Abstract
In this report, we demonstrate that chronic prenatal exposure to a moderate dose of caffeine disrupts novel object recognition and radial arm maze behaviors in adult male and female rats. Pregnant dams were administered either tap water or 75 mg/L caffeinated tap water throughout gestation. Oral self-administration in the drinking water led to an approximate maternal intake of 10 mg/kg/day, equivalent to 2–3 cups of coffee/day in humans based on a metabolic body weight conversion. In adulthood, the offspring underwent testing on novel object recognition, radial arm maze, and Morris water maze tasks. Prenatal caffeine exposure was found to impair 24-hour memory retention in the novel object recognition task and impair both working and reference memory in the radial arm maze. However, prenatal caffeine exposure did not alter Morris water maze performance in either a simple water maze procedure or in an advanced water maze procedure that included reversal and working memory paradigms. These findings demonstrate that chronic oral intake of caffeine throughout gestation can alter adult cognitive behaviors in rats.
Keywords: Caffeine, Prenatal, Learning and Memory, Novel Object Recognition, Radial Arm Maze, Morris Water Maze
1. Introduction
Caffeine, a methylxanthine, is one of the most widely consumed psychoactive drugs in the world due to its presence in coffee, tea, chocolate, soft drinks and numerous medications [17]. Caffeine is rapidly absorbed throughout the body and brain. At moderate doses, caffeine exerts its stimulatory effects on behavior directly via blockade of adenosine receptors. Of the four characterized adenosine receptors (A1, A2A, A2B, and A3), caffeine has its primary actions at the A1 and A2A receptors [16]. A1 receptors are the most abundant of the adenosine receptors and are highly expressed throughout the cerebral cortex, hippocampus, and cerebellum [10, 13, 24, 37, 47]. In the developing rat brain, the A1 receptor is one of the earliest receptors expressed [36] with mRNA detectable by gestational day 14 and adult patterns of distribution established by gestational day 20 [46]. A2A receptors are restricted in expression to dopaminergic regions of the brain [38], with mRNA expression detectable in the striatum by gestational day 14 in rats [47]. Developmentally, adenosine plays a key role in the inhibition of neuronal activity [15], formation of axons [43], and neuroprotection [36]. Gestational exposure to caffeine may inhibit the actions of adenosine within the fetal brain, thereby altering normal brain development.
Maternal consumption of caffeine during pregnancy is of concern due to the extended half-life of the drug in the fetus. Caffeine rapidly crosses both the placental and blood-brain barriers to reach the fetal brain [6, 23, 41]. In the fetus, the metabolism and elimination of caffeine are slowed due to a lack of cytochrome P-450 activity, resulting in a prolonged half-life [4]. The half-life of caffeine is estimated to be 50–100 hours at the end of gestation, which may lead to accumulation of caffeine in the fetal brain [5, 6, 34]. In the fetus, the half-life of caffeine is further protracted due to slowed maternal metabolism of caffeine during pregnancy. During the last trimester of pregnancy, the half-life of caffeine is estimated to be 10–15 hours in the expectant mother [3, 8, 26]. Thus, the altered half-life of caffeine in the fetus and expectant mother may greatly prolong the actions of caffeine within the fetal brain.
Perinatal exposure to caffeine causes persistent alterations in brain development. In the neonatal rat brain, concentrations of protein and cholesterol are decreased following low dose (1 or 2 mg/100 g body weight) caffeine exposure [30]. Caffeine injections (50 mg/kg/day) on postnatal days 1–12 cause an enduring increase in the dendritic length of pyramidal neurons within the rat prefrontal cortex [25]. In juvenile rats, caffeine exposure throughout gestation and lactation diminishes the stimulatory locomotor response to MK-801, an NMDA receptor antagonist, suggesting a persistent change in NMDA receptor function [9]. Thus, developmental exposure to caffeine may cause long-term alterations in the brain.
Perinatal exposure to caffeine also alters juvenile and adult locomotor behavior and emotional reactivity. Numerous studies have shown decreased levels of activity in both juvenile and adult rat offspring following developmental caffeine exposure [21, 50]. Caffeine treated offspring exhibit heightened emotional reactivity such that male offspring have increased defecation and decreased exploration in the open field and longer latencies to emerge from a light-dark box [21, 22]. However, increased activity and reduced anxiety following postnatal caffeine exposure has also been shown. Juvenile rats show increased pain sensitivity and reduced anxiety following caffeine exposure (15–20 mg/kg/day) across postnatal days 2–6 [33]. Further, juvenile rats exhibit hyperactivity, which persists into adulthood in an open field task after exposure to caffeine (20 mg/kg/day) across postnatal days 7–11 [42].
The long-term behavioral effects of chronic prenatal exposure to caffeine on measures of learning and memory have not been well investigated. Rat pups exposed to 1 or 9 mg/kg caffeine during the first week of life exhibit hypoactivity at two weeks of age and impairment in a spatial operant learning task during adulthood [50]. Following neonatal exposure, passive avoidance learning is significantly impaired in male and female juvenile rats [33]. In adult animals, neonatal caffeine (15–20 mg/kg/day on PN2–6) treated female rats have significantly improved retention of passive avoidance learning at 24 and 72 hours after training, while male rats have significantly reduced retention [14]. Thus, prenatal caffeine exposure may alter adult behavior in a sexually dimorphic fashion, although these sex differences may be undetectable in pre- or peri-pubertal animals. Importantly, sex differences in response to perinatal caffeine exposure may help determine whether males or females are more susceptible to caffeine induced developmental effects.
The aim of the present study was to document the effect of chronic prenatal exposure to caffeine on cognitive behavior in adult male and female rats. The study investigated whether daily oral administration of caffeine (approximately 10mg/kg) to pregnant rat dams could alter long-term learning and memory in offspring. Specifically, prenatal caffeine-treated and control offspring underwent testing in adulthood in three well-established learning and memory paradigms, including the novel object recognition task, radial arm maze, and Morris water maze.
2. Materials and Methods
2.1 Prenatal caffeine treatment
Female Sprague-Dawley rats 60–70 days of age were mated within our colony at Michigan State University. Males were housed with females for 4 days to ensure that one estrous cycle was completed. Males were removed after 4 days and this day was counted as gestational day 4 to approximate the day of birth. From gestational day 4 until the day of birth, pregnant dams were administered ad libitum tap water (n=4) or 75 mg/L caffeinated tap water (n#x02212;4) with intake measured daily. Caffeine administration was started early in gestation to determine the effects of chronic exposure since it has been shown previously that caffeine can accumulate in the fetal brain [48]. The dose was chosen after calculating metabolic body weight (=body weight3/4) [31]. Oral administration was used to maintain the human relevance of the study.
On postnatal day 2 (day of birth is postnatal day 0) the sex of each offspring was determined and litters were culled to 4 males and 4 females. No observational differences in litter size, sex ratio, or maternal nest building, retrieval, and nursing behavior were noted following birth. Each litter contained approximately 12–14 pups and there was no difference in mean body weights between control (6.57 ± 0.104 g) and caffeine-treated (6.75 ± 0.243 g) offspring. The pups were weaned on postnatal day 25 (PN25) to 2–3 animals per cage such that animals of the same litter and sex were housed together. Offspring were housed in a temperature and humidity controlled environment with a 12 h light/dark cycle (lights off at 5 p.m.) through adulthood. Ad libitum standard chow and tap water were available except during radial arm maze training. The experiments were approved by the Michigan State University Institutional Animal Use and Care Committee and followed NIH guidelines for animal use.
