgements
None
Biography
 Sirichai Pasadhika, MD, is a retinal specialist and Chief of the Uveitis Service at Chulalongkorn University, Bangkok, Thailand. His post-residency training included a vitreo-retinal fellowship in Bangkok, a Graham-Lovett clinical fellowship in vitreo-retinal surgery at the Sydney Eye Hospital in Australia, a clinical uveitis fellowship at Oregon Health & Science University in Portland, and a clinical -research fellowship in inherited retinal disease and electrophysiology at the University of Illinois at Chicago.
Sirichai Pasadhika, MD, is a retinal specialist and Chief of the Uveitis Service at Chulalongkorn University, Bangkok, Thailand. His post-residency training included a vitreo-retinal fellowship in Bangkok, a Graham-Lovett clinical fellowship in vitreo-retinal surgery at the Sydney Eye Hospital in Australia, a clinical uveitis fellowship at Oregon Health & Science University in Portland, and a clinical -research fellowship in inherited retinal disease and electrophysiology at the University of Illinois at Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. Medline. doi:10.1016/S0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 2.Ward JR. Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis. Am J Med. 1988;85:39–44. doi: 10.1016/0002-9343(88)90361-0. Medline. doi:10.1016/0002-9343(88)90361-0. [DOI] [PubMed] [Google Scholar]
- 3.Danovitch GM. Choice of immunosuppressive drugs and individualization of immunosuppressive therapy for kidney transplant patients. Transplant Proc. 1999;31:2S–6S. doi: 10.1016/s0041-1345(99)00840-4. Medline. doi:10.1016/S0041-1345(99)00840-4. [DOI] [PubMed] [Google Scholar]
- 4.Wise M, Callen JP. Azathioprine: a guide for the management of dermatology patients. Dermatol Ther. 2007;20:206–215. doi: 10.1111/j.1529-8019.2007.00134.x. Medline. doi:10.1111/j.1529-8019.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- 5.Gisbert JP, Nino P, Cara C, Rodrigo L. Comparative effectiveness of azathioprine in Crohn's disease and ulcerative colitis: prospective, long-term, follow-up study of 394 patients. Aliment Pharmacol Ther. 2008;28:228–238. doi: 10.1111/j.1365-2036.2008.03732.x. Medline. doi:10.1111/j.1365-2036.2008.03732.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Gladman DD, Schentag CT, Cook RJ. The long-term use of azathioprine in patients with psoriatic arthritis. J Clin Rheumatol. 2001;7:160–165. doi: 10.1097/00124743-200106000-00005. Medline. doi:10.1097/00124743-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Shakra M, Shoenfeld Y. Azathioprine therapy for patients with systemic lupus erythematosus. Lupus. 2001;10:152–153. doi: 10.1191/096120301676669495. Medline. doi:10.1191/096120301676669495. [DOI] [PubMed] [Google Scholar]
- 8.Polack FM. Effect of azathioprine (imuran) on corneal graft reaction. Am J Ophthalmol. 1967;64:233–244. doi: 10.1016/0002-9394(67)92517-2. Medline. [DOI] [PubMed] [Google Scholar]
- 9.Mathews JD, Crawford BA, Bignell JL, Mackay IR. Azathioprine in active chronic iridocyclitis. A double-blind controlled trial. Br J Ophthalmol. 1969;53:327–330. doi: 10.1136/bjo.53.5.327. Medline. doi:10.1136/bjo.53.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood AJ, Stanford MR, Graham EM. The role of azathioprine in the management of retinal vasculitis. Eye. 1998;12:783–788. doi: 10.1038/eye.1998.203. Medline. [DOI] [PubMed] [Google Scholar]
- 11.Yazici H, Pazarli H, Barnes CG, et al. A controlled trial of azathioprine in Behcet's syndrome. N Engl J Med. 1990;322:281–285. doi: 10.1056/NEJM199002013220501. Medline. [DOI] [PubMed] [Google Scholar]
- 12.Hamuryudan V, Ozyazgan Y, Hizli N, et al. Azathioprine in Behcet's syndrome: effects on long-term prognosis. Arthritis Rheum. 1997;40:769–774. doi: 10.1002/art.1780400425. Medline. doi:10.1002/art.1780400425. [DOI] [PubMed] [Google Scholar]
- 13.Moore CE. Sympathetic ophthalmitis treated with azathioprine. Br J Ophthalmol. 1968;52:688–690. doi: 10.1136/bjo.52.9.688. Medline. doi:10.1136/bjo.52.9.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper PL, Kaplan HJ. Triple agent immunosuppression in serpiginous choroiditis. Ophthalmology. 1991;98:944–951. doi: 10.1016/s0161-6420(91)32198-5. Medline. [DOI] [PubMed] [Google Scholar]
- 15.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. Medline. doi:10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. Medline. doi:10.1016/j.ajo.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DR, Oakes D. Analysis of survival data. Chapman & Hall; London: 1984. [Google Scholar]
- 18.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. doi:10.2307/2290085. [Google Scholar]
- 19.Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115:1826–1832. doi: 10.1016/j.ophtha.2008.04.026. Medline. doi:10.1016/j.ophtha.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Andrasch RH, Pirofsky B, Burns RP. Immunosuppressive therapy for severe chronic uveitis. Arch Ophthalmol. 1978;96:247–251. doi: 10.1001/archopht.1978.03910050115002. Medline. [DOI] [PubMed] [Google Scholar]
- 21.Gangaputra S, Newcomb CW, Liesegang T, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. doi: 10.1016/j.ophtha.2009.04.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saway PA, Heck LW, Bonner JR, Kirklin JK. Azathioprine hypersensitivity. Case report and review of the literature. Am J Med. 1988;84:960–964. doi: 10.1016/0002-9343(88)90079-4. Medline. doi:10.1016/0002-9343(88)90079-4. [DOI] [PubMed] [Google Scholar]
- 23.Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008;146:802–812. doi: 10.1016/j.ajo.2008.04.035. Medline. doi:10.1016/j.ajo.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempen JH, Daniel E, Dunn JP, et al. Risk of overall and cancer mortality among patients with ocular inflammation treated with immunosuppressive therapy: retrospective cohort study. Brit Med J. doi: 10.1136/bmj.b2480. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696–701. doi: 10.1002/bdra.20399. Medline. doi:10.1002/bdra.20399. [DOI] [PubMed] [Google Scholar]
- 26.Alstead EM, Ritchie JK, Lennard-Jones JE, Farthing MJ, Clark ML. Safety of azathioprine in pregnancy in inflammatory bowel disease. Gastroenterology. 1990;99:443–446. doi: 10.1016/0016-5085(90)91027-4. Medline. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.