Abstract
These experiments examined the effects of prior stress, corticosterone, or epinephrine on learning in mazes that can be solved efficiently using either place or response strategies. In a repeated stress condition, rats received restraint stress for 6 h/day for 21 days, ending 24 h before food-motivated maze training. In two single-stress conditions, rats received a 1-h episode of restraint stress ending 30 min or 24 h prior to training. Single stress ending 30 min prior to training resulted in a significant interaction of stress and learning on the two tasks, with significant enhancement of learning in the response task and non-significant impairment in the place task. Neither acute nor chronic stress significantly altered learning in either task when the stress ended 24 h before training. Thus, the anterograde effects of stress on maze learning ended within a single day. Two stress-related hormones, corticosterone and epinephrine, were tested for effects on learning parallel to those of acute stress. When administered 30 min prior to training, a corticosterone dose (40 mg/kg) that enhanced memory on a spontaneous alternation task did not significantly enhance or impair learning in either task. Two doses of epinephrine that modulate memory in other settings were used to test the effects of epinephrine on learning. Pretraining injections of 0.03 mg/kg epinephrine impaired place learning, while 0.1 mg/kg epinephrine impaired response learning. The epinephrine results mimicked those seen with acute stress on the place task, but were opposite those seen after acute stress on the response task. Thus, corticosterone does not appear to be a major factor mediating the effects of acute stress on place and response learning and epinephrine is, at most, a partial contributor to these effects.
Keywords: hippocampus, striatum, stress, epinephrine, corticosterone, spontaneous alternation, response learning, place learning, memory systems
1. Introduction
Acute and chronic stress treatments can have substantial influences on learning and memory in both humans and rodents [52, 53, 54,76, 79, 80, 83]. However, the direction of effects differs across studies, with some reports describing stress-induced enhancements [8, 21, 49,81, 84], impairments [8, 25, 26, 49, 78], or no effects [91] on learning, memory, and retrieval. Among the variables contributing to different effects of stress on learning and memory are age and sex [11, 12, 13, 20, 45, 82, 83, 93]. Task differences also contribute importantly to the nature of stress effects on learning and memory [8, 64, 76]. For example, stress prior to training resulted in effects of opposite direction on spatial and non-spatial variants of the swim task [37]. Of note, these results were obtained on tasks that are themselves highly stressful to rats, as evident from the magnitude of systemic release of epinephrine and corticosterone initiated by swimming procedures [7, 37, 48, 75]. One goal of the present experiment was to determine if stress similarly or differently affected place and response learning in appetitive tasks. The present experiments used two versions of a T-shaped maze to address this question. Lesions or inactivation of the hippocampus and striatum impair place and response learning, respectively, in tasks like these and sometimes enhance learning in the opposite task [15, 31, 35, 50, 62, 63, 78]. Such findings suggest that the respective neural systems may compete with each other for control over learning strategy [31, 40, 67, 91].
Another variable that may influence the effects of stress on learning and memory is whether the stress is administered in a single session, i.e. acute stress, or across many days, i.e. chronic stress. Although many studies have investigated the effects of acute stress on learning and memory [e.g., 20, 21, 25, 26, 36, 37, 38, 77, 82] and others have examined the effects of chronic stress on learning and memory [e.g., 11, 12, 13, 39, 44, 52, 53, 54], comparisons of effects of acute vs. chronic stress on learning and memory are generally lacking. One study compared different lengths of chronic stress administration, finding that a chronic stress regimen of 6 h/day for 21 days, but not shorter regimens, caused spatial memory deficits [58]. In that experiment, the stress sessions ended 24 h prior to training. In contrast, most assessments of the effects of acute stress on learning and memory involve administration of stress near the time of training, i.e. either posttraining manipulations of stress or stress hormones [26, 33, 73] or pretraining manipulations, ending within 1 h or less before training [37, 38, 66]. However, several studies have revealed proactive effects of stress that last at least 24 h after a stress session [21, 81, 95]. The present experiment compared the effects of repeated or single stress sessions, with repeated stress ending 24 h prior to training and single stress ending either 30 min or 24 h before training.
The overall hypothesis was that prior repeated and single stress would impair learning in a place task, but enhance learning in a response task. This hypothesis was supported only for a single 60-min stress treatment ending 30 min prior to training. A second hypothesis was that the stress-related hormones, corticosterone and epinephrine, would have effects on learning like those of stress. However, corticosterone had no effect on learning and epinephrine impaired learning in both place and response tasks, suggesting that these hormones are not the primary mediators of stress effects on place and response learning.
2. Experiment 1 – Stress and learning
The first experiment compared the effects of repeated or single stress sessions, with the latter ending either 30 min or 24 h before training.