2.2 Behavioral tests
To minimize the stress associated with behavioral testing, animals were handled for two minutes daily for two weeks prior to the start of testing. In the first set of behavior testing, twenty-nine male and female offspring from four litters (control male, n=5; control female, n=8; prenatal caffeine-treated male, n=8; and prenatal caffeine-treated female, n=8) underwent three neurobehavioral tests chosen to measure hippocampal-dependent and -independent learning and memory. Object recognition testing, radial arm maze, and Morris water maze were assessed between PN 110–150, respectively. The testing order was chosen so that less stressful behavior tasks were performed prior to more stressful tasks. The stress induced by each task was previously determined by the defecation and grooming behavior of animals during each task, with almost no defecation and grooming behaviors in the object recognition test and very high levels of defecation and grooming in the Morris water maze. In the second set of behavior tests, thirty-two male and female offspring from four litters (control male, n=8; control female, n=8; prenatal caffeine-treated male, n=8; and prenatal caffeine-treated female, n=8) underwent 10 consecutive days of testing in the advanced Morris water maze. Prior to each behavior test, the animals were placed in the testing room 30 min before testing to allow habituation to the environment. All testing was performed between noon and 4 p.m. During each test, the experimenter remained outside the testing room except between trials. The behavior room has numerous extra-maze cues such as shelving, counters, and signs. Testing was always performed under dim illumination. Each test was recorded and tracked using Smart tracking system (San Diego Instruments). Behavior was scored by at least two observers blind to the treatment groups.
2.2.1 Novel object recognition test
The novel object recognition task was used to assess non-spatial memory. The testing arena was a 40 cm × 60 cm open area to which rats were habituated for 10 min over two days prior to pre-training and training. During pre-training, the animals were allowed to explore two identical objects (A, A) for 3 min. Memory retention testing was performed at both 3 and 24 hrs after pre-training. To determine memory retention at 3 hrs, animals were allowed to investigate a familiar object (A) and a novel object (B) for 3 min. Memory retention was again tested at 24 hrs following pre-training with the familiar object (A) and a novel object (C). The objects used were small plastic toys of varying color and shape. Objects were placed 12 cm from each wall and 18 cm from each other in the maze. Objects were counterbalanced within the arena during each trial and rinsed with 70% alcohol between trials. Time exploring each object was assessed, which included approaching within 2 cm of the object with the face, sniffing, biting, and pawing the object. Sitting or standing on the object was not counted as exploration. The training exploration time was calculated as exploration of objects A1 + A2. The exploration time during the 3 and 24 hour retention tasks were calculated as A + B and A + C respectively. The discrimination index was calculated as the (exploration of novel object − exploration of familiar object) / (total exploration time). A discrimination index above zero indicates the animal explored the novel object more than the familiar object and retained a memory of the familiar object. The goal of this task is to determine the memory retention of the previously explored object (A) after a 3 or 24 hour delay from pre-training, which is assessed by exploration of the novel object. Therefore, in this task the delay between pre-training and the memory retention task is a key variable. As the delay is increased, the memory demands become more difficult.
2.2.2 Radial arm maze
The radial arm maze was used to assess hippocampal-dependent working and reference memory. The radial arm maze is a spatial navigation memory task involving primarily the hippocampus and prefrontal cortex [32]. Two weeks prior to behavior testing, animals were individually housed and began food deprivation until 90% of their starting body weight was reached. During this time, animals were handled, weighed, and habituated to a cereal reward daily. The eight-arm maze consisted of a 25 cm diameter round center platform from which 65 cm walled arms emerge. To reduce the stress of the novel environment, rats were pre-trained to the radial arm maze for one session per day over 7 consecutive days. During each pre-training session, a cereal reward was moved closer to the end of each arm to ensure the rats learned to search the entire length of the arm for the reward. Each animal was allowed 10 min to consume the rewards in all 8 arms.
Radial arm maze training occurred over 10 consecutive days, one trial per day. During training, food cups were placed at the end of each arm to ensure that the rats could not see the cereal reward from the center platform. Four of the eight food cups were baited with a reward and the sequence of baiting (arms 2, 3, 5, and 7) remained constant. Animals were placed onto the center platform at the beginning of each trial and were allowed 10 min to retrieve all cereal rewards. After eating all 4 cereal rewards or once 10 min had elapsed, animals were placed in their home cage. Each day animals were given food pellets (gram amount equaled 12–16% of their body weight) after the completion of the behavior session. The maze was cleaned with ethanol between each trial. Upon completion of radial arm maze testing, standard chow was again freely available to the rats. Memory acquisition was assessed as the decrease in the number of errors between each trial. Reference memory errors were scored as entries into an arm that was not baited such that animals would need to recall from previous days which arms were not baited to avoid error. Working memory errors were scored as reentries into an arm from which food had already been eaten such that animals would have to recall within a given trial which arms they had already visited.
2.2.3 Standard Morris water maze
The standard Morris water maze [29] was used as a measure of hippocampal-dependent spatial learning and memory. The maze was a 2 m diameter, 1 m tall circular pool in which the water was kept at 26 ± 1 °C. During pre-training, animals underwent three consecutive trials from separate starting points. Animals were allowed 60 sec per trial to find the non-submerged platform. Between each of the three trials, animals were allowed to sit upon the platform for 20 sec. If an animal did not find the platform within the allowed time, an experimenter would guide the animal to the platform.
Morris water maze training began two days after pre-training. Animals underwent 12 non-consecutive 60 sec trials starting at random from one of four cardinal directions. At the start of each trial, animals were placed into the water facing the wall of the maze. The platform (8 inch diameter) was submerged beneath water made opaque with non-toxic white paint and always remained in the same location within the maze. Animals were removed from the maze after finding the platform or once the allowed time had elapsed and were dried and returned to a holding cage. The inter-trial interval was approximately 30 min. Latency to find the platform and path length were recorded using the Smart tracking system.
A probe test was done on the same day after the completion of the 12 trials. During the 30 second probe test, the platform was completely removed from the water. The number of times the animals crossed the location where the platform was previously located was assessed.
2.2.4 Advanced Morris water maze
Three paradigms were tested in the Morris water maze across nine consecutive days; Days 1–3 examined place learning, days 4–6 examined reversal, and days 7–9 examined working memory. The water maze and recording software used was the same as described previously.
For the place learning task, animals underwent 4 non-consecutive trials per day starting once from each cardinal direction. At the start of each trial, animals were placed into the water facing the wall of the maze. The platform was submerged beneath the opaque water and remained in the same location all three days. Animals were given 60 s to find the hidden platform and if unsuccessful were guided to the platform location by an experimenter. All animals were allowed to rest for 20 s on the platform before being returned to their home cage. No pre-training occurred prior to day 1 of testing. A 30 s probe test in which the platform was completely removed from the water was performed on day 3 after the completion of the place learning trials.
For the reversal task, animals underwent 4 non-consecutive trials per day starting once from each cardinal direction and were again allowed 60 s per trial. However, the platform was located opposite to its position during the place learning task. The platform remained in the new location all three days.
During the working memory task, animals underwent 4 consecutive trials per day starting from the same location. The platform was moved to a new location each day and the starting location was also varied each day. The animals were allowed 60 s per trial to find the submerged platform and then were allowed a 20 s rest on the platform before beginning the next trial.
2.3 Data analysis
Statistical assessment of weekly water intake was performed using a two-way repeated measures analysis of variance (week, treatment). For assessment of the object recognition test, two-way analysis of variance (sex, treatment) was performed on the pre-training, 3 hr memory, and 24 hr memory trials. Radial arm maze and Morris water maze were assessed by two-way repeated measures analysis of variance (sex, treatment). All analyses were performed using the SYSTAT program (version 11.0).
3. Results
3.1 Water intake
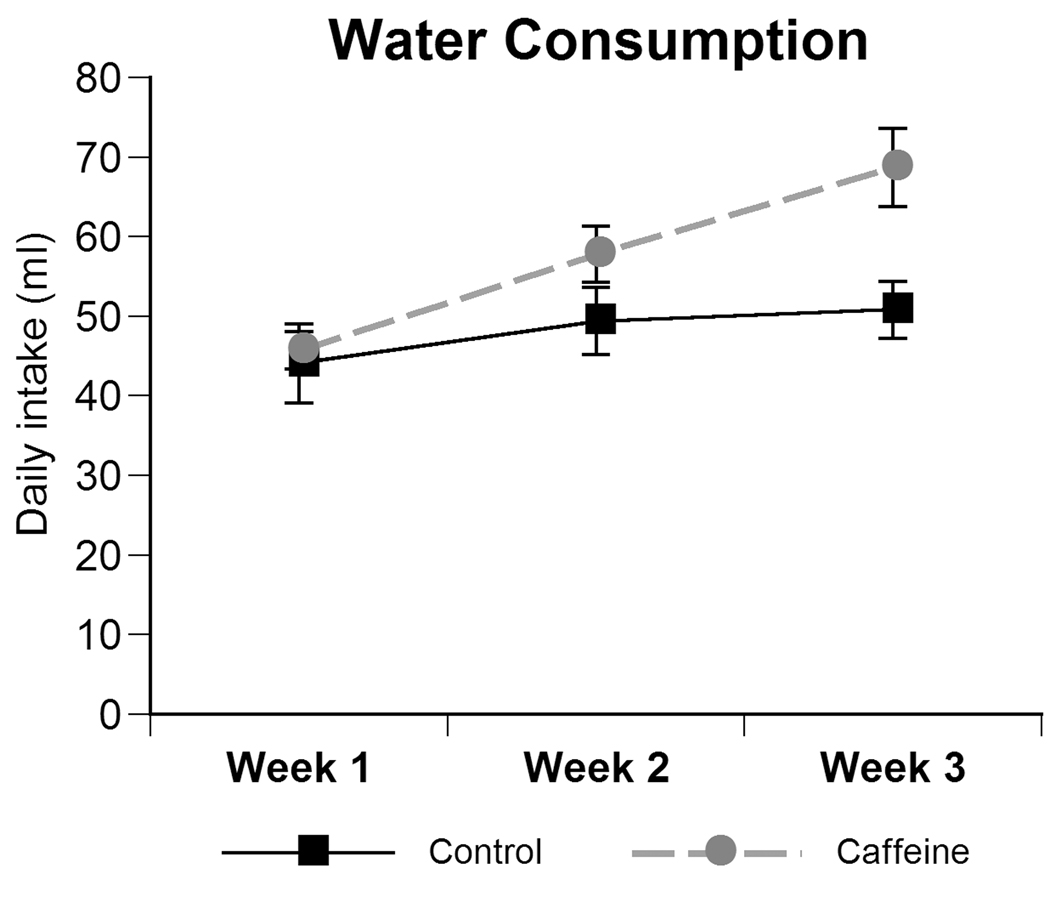
During the time of caffeine exposure, the average water intake in control and caffeine-treated dams significantly increased each week (Fig 1; within-group analyses – F(2,12)=23.054, p<0.001) and showed a significant week by treatment interaction [F(2,12)=6.885, p<0.01] with caffeine-treated animals having greater intake than controls during week 3. However, there was no significant main effect of treatment on water intake.
Fig. 1.
Maternal intake of either water or caffeinated tap water across each gestational week. Data represent the mean daily intake ± SEM for each week. Water intake increased throughout gestation but did not significantly differ by treatment.
3.2 Novel object recognition test
Total exploration time
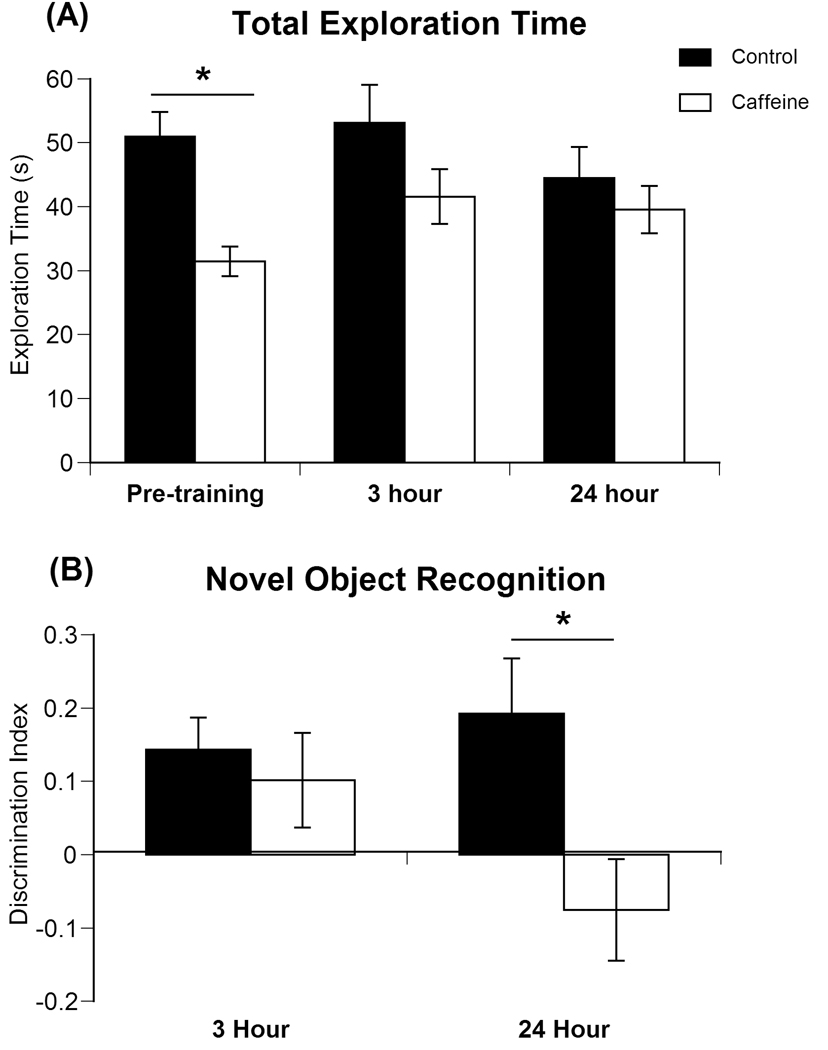
During pre-training, two-way analysis of variance revealed a significant effect of treatment [F(1,25)=18.94, p<0.001] on the total amount of time spent exploring the novel objects (Fig 2a). Caffeine-treated animals spent less total time exploring the novel objects during pre-training. However, the total time spent exploring the novel and familiar objects was not significantly different between treatments during the 3 hour and 24 hour recognition memory tests. At 3 hours, there was a significant effect of sex [F(1,25)=5.455, p<0.05] on total exploration time, with females spending more time (54.1 ± 5.3 sec) exploring than males (37.8 ± 3.8 sec) yet this effect was not seen at 24 hours.
Fig 2.
(A) Effects of prenatal caffeine on the total time spent exploring both objects in the novel object recognition test during each 3 min trial. Data represent the mean exploration time in seconds ± SEM for pre-training, 3 hour recognition memory, and 24 hour recognition memory. There was a significant effect of treatment on total exploration time during pre-training, with caffeine-treated animals exploring the objects less. (B) Effects of prenatal caffeine exposure on memory retention of the novel object at 3 and 24 hours after pre-training. Data represent the percent of time ± SEM spent exploring the novel object. Prenatal exposure to caffeine significantly reduced memory retention of the novel object at 24 hours after pre-training.
Memory Retention Testing
At 3 hours following pre-training, there was no significant effect of treatment, sex, or treatment by sex interaction on the novelty discrimination index (Fig 2b). At 24 hours following pre-training, two-way analysis of variance showed a significant effect of treatment [F(1,25)=7.652, p<0.02] but no significant effect of sex or treatment by sex interaction on the discrimination index of novel object exploration (Fig 2b). These findings indicate that at 24 hours following pre-training, the caffeine-treated animals do not distinguish between the novel and familiar objects as well as control animals.
3.3 Radial arm maze
Pre-training
Across the 7 consecutive days of pre-training in the radial arm maze, a two-way repeated measures ANOVA revealed no significant between-group effect of treatment, sex, or treatment by sex interaction on the total time required to retrieve all food rewards. However, there was a significant within-group effect of time [F(6,150)=49.879, P<0.001] indicating that animals learned the task (Table 1).
Table 1.