2.1. Materials and methods
2.1.1. Subjects
Male Sprague-Dawley rats (Harlan Laboratories, Oregon Barrier 236B) weighing approximately 300 g upon arrival were used as subjects. Rats were maintained on a 12/12 light/dark cycle with access to food and water ad libitum until food restriction began prior to training. The rats were individually housed in clear, plastic cages and were handled at least 5 times for 3 min each, starting one week prior to training. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.1.2. Stress
The 6-h duration stress procedures began between 0800 and 1200 each day. Rats were placed in cylindrical wire mesh restrainers (19 cm × 26.5 cm) secured with plastic clips at both ends. Repeatedly stressed rats were restrained for 6 h each day for 21 consecutive days. The final stress episode ended approximately 24 h before training. Single-stressed rats received similar stressors and were returned to their home cages in the colony room 30 min or 24 h prior to training on a place or response task during afternoon hours. During the acute stress treatment, the restrainers were placed on a plate shaker (Fisher Scientific, model no. 260301F) for 1 h. We had assumed it would take additional stress to observe effects of single stress that might match those of repeated stress. However, neither repeated nor single stress episodes ending 24 h before training had effects on learning. Therefore, the addition of the plate shaker treatment was not sufficient to induce an effect on learning that persisted for 24 h and the difference in treatments seems not to have been an important variable. Ten min prior to training, rats from all groups were placed in clean cages in the training room. Control rats received no treatment other than handling prior to training. Due to the length of the training sessions and the number of rats involved, rats from control and treatment groups were interspersed across training days within each squad of rats.
2.1.3. Place and Response Training
One week before testing, rats were placed on a food restriction diet that reduced body weights to 80-85% of original weight. Body weights were monitored daily and an aliquot of food was provided each day to maintain body weight at the target level. To avoid possible neophobia on the training day, rats received three Frosted Cheerios® (General Mills), the reward used in maze training, in their home cages for at least three days prior to training.
The training apparatus was comprised of black Plexiglas and was situated 72 cm above the floor. The plus-shaped maze was composed of four arms (46 cm long × 12.5 cm wide × 7 cm high), each separated by 90°, radiating from the center (12.5 cm × 12.5 cm) of the maze. A removable barrier formed a T-maze by blocking the arm opposite the start arm. The testing room (350 cm × 320 cm) contained 2 desks, a chair, and various posters on the walls of the testing room, providing a moderate extramaze cue environment.
The start of training occurred between 1100 and 1600 h, i.e. 3-8 h, after the start of the light cycle. On each trial, the start arm was positioned 90° from the goal arm in a semi-random assignment starting from either the north or south arms. There were no instances of more than two consecutive starts from the same arm. To eliminate odor cues within the maze, the maze was rotated so that the physical goal arms differed between trials. Food wells were located at the end of every arm so that rats could not visually identify which arm contained the food reward. In addition, to avoid the use of odor cues to identify the goal arm, the non-goal cups had inaccessible food crumbs underneath a screen in the cups. The criterion for an arm entry was entry of all four feet into an arm. Rats received 100 trials within a single day, with a maximum trial duration of 1 min and an inter-trial interval of 1 min. If rats did not find the food within 1 min on the maze, the trial was scored as an error. On rare occasions, rats that ventured outside the maze were returned to the start arm, and the trial was continued.
For training on the place version of the maze, the goal arm, which contained a small portion of a Cheerio, was always located in a specific spatial location in the room regardless of the start arm position. Rats trained on the place version of the task received no treatment (n=10), acute stress ending 30 min prior to training (n=8), acute stress ending 24 h prior to training (n=10), or chronic stress ending 24 h before training (n=10).
For training on the response version of the maze, the goal arm, which contained a small portion of a Cheerio, was always in the same right/left position relative to the start arm. Rats trained on the response version of the task received no treatment (n=16), acute stress ending 30 min prior to training (n=12), acute stress ending 24 h prior to training (n=11), or chronic stress ending 24 h prior to training (n=10).
2.1.4. Statistical Analysis
Learning in both tasks was assessed using trials to reach a criterion of 9/10 correct arm choices and using learning curves with 10 blocks of 10 trials. Two-way AVOVAs were used to test interactions (stress × task) and planned posthoc t-tests were used to test differences in trials to criterion within task for each stress condition vs. controls. Repeated-measure ANOVAs were used to test the same interactions and differences for the learning curves.
2.2. Results
A single stress session, ending 30 min before training, impaired learning of the place task and enhanced learning of the response task (Figure 1). Neither a single stress session nor repeated stress sessions ending 24 h before training significantly altered learning of the tasks.
Figure 1.
Number of trials to reach criterion on the place and response task after no treatment or single stress administration ending 30 prior to training. ANOVAS revealed a significant interaction of task by treatment. Pre-training single stress impaired learning on the place task and significantly enhanced learning on the response task.
Figure 1 shows the trials to criterion for rats receiving a single stress session ending 30 min before training on the two tasks as compared to non-stressed controls. There was a significant interaction of stress by task (F1,42 = 8.16, p<0.01), in which stress impaired place learning and enhanced response learning. The individual posthoc t-tests revealed a significant effect of stress on response learning (p<0.05), but not place learning (p>0.05).
Analyses of learning curves for these four groups (Figure 2) were consistent with the results obtained with trials to criterion. Although rats do not maintain perfect performance after reaching trials to criterion, their performance remained above chance during these blocks. There was a significant interaction of task by treatment (F1,42 = 5.15, p<0.05) in which stress affected learning in the two tasks in opposite directions, impairing learning of the place task and enhancing learning of the response task.