Mean (± SEM) values of radial arm maze pre-training time (s), training path lengths (cm), time to retrieve all cereal rewards (s), and body weights before and after food deprivation.
| Control Male | Control Female | Caffeine Male | Caffeine Female | |
|---|---|---|---|---|
| (n=5) | (n=8) | (n=8) | (n=8) | |
| Pre-Training Time (s) | ||||
| Day 1 | 482.8 ± 68.5 | 569 ± 31 | 361.5 ± 36.5 | 551.5 ± 38.1 |
| Day 2 | 285.4 ± 56.2 | 385.2 ± 53.1 | 255.7 ± 54.4 | 315.7 ± 32.9 |
| Day 3 | 215 ± 19.8 | 266.3 ± 64.2 | 212 ± 39.2 | 180 ± 29.8 |
| Day 4 | 230 ± 41.8 | 253 ± 64.1 | 185.2 ± 31.4 | 194.5 ± 41.8 |
| Day 5 | 151.2 ± 15.4 | 127.1 ± 24.2 | 124.7 ± 12 | 243.8 ± 62.7 |
| Day 6 | 171.2 ±33.7 | 189 ± 31.5 | 136.4 ± 22.1 | 139.4 ± 16.4 |
| Day 7 | 228.2 ± 42.3 | 208.7 ± 19.1 | 137.1 ± 8.9 | 181 ± 28.9 |
| RAM Training – Path Length (cm) | ||||
| Block 1 | 3862 ± 658 | 5042 ± 795 | 4129 ± 325 | 5857 ± 785 |
| Block 2 | 3546 ± 309 | 4272 ± 310 | 5354 ± 589 | 4705 ± 389 |
| Block 3 | 4244 ± 720 | 3724 ± 369 | 4434 ± 567 | 4555 ± 440 |
| Block 4 | 3598 ± 264 | 3626 ± 569 | 3523 ± 450 | 3065 ± 222 |
| Block 5 | 3278 ± 167 | 2793 ± 445 | 2908 ± 239 | 3270 ± 253 |
| RAM Training – Time to Retrieve Rewards (s) | ||||
| Block 1 | 116.1 ± 11.9 | 164.6 ± 40.2 | 115.6 ± 12.8 | 171.5 ± 12 |
| Block 2 | 99.9 ± 3.9 | 119.4 ± 10.9 | 147.2 ± 22.6 | 115.9 ± 15.4 |
| Block 3 | 133.7 ± 31.1 | 112.8 ± 10.2 | 104.1 ± 6.6 | 112.8 ± 9.9 |
| Block 4 | 115.6 ± 5.9 | 116.1 ± 16.6 | 93.4 ± 12.4 | 84.2 ± 6.9 |
| Block 5 | 113.6 ± 8.6 | 106.5 ± 11.7 | 93.4 ± 16.2 | 96.9 ± 9.7 |
| Body Weights (g) | ||||
| Start Weight | 435.4 ± 8.6 | 418 ± 12 | 247.5 ± 6 | 238.8 ± 10.3 |
| End Weight | 393.4 ± 8.9 | 378.3 ± 10.1 | 227.63 ± 4.4 | 226 ± 7.8 |
Training
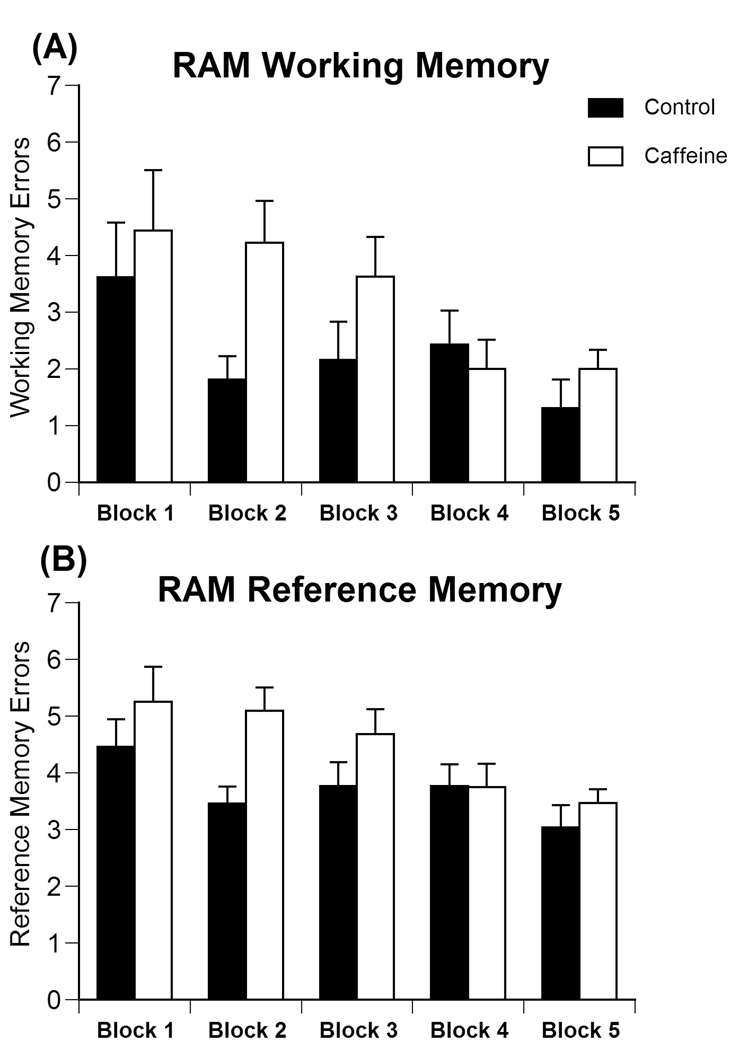
There was a significant between-group effect of treatment on the number of working memory errors [F(1, 25)=4.357, P<0.05] across the five trial blocks (trials 1–2, 3–4, 5–6, 7–8, 9–10) of the radial arm maze. Working memory errors were assessed as the number of reentries into arms already entered during the same trial. Caffeine-treated animals made more errors than controls (Fig. 3a). A significant within-group difference by trial block was observed [F(4,100)=2.988, p<0.03] indicating that animals learned the task across the five blocks.
Fig 3.
Effects of prenatal caffeine exposure on the number of (A) working memory errors and (B) reference memory errors performed during Radial arm maze testing, with caffeine-treated animals performing significantly more memory errors. Data represent the average number of errors ± SEM performed during each block (Trials 1–2, 3–4, 5–6, 7–8, 9–10).
Between-group analyses also revealed a significant main effect of treatment on the number of reference memory errors [F(1, 25)=6.503; P<0.02] with caffeine-treated animals making more errors than controls (Fig. 3b). Reference memory errors were assessed as entries into an arm that never contained a reward. A significant within-group difference was also found across the 5 trial blocks [F(4,100)=3.201, P<0.02] representing a decrease in reference memory errors in both treatment groups as the task was acquired.
There was no significant between-group effect of treatment, sex, or treatment by sex interaction on path length. Path length was measured as the total distance in cm covered across each of the 5 trial blocks. There was a significant within-group effect of path length [F(4,100)=8.601, P<0.001] indicating that as the rats learned the task they decreased their traveling distance to obtain the rewards (Table 1).
Across the 10 consecutive days of testing, there was no significant between-group effect of treatment, sex, or treatment by sex in the total time required for animals to retrieve all 4 food rewards. As in pre-training, there was a significant within-group effect of time [F(9,225)=3.672, P<0.003] across the trial blocks indicating acquisition of the task (Table 1).
Two-way ANOVA revealed no significant effect of treatment or treatment by sex interaction on body weights at the start of food deprivation and at the conclusion of food deprivation (Table 1). However, a significant effect of sex was observed for body weights prior to food deprivation [F(1,25)=335.97, p<0.001] and at the conclusion of food deprivation [F(1,25)=374.42, p<0.001]. Thus, both male and female rats began food deprivation at a healthy weight and lost weight to the same extent during radial arm maze testing.
3.4 Standard Morris water maze
Pre-training
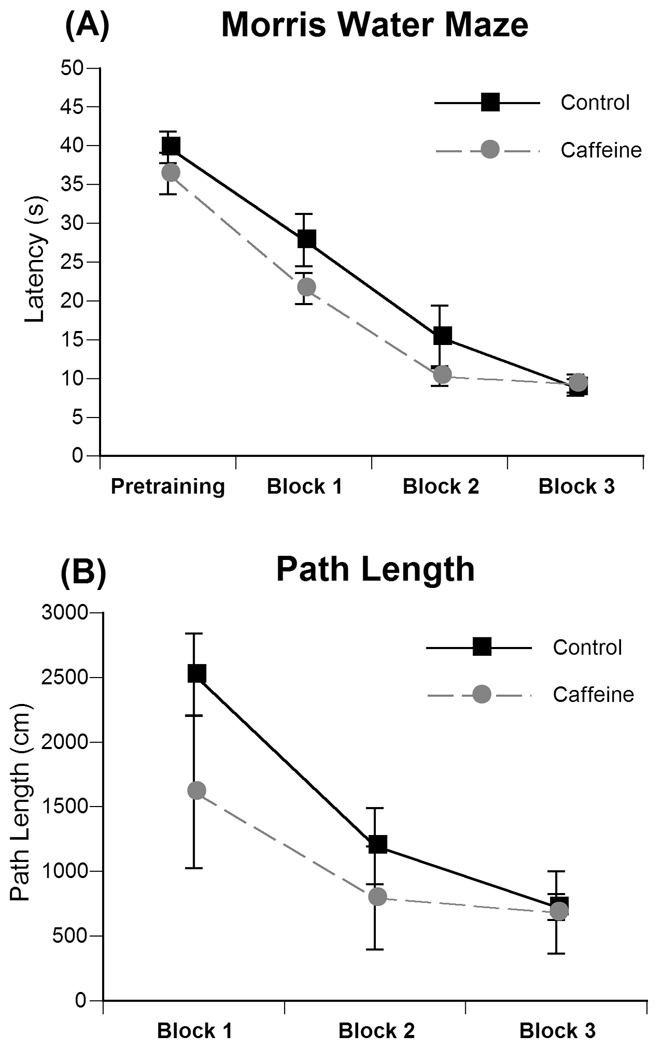
Two-way analysis of variance revealed no significant effect of treatment, sex, or treatment by sex interaction on the pre-training component of the Morris water maze (Fig. 4a). This indicates that the animals did not exhibit motor or sensory deficits that would interfere with the procedural learning of the task.
Fig. 4.
(A) Effects of prenatal caffeine on latency to find the hidden platform during pretraining and across the three trial blocks (block 1=trials 1–4, block 2 = trials 5–8, block 3 = trials 9–12) in the Morris water maze. Data represent the average latency ± SEM needed to find the platform during each 60 s trial. (B) Effects of prenatal caffeine on path lengths in the Morris water maze. Path length was measured as the total distance in centimeters ± SEM swam during each trial averaged across the 3 trial blocks.
Training
Two-way repeated measures ANOVA revealed no significant effect of treatment, sex, or treatment by sex interaction across the 3 trial blocks (trials 1–4, trials 5–8, trial 9–12; Fig 4a) on water maze latency. There was a significant within-group difference across the three trial blocks [F(2,50)=32.65, P<0.001] indicating that all animals were able to decrease their latency to find the platform across the trial blocks. Additionally, no significant effect of treatment, sex, or treatment by sex interaction was observed in the probe trial, indicating that all animals were successfully able to spatially locate the region of the platform by the final trial.
Between-group analyses revealed a significant main effect of treatment on path length [F(1,25)=6.992; P<0.02] across the 3 trial blocks, with caffeine-treated animals swimming less distance than controls (Fig 4b). Path length was measured as the total distance (cm) swam in each trial averaged across the 3 trial blocks. A significant within-group difference was also found across the 3 trial blocks [F(2,50)=37.372, P<0.001] along with a significant within-group block by treatment effect [F(2,50)= 3.466, P<0.04] due to the increased path length of both groups in the first trial block when compared to path length in the second and third trial blocks.
3.5 Advanced Morris Water Maze
Place Learning Task
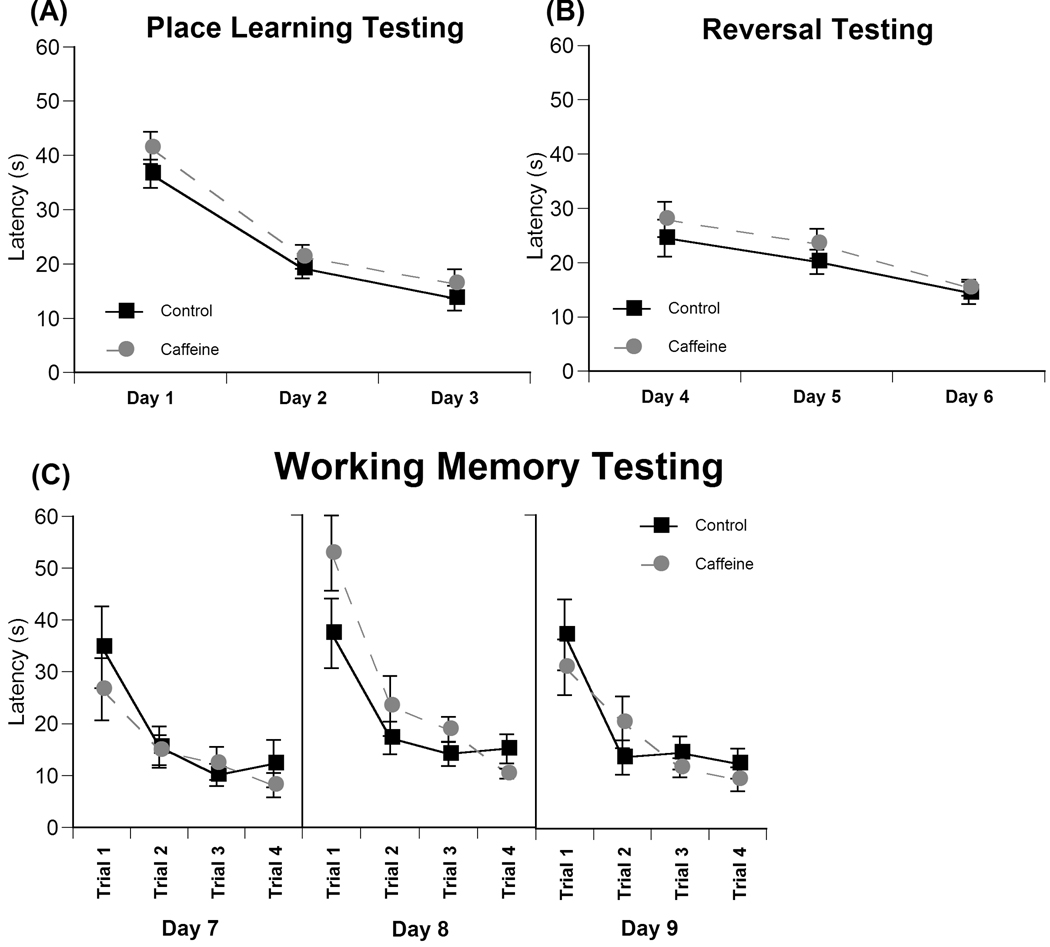
Two-way repeated measures ANOVA revealed no significant effect of treatment, sex, or treatment by sex interaction across the 3 days of place learning (Day 1: trials 1–4, Day 2: trials 5–8, Day 3: trial 9–12; Fig 5a) on latency to find the platform. There was a significant within-group difference across the three days [F(2,54)=88.05, P<0.001] indicating that all animals were able to decrease their latency to find the platform as they learned the task. Additionally, no significant effect of treatment, sex, or treatment by sex interaction was observed in the probe trial conducted on Day 3, indicating that all animals were successfully able to spatially locate the region of the platform by the final trial.
Fig 5.
Effects of prenatal caffeine on latency to find the hidden platform during (A) place learning, (B) reversal, and (C) working memory tasks in the Morris water maze across the four trials conducted each day over 9 days of testing. Data represent the average latency ± SEM needed to find the platform during each 60 s trial.
Between-group analyses revealed no significant difference of treatment, sex, or treatment by sex interaction on place learning path length. Path length was measured as the total distance (cm) swam averaged across the 4 trials swam each day. A significant within-group difference was also found across the 3 days [F(2,54)=62.54, P<0.001] indicating that the path length decreased as animals learned to find the platform (Table 2).
Table 2.