Figure 2.
Learning curves for acquisition of the place or response task after no treatment of single stress administration ending 30 prior to training. Administration of pre-training single stress resulted in slower learning in the place task and faster learning in the response task when compared to controls.
In contrast to the effects of single stress ending 30 min prior to training, neither repeated nor single stress episodes ending 24 h before training significantly affected learning in either the place or response task. The trials to criterion for the two stress-24 h groups are shown in Figure 3; for comparison, the controls are repeated from Figure 1. There were no significant effects of stress, task, or interaction of stress by task (all ps > 0.2). Similarly, the learning curves did not differ significantly across conditions or by interaction with stress (data not shown; all ps >0.2)
Figure 3.
Number of trials to reach criterion on the place or response task after no treatment or single or repeated stress ending 24 h prior to training. Single or repeated stress ending 24 h prior to training had no effect on place or response learning.
2.3. Discussion
A single 60-min stress episode ending 30 min prior to training resulted in impaired learning in the place task and enhanced learning in the response task. These findings are consistent with past results indicating that acute stress and anxiogenic drugs shift rats towards the use of striatal-sensitive strategies and away from the use of hippocampal-sensitive strategies [37, 64] in dual solution tasks that can be solved with either place- or response-based behaviors. Although the task used in the current study placed rats into either place or response trained groups, evidence suggests that results obtained using dual-solution tasks in which the rats choose their preferred place or response strategy and results obtained using single-solution place or response tasks are related. For example, shifts in preferred learning strategies towards place tasks occur across the estrous cycle when rats are tested in a dual solution (place or response) task, while injections of estrogen enhance place learning and impair response learning [40, 42]. Therefore, based on previous [37, 64] and present results, the effects of acute stress on learning are similar when assessed with either active avoidance swim tasks or appetitive tasks, impairing learning in the hippocampal-sensitive form of learning and enhancing learning for the striatal-sensitive form of learning, similar to findings of other studies [15, 35, 62, 63]. The reciprocal stress effects of hippocampal- and striatal-sensitive tasks are consistent with other findings suggesting that these neural systems compete for control over learning and memory, biasing learning strategies toward response learning and away from place learning [30, 40, 67, 91].
Neither repeated nor single stress ending 24 h prior to training significantly influenced learning in either task, although learning in the chronic stress was in the direction of impairment as compared to controls. The failure to observe significant effects of chronic stress on learning in this experiment is not consistent with the findings several studies showing such effects on learning and memory [39, 85, 92], including reports that used chronic stress procedures similar to those used here [e.g.,19, 47, 58, 95]. Past studies have used a range of tasks to study stress effects on learning and memory, including classical eyeblink conditioning, object recognition, swim tasks, and fear conditioning [3, 4, 19, 21, 71, 85, 93]. Additional work is needed to identify the training conditions sensitive to long-lasting effects of acute and chronic stress, for example including possible differential susceptibility to stress of learning vs. memory measures.
3. Experiment 2- Corticosterone, learning, and spontaneous alternation
The adrenal stress hormone, corticosterone, has both enhancing and impairing effects on memory and may contribute to the anterograde effects of stress on learning and memory [1, 9, 18, 21, 65, 66, 80]. The present experiment explored the possible effects of corticosterone on learning in the place and response mazes. Prior to beginning this phase of the experiment, we used a spontaneous alternation task, a test of spatial working memory, to establish a dose of corticosterone that affected memory. That dose was then used in the tests of place and response learning. It was hypothesized that corticosterone would show effects similar to those of stress, i.e., an impairment in place learning and an enhancement in response learning.
3.1. Materials and methods
3.1.1. Subjects
Subjects, housing, and general testing conditions were as described for Experiment 1.
3.1.2. Corticosterone
Corticosterone (Sigma-Aldrich, St. Louis, MO) was suspended in sesame oil at concentrations of 20, 40, or 80 mg/ml. One set of rats received injections (1 ml/kg s.c.) of sesame oil or corticosterone 30 min prior to spontaneous alternation testing. In this and later experiments, the time between injection and start of behavioral training and testing matched the time between cessation of single stress and behavioral tests. Other times might certainly be useful in this context, particularly because the hormonal response to the stressor might dissipate during the time of stress administration [cf.22]. On the other hand, the biological underpinnings of the effects of stress on learning might reflect processes initiated by the onset, offset, peak amplitude, etc. of the hormonal response. While the timing of hormones in relation to behavioral tests is quite important [24], there is no clear guidance from the literature regarding how to match the temporal aspects of hormone injection to stress as applied to learning. The present results should be viewed with these caveats.
Rats were tested on a spontaneous alternation task after receiving vehicle or corticosterone doses of 20 mg/kg, 40 mg/kg, or 80 mg/kg. On the basis of the results of this dose-response curve, the 40 mg/kg dose was tested for effects on learning in place and response tasks. Rats were returned to the colony room prior to behavioral testing and were then placed in clean cages in the testing room 10 min prior to alternation testing or maze training.