Mean (± SEM) values for advanced Morris water maze path length (cm) in the place learning, reversal, and working memory tasks.
| Control Male | Control Female | Caffeine Male | Caffeine Female | |
|---|---|---|---|---|
| (n=7) | (n=8) | (n=8) | (n=8) | |
|
Place Learning Path Length (cm) |
||||
| Day 1 | 2182 ± 182 | 2571 ± 257 | 2732 ± 161 | 2125 ± 186 |
| Day 2 | 1524 ± 181 | 1252 ± 189 | 1360 ± 247 | 1320 ± 167 |
| Day 3 | 770 ± 156 | 1232 ± 268 | 1171 ± 205 | 890 ± 224 |
| Reversal Path Length (cm) | ||||
| Day 4 | 1449 ± 249 | 2249 ± 408 | 1911 ± 255 | 1613 ± 325 |
| Day 5 | 1264 ± 227 | 1884 ± 245 | 1927 ± 279 | 1327 ± 326 |
| Day 6 | 968 ± 237 | 1188 ± 198 | 737 ± 91 | 1148 ± 175 |
| Working Memory Path Length (cm) | ||||
| Day 7 - Trial 1 | 2753 ± 973 | 2318 ± 636 | 2026 ± 744 | 1658 ± 403 |
| Day 7 - Trial 2 | 930 ± 372 | 1722 ± 603 | 1269 ± 296 | 1023 ± 395 |
| Day 7 - Trial 3 | 854 ± 219 | 788 ± 300 | 548 ± 129 | 1233 ± 475 |
| Day 7 - Trial 4 | 591 ± 290 | 583 ± 193 | 473 ± 130 | 713 ± 303 |
| Day 8 - Trial 1 | 3192 ± 597 | 2905 ± 975 | 3802 ± 762 | 3639 ± 812 |
| Day 8 - Trial 2 | 1247 ± 202 | 1549 ± 443 | 2886 ± 809 | 729 ± 125 |
| Day 8 - Trial 3 | 1310 ± 318 | 1102 ± 266 | 1839 ± 196 | 1114 ± 308 |
| Day 8 - Trial 4 | 1647 ± 401 | 944 ± 221 | 841 ± 75 | 814 ± 191 |
| Day 9 - Trial 1 | 1684 ± 273 | 3650 ± 734 | 2301 ± 604 | 2115 ± 537 |
| Day 9 - Trial 2 | 814 ± 180 | 1069 ± 349 | 1553 ± 399 | 1338 ± 525 |
| Day 9 - Trial 3 | 1083 ± 457 | 1352 ± 369 | 777 ± 297 | 1029 ± 199 |
| Day 9 - Trial 4 | 1117 ± 391 | 798 ± 137 | 869 ± 358 | 544 ± 100 |
Reversal Task
Two-way repeated measures ANOVA revealed no significant effect of treatment, sex, or treatment by sex interaction across the 3 days of reversal testing (Day4: trials 1–4, Day 5: trials 5–8, Day6: trial 9–12; Fig 5b) on latency to find the hidden platform. There was a significant within-group difference across the three days [F(2,54)=16.14, P<0.001] indicating that all animals were able to decrease their latency to find the platform as they learned the task. Additionally, there was a significant within-group day by treatment by sex.interaction [F(2,54)=3.578, P<0.035].
Between-group analyses revealed no significant difference of treatment, sex, or treatment by sex interaction on reversal path length. A significant within-group difference was also found across the 3 days of reversal testing [F(2,54)=15.297, P<0.01] along with a significant within-group day by treatment by sex effect [F(2,54)= 3.432, P<0.04] (Table 2).
Working Memory Task
Two-way repeated measures ANOVA revealed no significant effect of treatment, sex, or treatment by sex interaction on latency to find the platform across the 4 trials of each of the three days of repeated acquisition working memory testing. There was a significant within-group difference across the four trials each day (Day 7: [F(3,81)=8.65, P<0.001], Day 8: [F(3,81)=21.41, P<0.001], Day 9: [F(3,81)=13.694, P<0.001] indicating that animals were able to find the platform more rapidly each day during the consecutive trials.
Two-way repeated measures ANOVA revealed no significant effect of treatment, sex, or treatment by sex interaction on path length across the 4 trials of each of the three days of repeated acquisition working memory testing. There was a significant within-group difference across the four trials each day (Day 7: [F(3,81)=6.82, P<0.001], Day 8: [F(3,81)=17.15, P<0.001], Day 9: [F(3,81)=13.16, P<0.001] indicating that the path length shortened during each consecutive trial as the animals learned the location of the hidden platform (Table 2).
4. Discussion
In this report, we demonstrate that chronic prenatal exposure to a moderate dose of caffeine disrupts learning and memory behaviors in adult male and female rats. Specifically, we have shown that prenatal caffeine exposure impairs 24 hour memory retention in the novel object recognition task and impairs spatial learning in the radial arm maze. Previous studies in rats have shown that prenatal caffeine exposure alters the concentration of DNA, protein, and cholesterol in the newborn brain [30], decreases fetal brain weight [40], delays developmental milestones [42], and alters juvenile behavior [33]. However, the long-term effects of prenatal caffeine exposure have not been well studied. Unlike many previous studies that used a high dose of caffeine and an injection or gavage administration paradigm, we used a low dose of approximately 10 mg/kg/day and allowed self-administration in the drinking water. We chose this dose and method of administration to maintain human relevance and to avoid the stress associated with injections or gavage. We did not find any main effects of treatment on water intake between control and caffeine-treated dams indicating that caffeine-treated dams were not averse to the taste of the caffeinated water. However, there was a significant week by treatment interaction such that caffeine treated animals drank more than control animals during the latter weeks of gestation. In pregnant rat dams, water consumption usually increases throughout gestation. In dams treated with caffeine, this increase in consumption may be more pronounced due to the diuretic effects of the drug. Overall, the findings presented demonstrate that chronic oral intake of caffeine throughout gestation can lead to subtle alterations in adult cognitive behavior in rat offspring.
The novel object recognition test, which measures the natural propensity of a rat to explore a novel versus familiar object [11], revealed that after a 24 hour inter-trial interval, exploration of the novel object is reduced in animals exposed to prenatal caffeine. This task has both an exploratory behavior component as well as a memory retention component such that an animal must have sufficiently explored the familiar object during the pretest phase in order to distinguish between it and a novel object later during the test phase [11, 12]. Caffeine-treated animals exhibited less total exploration time during pre-training than controls. This is similar to previous findings in other behavioral paradigms showing prenatal caffeine-treated adult animals exhibit decreased exploratory behavior, which may be due to altered emotional reactivity in caffeine treated animals [22]. However, the total exploration time of control and caffeine-treated animals during the 3 hour and 24 hour tests were not significantly different. At 3 hours, both groups had a discrimination index greater than zero indicating preference for the novel over the familiar object. After a 24 hour delay, caffeine-treated animals had a discrimination index less than zero indicating that they could no longer distinguish between the familiar and novel objects indicating a deficit in memory, exploratory behavior, or attentional processes.
We also found a deficit in the performance of caffeine-treated animals in the radial arm maze with caffeine-treated animals performing significantly more reference and working memory errors than control animals. Increased errors performed by caffeine-treated animals indicate that prenatal caffeine exposure may alter the developing hippocampus and prefrontal cortex affecting both working and reference memory in adults.
Unlike our findings in the radial arm maze, we did not find a deficit in the Morris water maze. In the standard version of the Morris water maze employed, there was approximately a 30-minute delay between trials, with all 12 trials and the probe trial run on the same day. Thus, the memory retention demands of this version of the water maze task were greatly diminished. However, in the advanced Morris water maze task, the memory demands were increased in both the reversal and working memory paradigms and still there were no differences between control and caffeine-treated animals. This may be due to many differences between the Morris water maze and radial arm maze tasks, including level of difficulty, delay between trials, appetitive vs. aversive motivation used, and the type of memory tested.
In the simple version of the water maze, the caffeine-treated animals had a significantly shorter path length when compared to controls, indicating that caffeine-treated animals performed as well as, or better, than controls in directly finding the platform. The decrease in path length in caffeine-treated animals may be due to the aversive motivation used in the water maze. If prenatal caffeine exposure increases anxiety in juveniles and adults, as has been shown previously [21, 22], these animals may utilize a more direct escape route to avoid the stressful conditions inherent to the water maze. Previous studies have shown that animals with prior exposure to stressful experiences have enhanced learning and memory in the water maze when compared to unstressed animals [49].
It is also possible that prenatal caffeine exposure alters motivational or exploratory aspects of behavior. It has been shown previously that perinatal caffeine exposure alters locomotor activity, increases emotional reactivity, and increases adrenal weight, particularly in males [21, 22]. Each of the tasks presented in this study has a motivational aspect such that caffeine-treated animals may have differences in motivation to explore a novel object or a novel arena. As previously discussed, in the simple version of the Morris water maze, there is a facilitating effect of caffeine treatment on path length. The caffeine-treated animals appear to be more motivated to find the hidden platform than control animals, with the largest difference in path length occurring during the first trial. Perhaps this difference may be attributed to decreased exploratory behavior such that caffeine-treated animals are less motivated to explore the maze in their initial exposure to the novel area. However, we did not see a difference in path length or latency to find the platform on any of the advanced Morris water maze tasks. Nevertheless, it is possible that prenatal caffeine exposure may be altering motivational and exploratory aspects of behavior making it difficult to know whether and how these changes may alter learning and memory testing.
Another concern is that gestational caffeine intake may alter maternal behavior and the intrauterine environment of dams, either of which may alter behavior of the offspring. Recently, it has been shown that wildtype mice born to dams heterozygous for the A1 adenosine receptor exhibit hyperactivity as adults, similar to wildtype mice exposed to caffeine (0.3g/L) from gestational day 1 to postnatal day 21 [7]. Dams heterozygous for the A1 receptor express approximately half the number of A1 receptors in the cortex and hippocampus. Similarly, chronic caffeine exposure leads to a blockade of approximately half the A1 adenosine receptors in the brain. These studies suggest that alterations in maternal A1 receptor function may have long-lasting changes on offspring behavior. Bjorklund et al. (2008) hypothesize that the alterations in offspring behavior are likely due to changes in the prenatal intrauterine environment rather than maternal behavior, since they observed no differences in maternal behavior. This hypothesis aligns with other behavioral teratogen studies, in which the effects on the offspring have been found to result from prenatal drug exposure rather than postnatal maternal influences [45]. Similarly, others have shown no changes in maternal behavior, as assessed by nesting activity as well as licking and grooming behaviors, in dams exposed to caffeine [35] and no effects of cross-fostering on reduced effects of caffeine on the offspring [2]. Therefore, it seems unlikely that the behavioral changes reported are due to alterations in maternal behavior, however it is not possible to completely rule out the effects of the intrauterine environment and maternal behavior as important factors.
The mechanisms by which prenatal caffeine exposure may alter the brain are not well understood. It is known that caffeine targets the adenosine receptor in both the developing and adult brain [17], however it is unknown whether caffeine exerts the same action at these receptors in the fetus and adult. Prenatal exposure to caffeine decreases A1 adenosine receptor expression in the fetal rat brain [1, 27]. In the brain of young adult rats, A1 receptor levels are up-regulated in the cortex, hippocampus, and cerebellum following postnatal caffeine treatment [20]. Thus, exposure to caffeine during development can have long-term effects on adenosine receptor expression. Further, the levels of mGluRs are decreased in the fetal brain following caffeine and theophylline treatment [28]. Enduring changes in adenosinergic and glutamatergic receptor expression following perinatal caffeine exposure may contribute to changes in learning and memory.
Recent studies in transgenic animals have shown that altered adenosine receptor expression impairs learning and memory behavior. Similar to our findings, transgenic rats overexpressing the adenosine A2A receptor were found to have impaired novel object recognition and increased errors in a 6-arm radial tunnel maze while showing no impairments in place learning and reversal tasks in the Morris water maze [19]. A1 receptor knockout animals also show no impairments in the Morris water maze, while showing slowed habitutation to the 6-arm tunnel maze and impaired paired pulse facilitation of long-term potentiation [18]. Thus, if chronic prenatal caffeine is altering adenosine receptor expression in adults, it seems likely that animals may have impaired learning and memory which is not detected using the Morris water maze alone.
In the current study, no sex differences were found in learning and memory behaviors as a result of prenatal caffeine exposure. This finding was surprising given the previous literature on sex dependent effects of developmental caffeine exposure. In adult rats, it has been shown that neonatal caffeine exposure (15–20 mg/kg/day on PN2–6) has differential sex effects on passive avoidance learning with females exhibiting enhanced memory retention at 24 and 72 hours after training, while males exhibit significantly reduced retention at both time periods [14]. Interestingly, the same neonatal caffeine treatment did not cause gender specific effects in juvenile passive avoidance memory tests [14]. Prenatal caffeine exposure has also been shown to alter passive avoidance learning in a sex dependent fashion. Adult female offspring of caffeine treated dams (60 mg/kg/day on GD13–19) showed significantly enhanced retention at 25 days after training when compared to placebo treated controls [39]. Sexually dimorphic effects of prenatal caffeine exposure have also been shown on measures of locomotion and anxiety in the open field, with male animals exhibiting decreased activity and increased defecation [21]. In humans, mean intake of approximately 300 mg/day caffeine during the third trimester of pregnancy was associated with an increased risk of lowered birth weight in males, but not in females [44]. Thus, the current literature suggests that males may be more susceptible to the detrimental effects of developmental caffeine exposure. However, our study and others [50] suggest that both males and females may be affected in specific learning and memory paradigms. Future research is needed to determine why developmental caffeine exposure renders males more susceptible to some detrimental effects of caffeine, while affecting other behaviors equally in both sexes.
In summary, prenatal caffeine exposure alters some learning and memory behaviors in adult male and female rats. Caffeine’s ability to cross both the placental and blood-brain barrier allows it to enter the fetal brain where it has actions at adenosine receptors. Developmental changes in the expression or function of adenosine receptors within the hippocampus and cortex following prenatal exposure to caffeine may contribute to the long-term alterations in learning and memory reported here. Thus, pregnant women should consume caffeine with caution as it may have long-term developmental consequences on multiple facets of behavior including cognition.