Because repeated stress episodes ending 24 hr before training did not alter learning in Experiment 1, repeated corticosterone injections were not tested here. Of note, several previous experiments have reported an absence of effects on learning in some tasks after long-term treatment with corticosterone [e.g., 5, 10, 17, 46] as well as dissociation of plasma corticosterone levels and effects on memory across arousing treatments [95].
3.1.3. Spontaneous Alternation Testing
Spontaneous alternation testing was conducted in the same maze as that used for place and response learning. All four arms were open in a plus-shaped configuration. Rats were placed in one arm of the maze and were allowed to explore the maze freely for 20 min. Both the number and choice of arm entries were recorded. The criterion for an arm entry was entry of all four limbs into an arm. An alternation was defined as entry into all four arms within 5 consecutive arm choices (e.g. entry into A, C, A, B, D would be recorded as an alternation and entry into B, C, B, A, C would not). Percent alternation scores were calculated using the formula (# of alternations / total # of possible alternations) × 100. The chance alternation score on this measure is 44%. Rats received sesame oil (n=8), 20 mg/kg (n=7), 40 mg/kg (n=5), or 80 mg/kg (n=6) corticosterone prior to training.
3.1.4. Place and Response Training
Rats were trained on response or place tasks as before. Thirty min prior to training, rats received sesame oil or corticosterone (40 mg/kg) injections. Thus, there were four groups: Place task: oil (n=11) or corticosterone (n=10); Response task: oil (n=10) or 40 mg/kg corticosterone (n=9).
3.1.5. Statistical Analyses
Two-tailed student t-tests were utilized to compare differences in averages of spontaneous alternations and numbers of arm choices. Learning in the place and response mazes was assessed as in Experiment 1.
3.2. Results
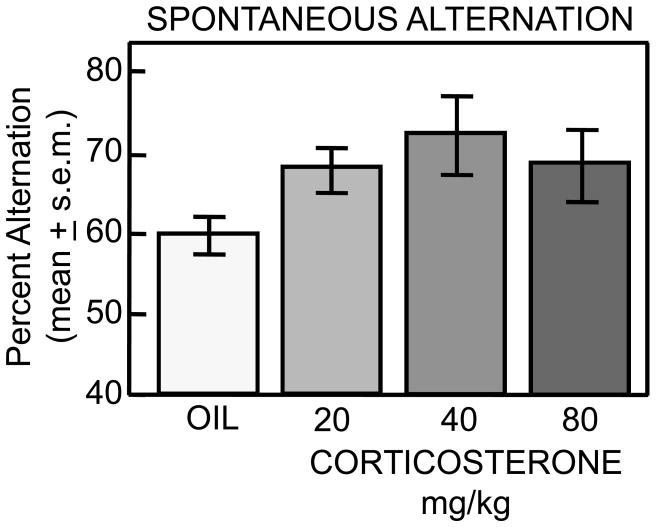
As shown in Figure 4, corticosterone enhanced alternation scores in an inverted-U dose-response manner. Rats that received the 40 mg/kg dose of corticosterone showed enhanced spontaneous alternation scores compared to those that received vehicle injections (p<0.01). Rats that received either 20 mg/kg or 80 mg/kg injections of corticosterone also showed higher alternation scores compared to controls, but these scores were not significantly different than those of vehicle-treated rats (ps<0.2). No significant differences in total arm choices were observed for any treatment group (ps>0.4) compared to controls (data not shown). Based on these spontaneous alternation results, the 40 mg/kg dose was used in tests of corticosterone effects on place and response learning. Note that all doses, including the 40 mg/kg dose, may have saturated classical glucocorticoid receptors. However, the relationship of these doses to non-classical steroid actions and membrane receptors [e.g., 6, 59, 60, 74] is not clear.
Figure 4.
Pre-testing corticosterone significantly enhanced spontaneous alternation behavior on a plus-shaped maze in a dose-dependent manner, revealing an inverted-U dose-response curve.
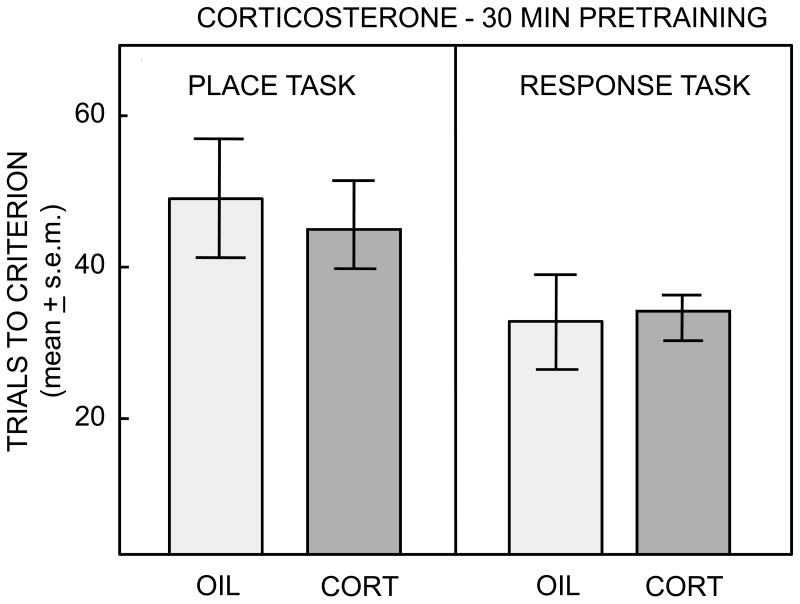
As shown in Figure 5, corticosterone did not significantly affect learning on either task as measured by trials to criterion or by learning curves. Neither treatment effects nor interactions were statistically significant (ps>0.2).
Figure 5.
Number of trials to reach criterion on the place or response task after pre-training administration of corticosterone. There were no significant differences between control and corticosterone groups in either the place or response task.
3.3. Discussion
When administered prior to testing on a spontaneous alternation task, corticosterone enhanced alternation scores in an inverted-U dose-response function. The inverted-U dose-response function is similarly observed with corticosterone and many other drugs in studies of learning, memory and neural plasticity [cf., 2, 18, 23, 32, 34, 41, 72]. However, the dose optimally effective at modulating memory in the spontaneous alternation task failed to enhance or to impair learning on the place and response tasks. These negative results suggest that corticosterone is not a principle mediator of the reciprocal effects of a single stress episode on place and response learning tasks, in contrast to effects of stress and cortisol reported in humans [80].
Note that learning in the oil control group was faster in this experiment than in Experiment 1, reducing the parametric space available for enhancement of learning by corticosterone. The reason for the difference in control scores is unclear, but may reflect either the use of a different set of rats or the sesame oil injection (vs. no injection in Experiment 1). Nonetheless, there remains potential room for enhancement of learning, which was not observed in this experiment.
4. Experiment 3- Epinephrine and learning
Epinephrine, a hormone released in response to stress, enhances memory in a variety of tasks [cf., 29, 32, 33, 41, 55], but has not been studied in the context of appetitively motivated place and response mazes. To determine whether epinephrine may serve as a mediator of the effects of acute stress on place and response learning, Experiment 3 compared the effects systemic epinephrine on learning in a place or response task. It was hypothesized that epinephrine would alter both place and response learning, although the direction of the effects could be similar to memory enhancement findings or previous results found in Experiment 1.
4.1. Materials and methods
4.1.1. Subjects
Subjects, housing, and general testing conditions were as described for Experiment 1.
4.1.2. Epinephrine
Epinephrine bitartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in saline. Rats received injections of saline or epinephrine (0.03 or 0.1 mg/kg) 30 min prior training on a place or response task. Rats were returned to the colony room prior to behavioral testing and were then placed in clean cages in the testing room 10 min prior to alternation testing or maze training.
4.1.4. Place and Response Training
Rats were trained on response or place tasks as before. There were six groups: Place task: saline (n=6), 0.03 mg/kg epinephrine (n=5), or 0.1 mg/kg epinephrine (n=6); Response task: saline (n=6), 0.03 mg/kg epinephrine (n=5) or 0.1 mg/kg epinephrine (n=7).
4.1.5. Statistical Analyses
Learning in the place and response tasks was assessed as in Experiment 1.
4.2. Results
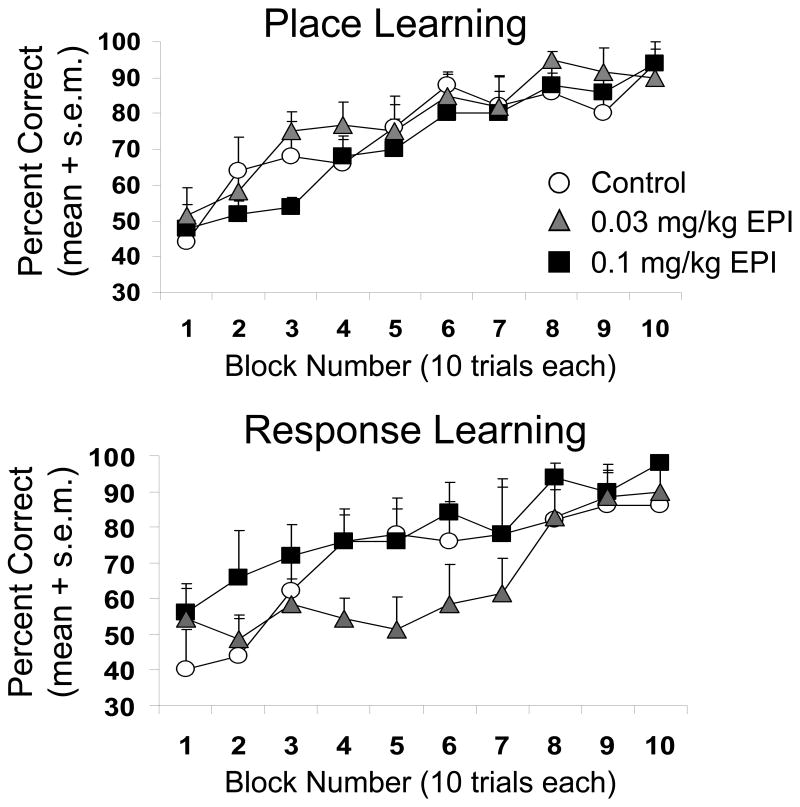
Epinephrine, injected 30 min before training, impaired learning in rats trained in either the place or response tasks (Figure 6). Figure 6 shows the trials to criterion for rats receiving epinephrine on the two tasks as compared to saline controls. Epinephrine impaired learning in both tasks, at a different dose in each, resulting in a significant treatment effect (F2,29 = 4.32, p<0.05). Posthoc t-tests revealed that epinephrine significantly impaired place learning at the 0.03 mg/kg dose, but not at the 0.1 mg/kg dose (p>0.2). Epinephrine significantly impaired learning in the response task at the 0.1 mg/kg dose, but not at the 0.03 mg/kg dose (p>0.2). Repeated measures ANOVAs were used to test the effects of epinephrine on learning curves (Figure 7). A significant treatment effect was not evident for learning curves on either task (ps>0.05).