Acknowledgements
This work was supported by the Predoctoral NRSA F31DA023785 from NIDA as well as NIMH grant MH68347. The authors wish to thank Dr. Cheryl Sisk for helpful revisions and Jacob McClean for his assistance setting up the behavior recording software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aden U, Herlenius E, Tang LQ, Fredholm BB. Maternal caffeine intake has minor effects on adenosine receptor ontogeny in the rat brain. Pediatr Res. 2000;48:177–183. doi: 10.1203/00006450-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Ajarem JS, Brain PF. Prenatal caffeine exposure modifies behavioural responses in mice. Behav Pharmacol. 1993;4:541–544. [PubMed] [Google Scholar]
- 3.Aldridge A, Bailey J, Neims AH. The disposition of caffeine during and after pregnancy. Semin Perinatol. 1981;5:310–314. [PubMed] [Google Scholar]
- 4.Aranda JV, Collinge JM, Zinman R, Watters G. Maturation of caffeine elimination in infancy. Arch Dis Child. 1979;54:946–949. doi: 10.1136/adc.54.12.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aranda JV, Gorman W, Bergsteinsson H, Gunn T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr. 1977;90:467–472. doi: 10.1016/s0022-3476(77)80718-x. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud MJ. Metabolism of caffeine and other components of coffee. In: Garattini S, editor. Caffeine, Coffee, and Health. New York: Raven Press; 1993. pp. 43–95. [Google Scholar]
- 7.Bjorklund O, Kahlstrom J, Salmi P, Fredholm BB. Perinatal caffeine, acting onmaternal adenosine A(1) receptors, causes long-lasting behavioral changes in mouse offspring. PLoS ONE. 2008;3:e3977. doi: 10.1371/journal.pone.0003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazier JL, Ritter J, Berland M, Khenfer D, Faucon G. Pharmacokinetics of caffeine during and after pregnancy. Dev Pharmacol Ther. 1983;6:315–322. doi: 10.1159/000457332. [DOI] [PubMed] [Google Scholar]
- 9.da Silva RS, Hoffman A, de Souza DO, Lara DR, Bonan CD. Maternal caffeine intake impairs MK-801-induced hyperlocomotion in young rats. Eur J Pharmacol. 2005;509:155–159. doi: 10.1016/j.ejphar.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 12.Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- 13.Fastbom J, Pazos A, Probst A, Palacios JM. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 1987;22:827–839. doi: 10.1016/0306-4522(87)92962-9. [DOI] [PubMed] [Google Scholar]
- 14.Fisher S, Guillet R. Neonatal caffeine alters passive avoidance retention in rats in an age- and gender-related manner. Brain Res Dev Brain Res. 1997;98:145–149. doi: 10.1016/s0165-3806(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 15.Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm BB. Are methylxanthine effects due to antagonism of endogenous adenosine? Trends Pharmacol Sci. 1980;1:129–132. [Google Scholar]
- 17.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 18.Gimenez-Llort L, Masino SA, Diao L, Fernandez-Teruel A, Tobena A, Halldner L, Fredholm BB. Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse. 2005;57:8–16. doi: 10.1002/syn.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimenez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camon L, Wassholm M, Canals M, Terasmaa A, Fernandez-Teruel A, Tobena A, Popova E, Ferre S, Agnati L, Ciruela F, Martinez E, Scheel-Kruger J, Lluis C, Franco R, Fuxe K, Bader M. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol Learn Mem. 2007;87:42–56. doi: 10.1016/j.nlm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Guillet R, Kellogg C. Neonatal exposure to therapeutic caffeine alters the ontogeny of adenosine A1 receptors in brain of rats. Neuropharmacology. 1991;30:489–496. doi: 10.1016/0028-3908(91)90011-y. [DOI] [PubMed] [Google Scholar]
- 21.Hughes RN, Beveridge IJ. Behavioral effects of exposure to caffeine during gestation, lactation or both. Neurotoxicol Teratol. 1991;13:641–647. doi: 10.1016/0892-0362(91)90048-2. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RN, Beveridge IJ. Behavioral effects of prenatal exposure to caffeine in rats. Life Sci. 1986;38:861–868. doi: 10.1016/0024-3205(86)90253-5. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda GJ, Sapienza PP, McGinnis ML, Bragg LE, Walsh JJ, Collins TF. Blood levels of caffeine and results of fetal examination after oral administration of caffeine to pregnant rats. J Appl Toxicol. 1982;2:307–314. doi: 10.1002/jat.2550020609. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis MF, Jacobson KA, Williams M. Autoradiographic localization of adenosine A1 receptors in rat brain using [3H]XCC, a functionalized congener of 1,3- dipropylxanthine. Neurosci Lett. 1987;81:69–74. doi: 10.1016/0304-3940(87)90342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juarez-Mendez S, Carretero R, Martinez-Tellez R, Silva-Gomez AB, Flores G. Neonatal caffeine administration causes a permanent increase in the dendritic length of prefrontal cortical neurons of rats. Synapse. 2006;60:450–455. doi: 10.1002/syn.20318. [DOI] [PubMed] [Google Scholar]
- 26.Knutti R, Rothweiler H, Schlatter C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur J Clin Pharmacol. 1981;21:121–126. doi: 10.1007/BF00637512. [DOI] [PubMed] [Google Scholar]
- 27.Leon D, Albasanz JL, Ruiz MA, Fernandez M, Martin M. Adenosine A1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem. 2002;82:625–634. doi: 10.1046/j.1471-4159.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- 28.Leon D, Albasanz JL, Ruiz MA, Iglesias I, Martin M. Effect of chronic gestational treatment with caffeine or theophylline on Group I metabotropic glutamate receptors in maternal and fetal brain. J Neurochem. 2005;94:440–451. doi: 10.1111/j.1471-4159.2005.03211.x. [DOI] [PubMed] [Google Scholar]
- 29.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Nakamoto T, Joseph FJ, Yazdani M, Hartman AD. Effects of different levels of caffeine supplemented to the maternal diet on the brains of newborn rats and their dams. Toxicol Lett. 1988;44:167–175. doi: 10.1016/0378-4274(88)90143-9. [DOI] [PubMed] [Google Scholar]
- 31.Nehlig A, Derby G. Potential teratogenic and neurodevelopmental consequences of coffee and caffeine exposure: a review on human and animal data. Neurotoxicol Teratol. 1994;16:531–543. doi: 10.1016/0892-0362(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 32.Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physiol Behav. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- 33.Pan HZ, Chen HH. Hyperalgesia, low-anxiety, and impairment of avoidance learning in neonatal caffeine-treated rats. Psychopharmacology (Berl) 2007;191:119–125. doi: 10.1007/s00213-006-0613-y. [DOI] [PubMed] [Google Scholar]
- 34.Parsons WD, Neims AH. Prolonged half-life of caffeine in healthy tem newborn infants. J Pediatr. 1981;98:640–641. doi: 10.1016/s0022-3476(81)80784-6. [DOI] [PubMed] [Google Scholar]
- 35.Picard N, Guenin S, Larnicol N, Perrin Y. Maternal caffeine ingestion during gestation and lactation influences respiratory adaptation to acute alveolar hypoxia in newborn rats and adenosine A2A and GABA A receptor mRNA transcription. Neuroscience. 2008;156:630–639. doi: 10.1016/j.neuroscience.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Genet Metab. 2001;74:160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- 37.Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997;27:332–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 39.Swenson RR, Beckwith BE, Lamberty KJ, Krebs SJ, Tinius TP. Prenatal exposure to AVP or caffeine but not oxytocin alters learning in female rats. Peptides. 1990;11:927–932. doi: 10.1016/0196-9781(90)90011-s. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Nakazawa K, Arima M. Effects of maternal caffeine ingestion on the perinatal cerebrum. Biol Neonate. 1987;51:332–339. doi: 10.1159/000242672. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Nakazawa K, Arima M, Iwasaki S. Caffeine and its dimethylxanthines and fetal cerebral development in rat. Brain Dev. 1984;6:355–361. doi: 10.1016/s0387-7604(84)80111-4. [DOI] [PubMed] [Google Scholar]
- 42.Tchekalarova J, Kubova H, Mares P. Postnatal caffeine exposure: effects on motor skills and locomotor activity during ontogenesis. Behav Brain Res. 2005;160:99–106. doi: 10.1016/j.bbr.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Turner CP, Yan H, Schwartz M, Othman T, Rivkees SA. A1 adenosine receptor activation induces ventriculomegaly and white matter loss. Neuroreport. 2002;13:1199–1204. doi: 10.1097/00001756-200207020-00026. [DOI] [PubMed] [Google Scholar]
- 44.Vik T, Bakketeig LS, Trygg KU, Lund-Larsen K, Jacobsen G. High caffeine consumption in the third trimester of pregnancy: gender-specific effects on fetal growth. Paediatr Perinat Epidemiol. 2003;17:324–331. doi: 10.1046/j.1365-3016.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 45.Vorhees CV. Principles of behavioral teratology. In: Riley EP, Vorhees CV, editors. Handbook of Behavioral Teratology. New York: Plenum Press; 1986. pp. 23–48. [Google Scholar]
- 46.Weaver DR. A1-adenosine receptor gene expression in fetal rat brain. Brain Res Dev Brain Res. 1996;94:205–223. [PubMed] [Google Scholar]
- 47.Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res. 1993;20:313–327. doi: 10.1016/0169-328x(93)90058-w. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson JM, Pollard I. Accumulation of theophylline, theobromine and paraxanthine in the fetal rat brain following a single oral dose of caffeine. Brain Res Dev Brain Res. 1993;75:193–199. doi: 10.1016/0165-3806(93)90023-4. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Cao J, Xiong W, Zhang J, Zhou Q, Wei H, Liang C, Deng J, Li T, Yang S, Xu L. Both stress experience and age determine the impairment or enhancement effect of stress on spatial memory retrieval. J Endocrinol. 2003;178:45–54. doi: 10.1677/joe.0.1780045. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerberg B, Carr KL, Scott A, Lee HH, Weider JM. The effects of postnatal caffeine exposure on growth, activity and learning in rats. Pharmacol Biochem Behav. 1991;39:883–888. doi: 10.1016/0091-3057(91)90048-7. [DOI] [PubMed] [Google Scholar]