Figure 6.
Number of trials to reach criterion on the place or response task after pre-training administration of epinephrine. Pre-training epinephrine impaired learning on the place task and response task at different doses, with a significant interaction of task × treatment.
Figure 7.
Learning curves for acquisition of the place or response task after administration of saline or epinephrine ending 30 prior to training. Learning curves did not differ significantly between saline and epinephrine groups.
4.3. Discussion
Epinephrine impaired learning on the place task, a result similar to that observed when a single 60-min stress episode was administered ending 30 min before training. Therefore, stress-induced release of epinephrine may contribute to the impairment of learning on a task that requires an intact and functional hippocampus. However, opposite to the results found with acute stress, epinephrine impaired response learning. These differences between the effects on learning of acute stress and epinephrine may reflect different actions of the treatments on multiple memory systems or different hormonal profiles that result from acute stress and epinephrine administration.
The dose that impaired learning was 0.1 mg/kg for the response task, but was 0.03 mg/kg for the place task. The different effective doses suggest that the mechanisms underlying the impairments may be different for the hippocampus- and striatum-sensitive tasks. The impairments contrast with past findings showing that epinephrine enhances memory for a variety of tasks including inhibitory avoidance, active avoidance, spontaneous alternation, and object recognition [e.g., 27, 33, 86, 87, 88]. Generally, the enhancement is seen at moderate doses, such as those tested here; higher doses produce amnesia [e.g., 32, 33]. In these previous experiments, epinephrine was given shortly before or after training, as compared to the 30-min injection-training procedure here. Also, the prior tasks did not involve food deprivation prior to training. The ability of epinephrine to enhance memory decreased in food-deprived rats; food deprivation also blunted the increase in blood glucose levels subsequent to depletion of hepatic stores of glucose [87]. These findings are consistent with the view that glucose is a key mediator of epinephrine enhancement of memory. The present results suggest that the ability of epinephrine to impair memory may not depend on increases in blood glucose levels, in contrast to the apparent role of increase in glucose levels in memory enhancement. This is a possibility that will need additional attention.
5. General Discussion
The main finding of these experiments is that a single stress session ending 30 min prior to training enhanced learning on a response task but impaired learning on a place task. Both versions of the maze involve similar motivational and sensory-motor components, suggesting that the opposite direction of results is likely due to the different cognitive demands of the tasks and not to performance variables.
When administered 24 h prior to place or response training, neither repeated nor single stress treatments significantly enhanced or impaired learning in either task. The failure to observe 24 h anterograde effects of repeated or single stress episodes on learning is not consistent with many studies showing such effects [cf., 11, 12, 13, 44, 52, 53, 54]. One possible reason for the failure to see effects of chronic stress on learning is that the rats may have become habituated to the stressor during the 21 days of treatment. However, other studies using similar stressors have shown 24 h anterograde effects on memory and on brain function [e.g., 20, 43, 47, 57, 58]. It is also possible that the two tasks used here are not as sensitive to stress history as are the tasks used in prior studies, e.g. eyeblink conditioning, spatial and non-spatial versions of the swim task, novelty motivated spatial memory, inhibitory avoidance, object recognition, and fear conditioning [3, 4, 13, 19, 39, 71, 92].
The ability of a single stress episode near the time of training to enhance response learning and to impair place learning agrees with results of several prior reports showing that acute stress or anxiogenic drugs bias rats towards the use of a striatal-strategy and away from a hippocampal-strategy when trained on a water maze task [37, 64]. Preference for place or response solutions to tasks like those used here has also been observed in several other contexts. For example, estrogen and ethanol enhance learning on a maze optimally solved using a place strategy and impair learning on a task optimally solved using a response strategy [40, 42, 51, 96]. These findings are also similar to those observed after lesions of the fimbria-fornix [50] and after pharmacological inactivation of the hippocampus [15, 63, 78], conditions that impair spatial learning and enhance response learning. In addition, injections of glutamate and glucose into the hippocampus or striatum can alter the use of place or response strategies rats express on a dual-solution T-maze [14, 61]. In other studies, acetylcholine release measured before and during training is correlated with the relative participation of the hippocampus and striatum in learning processes. In particular, the ratio of acetylcholine release in the hippocampus and striatum immediately before and during training is associated with selection of place or response strategies used to solve a dual solution task and also predicts better place learning and poorer response learning in single-solution tasks, such as those used in the present experiment [16, 56, 68, 69]. The present findings suggest that recent stress may alter the ratio of acetylcholine release in the hippocampus and striatum in a direction that favors the striatum, a possibility amenable to direct tests in the future.
Evidence that place and response learning are modified in opposite directions by lesions, drugs, and stress has been interpreted as reflecting competition between hippocampal and striatal memory systems [31, 40, 67, 91]. The present findings do not reveal whether these effects reflect down-regulation of hippocampal contribution to learning, up-regulation of striatal contributions, or both. Under some conditions, the reciprocal enhancements and impairments seen across tasks, and presumably neural systems, may be somewhat independent of each other. For example, glucose injections into the striatum impair place learning, but do not enhance response learning [70]. Estradiol injections into the hippocampus enhance place learning, but do not impair response learning, and estradiol injections into the striatum impair response learning, while having no effect on place learning [96]. These findings suggest that apparent competition may reflect, in some cases, independent actions at each neural system rather than interacting effects.
In addition to evidence of stress-induced changes in the balance between memory systems, there is related information regarding the neural systems that mediate these stress effects. Pertinent to the present findings is evidence that hippocampal lesions block both enhancing (in male rats) and impairing (in female rats) influences of stress on delayed eye-blink conditioning, a task that does not require the hippocampus for learning per se [3]. Besides the hippocampus and striatum, other brain memory systems, such as the amygdala [28], may mediate acute stress effects on learning and memory. Administration of anxiogenic drugs into the amygdala results in a shift to the use of a response strategy over a place strategy [64]. Also, inactivation of the amygdala with muscimol blocks the effects of stress on classical eyeblink conditioning and spatial swim task learning after a single stress episode [36, 37, 89]. Therefore, in addition to interpreting the findings of the present experiment in terms of hippocampal and striatal memory systems, it is also important to identify the neural systems that mediate stress alterations of preferred learning strategies.
The change in preferred strategy with estrogen status [40] suggested the possibility that other hormones, including the stress hormones corticosterone and epinephrine, might shift the relative participation of different neural systems during learning. However, corticosterone did not result in modulation of learning either task. Thus, it does not appear that corticosterone is the basis for the effects of stress on the place and response tasks. Epinephrine mimicked effects of stress in the place task and may contribute to the stress-related impairment of learning in that task. However, epinephrine impaired learning of the response task, an effect opposite that of stress, suggesting that epinephrine at best only partially contributes to the effects of stress on learning.
In conclusion, the short-lasting effects of a single stress episode do not appear to be simply instances of enhancement or impairment of learning, but are characterized by impairment of learning on one task, place learning, and enhancement on another task, response learning. These findings support the view that acute stress alters the balance between memory systems.
Acknowledgments
This work was supported by research grants from NIA (AG 07648) and NIDA (DA 016951 and DA 024129).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn Mem. 2004;11:188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldi E, Bucherelli C. The inverted U-shaped dose-effect relationships in learning and memory : modulation of arousal and consolidation. Nonlinearity in Biol Toxicol Med. 2005;3:9–21. doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardgett ME, Newcomer JW, Taylor GT. The effects of chronic corticosterone on memory performance in the platform maze task. Physiol Behav. 1996;59:1111–1115. doi: 10.1016/0031-9384(95)02172-8. [DOI] [PubMed] [Google Scholar]
- 6.Baulieu EE. Cell membrane, a target for steroid hormones. Mol Cell Endocrinol. 1978;12:247–254. doi: 10.1016/0303-7207(78)90083-7. [DOI] [PubMed] [Google Scholar]
- 7.Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Beylin AV, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav Neurosci. 1998;112:1327–1338. doi: 10.1037//0735-7044.112.6.1327. [DOI] [PubMed] [Google Scholar]
- 9.Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowman RE. Stress-induced changes in spatial memory are sexually differentiated and vary across the lifespan. J Neuroendocrinol. 2005;17:526–535. doi: 10.1111/j.1365-2826.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 12.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 13.Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–166. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 14.Canal CE, Stutz SJ, Gold PE. Glucose injections into the dorsal hippocampus or dorsolateral striatum of rats prior to T-maze training: modulation of learning rates and strategy selection. Learn Mem. 2005;12:367–374. doi: 10.1101/lm.88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res. 2003;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 16.Chang Q, Gold PE. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 18.Conrad CD. The relationship between acute glucocorticoid levels and hippocampal function depends on task aversiveness and memory processing stage. Nonlinearity Biol Toxicol Med. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 20.Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of the estrous cycle. Pharmacol Biochem Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 22.Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels--a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- 23.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 24.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;60803:1–33. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 26.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Dornelles A, de Lima MN, Grazziotin M, Pesti-Torres J, Garcia VA, Scalco FS, Roester B, Schrëder N. Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem. 2007;88:137–142. doi: 10.1016/j.nlm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol Sin. 2000;21:481–493. [PubMed] [Google Scholar]
- 29.Gold PE. Modulation of emotional and non-emotional memories: Same pharmacological systems, different neuroanatomical systems. In: McGaugh JL, Weinberger NM, Lynch GS, editors. Brain and Memory: Modulation and Mediation of Neural Plasticity. New York: Oxford Press; 1995. pp. 41–74. [Google Scholar]
- 30.Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82:230–242. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Gold PE. Memory enhancing drugs. In: Eichenbaum H, editor. Memory Systems Vol 3 of J Byrne, editor Learning and Memory: A Comprehensive Reference. Oxford: Elsevier; 2008. pp. 555–576. [Google Scholar]
- 33.Gold PE, Van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- 34.Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;45:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Kesner RP, Bolland BL, Dakis M. Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp Brain Res. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- 36.Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JJ, Lee HJ, Welday AC, Song E, Cho J, Sharp PE, Jung MW, Blair HT. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc Natl Acad Sci U S A. 2007;104:18297–18302. doi: 10.1073/pnas.0708644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Korol DL, Gold PE. Modulation of learning and memory by adrenal and ovarian hormones. In: Kesner RP, Martinez JL, editors. Neurobiology of Learning and Memory. New York: Elsevier; 2007. pp. 243–268. [Google Scholar]
- 42.Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- 43.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luine VN. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 45.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 46.Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Res. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 47.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 48.Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine responses to acute swim stress. Neurobiol Learn Mem. 1995;63:260–268. doi: 10.1006/nlme.1995.1030. [DOI] [PubMed] [Google Scholar]
- 49.Maroun M, Akirav I. Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology. 2008;33:394–405. doi: 10.1038/sj.npp.1301401. [DOI] [PubMed] [Google Scholar]
- 50.Matthews DB, Best PJ. Fimbria/fornix lesions facilitate the learning of a nonspatial response task. Psychon Bull Rev. 1995;2:113–116. doi: 10.3758/BF03214415. [DOI] [PubMed] [Google Scholar]
- 51.Matthews DB, Ilgin M, White AM, Best PJ. Acute ethanol administration impairs spatial performance while facilitating nonspatial performance in rats. Neurobiol Learn Mem. 1999;72:169–179. doi: 10.1006/nlme.1998.3900. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 53.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 54.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 55.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Op Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 56.McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 57.McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 60.Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- 61.Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci U S A. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 64.Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem. 2004;82:243–252. doi: 10.1016/j.nlm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Park CR, Campbell AM, Woodson JC, Smith TP, Fleshner M, Diamond DM. Permissive influence of stress in the expression of a U-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose Response. 2006;4:55–74. doi: 10.2203/dose-response.004.01.005.Park. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15:271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 68.Pych JC, Chang Q, Colon-Rivera C, Gold PE. Acetylcholine release in hippocampus and striatum during training on a rewarded spontaneous alternation task. Neurobiol Learn Mem. 2005;84:93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. Acetylcholine release in the hippocampus and striatum during place and response training. Learn Mem. 2005;12:564–572. doi: 10.1101/lm.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pych JC, Kim M, Gold PE. Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behav Brain Res. 2006;167:373–378. doi: 10.1016/j.bbr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- 72.Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 73.Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:575–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 74.Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000;57:1337–1345. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- 75.Sandi C, Loscertales M, Gauza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 76.Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007;78970:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, Venero C, Diamond DM. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry. 2005;57:856–864. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 78.Schroeder JP, Wingard JC, Packard MG. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus. 2002;12:260–264. doi: 10.1002/hipo.10024. [DOI] [PubMed] [Google Scholar]
- 79.Schwabe L, Dalm S, Schächinger H, Oitzl MS. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiol Learn Mem. 2008;90:495–503. doi: 10.1016/j.nlm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 80.Schwabe L, Oitzl MS, Richter S, Schachinger H. Modulation of spatial and stimulus-response learning strategies by exogenous cortisol in healthy young women. Psychoneuroendocrinology. 2009;34:358–366. doi: 10.1016/j.psyneuen.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 82.Shors TJ. Learning during stressful times. Learn Mem. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shors TJ. Stressful experience and learning across lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 85.Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Sternberg DB, Isaacs KR, Gold PE, McGaugh JL. Epinephrine facilitation of appetitive learning: attenuation with adrenergic receptor antagonists. Behav Neural Biol. 1985;44:447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- 87.Talley CE, Kahn S, Alexander LJ, Gold PE. Epinephrine fails to enhance performance of food-deprived rats on a delayed spontaneous alternation task. Neurobiol Learn Mem. 2000;73:79–86. doi: 10.1006/nlme.1999.3920. [DOI] [PubMed] [Google Scholar]
- 88.Torras-Garcia M, Costa-Miserachs D, Portelli-Cortés I, Morgado-Bernal I. Posttraining epinephrine and memory consolidation in rats with different basic learning capacities. The role of the stria terminalis. Exp Brain Res. 1998;121:20–28. doi: 10.1007/s002210050432. [DOI] [PubMed] [Google Scholar]
- 89.Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci. 2008;28:5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Warren DA, Castro CA, Rudy JW, Maier SF. No spatial learning impairment following exposure to inescapable shock. Psychobiology. 1991;19:127–134. [Google Scholar]
- 91.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 92.Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- 93.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10:326–336. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav Brain Res. 2008;187:41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]