Abstract
Aluminum based adjuvants (alum), including aluminum hydroxide (Alhydrogel®) and aluminum phosphate are the most commonly used adjuvant in the US. In order to ensure quality of vaccines, regulatory authorities require evaluation of antigen content in final vaccine products. Currently, there are no generic methods available for the determination of protein content in alum based vaccines. Aluminum hydroxide gels exist as particles in solution, which interfere with direct quantitation of protein content in formulations using assays such as Lowry, BCA or Bradford protein assay. The present study adapts a simple fluorescent assay to directly (without the need for antigen extraction) determine antigen content on Alhydrogel® with accuracy and sensitivity using the o-phthalaldehyde (OPA) reagent. Malaria vaccine candidates AMA1- C1/Alhydrogel, AMA1-C2/Alhydrogel, MSP142-3D7/Alhydrogel, MSP142-C1/Alhydrogel or BSAM-2/Alhydrogel were used as model formulations. The results of the present study show that the OPA assay is highly accurate (87–100%), reproducible, and simple with a linear detection range of 25–400 μg/mL for Alhydrogel® vaccines (except for MSP142-C1, which has a linear detection range of 31.25–500 μg/mL). This assay has proven to be highly useful in our laboratory and been used in routine vaccine quality control processes.
Keywords: OPA, o-phthalaldehyde, Aluminum hydroxide, alum, vaccine, protein assay
1. Introduction
Aluminum containing adjuvants (alum), including aluminum hydroxide (Al(OH)3) (e.g. Alhydrogel®) and aluminum phosphate (AlPO4) are the most frequently used adjuvants in the USA1–5. To ensure vaccine quality, regulatory authorities require the quantitation of antigen content in final vaccine products before they are released for administration in humans. Although aluminum containing adjuvants have been used since 1926 6, there are no generic evaluation methods available for the determination of antigen content on alum-based vaccines. Katz et al. reported a dot-blot method to evaluate the desorbed porcine parvovirus from Alhydrogel® and an ELISA for diluted porcine parvovirus formulations7, 8. However, these methods are semi-quantitative and lack sensitivity and accuracy, not to mention that they are laborious and time consuming. The vaccine research and development fields need a simple assay that can quickly determine the protein content of alum-based vaccines with accuracy, sensitivity and reproducibility.
As early as 1959, Shore et al. reported that the reaction of o-phthalaldehyde (OPA) with histamine yielded a highly fluorescent product which could be used to assay submicrogram amounts of histamine in biological tissues 9, 10. Roth demonstrated that OPA reacted with amino acids under alkaline conditions in the presence of 2-mercaptoethanol by giving rise to strongly fluorescent compounds 11. Because OPA only gives a fluorescence signal after reacting with the N-terminal amine group or amine groups in side chains of lysine, it produces extremely low background and has been used in the determination of peptides, enzymes or proteins in many application areas 10–19. However, use of an OPA assay to directly determine protein content in alum-based vaccines has never been reported.
This paper describes the use of an OPA reagent to quantitate protein content for Alhydrogel®-based vaccines. Our results demonstrate that the OPA assay is simple to use and could directly determine protein content in alum-based vaccines with high accuracy, sensitivity, and reproducibility. This assay can be used as a generic assay for the quality control evaluation of Alhydrogel® based vaccines.
2. Materials and methods
2.1 Reagents
Recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1) from three parasite lines, FVO, 3D7 and L32 (AMA1-FVO, AMA1-3D7, and AMA1-L32, respectively) and P. falciparum 42 kDa fragment of merozoite surface protein 1 from parasite lines FVO and 3D7 (MSP142-FVO and MSP142-3D7, respectively) were purified and characterized by the Malaria Vaccine Development Branch (MVDB), National Institute of Allergy and Infectious Disease, National Institutes of Health, with the methods developed in the MVDB20,21.
Saline (0.9% Sodium Chloride Injection USP) was purchased from Baxter Healthcare (Deerfield, IL), PBS (10X Phosphate Buffered Saline, pH 7.4) from Invitrogen (Carlsbad, CA), Borate Buffer Solution (pH 9.5) from GFS Chemicals (Powell, OH), Alhydrogel® (Aluminum Hydroxide Gel Adjuvant) from Brenntag Biosector (Denmark), o-Phthalaldehyde (OPA) reagent from Pierce (Rockford, IL), and 96-well black flat bottom plate from Thermo Fisher Scientific (Waltham, MA).
2.2 Preparation of standards and formulations
Protein concentrations of AMA1-FVO, AMA1-3D7, AMA1-L32, MSP142-FVO, or MSP142-3D7 were measured by UV at 280 nm and calculated using the extinction coefficients of 1.206, 1.205, 1.096, 0.624, or 0.45, respectively. For preparation of standard curves, purified MSP142-3D7, AMA1-C1 (1:1 mixture of AMA1-FVO and AMA1-3D7), AMA1-C2 (1:1:1 mixture of AMA1-FVO, AMA1-3D7 and AMA1-L32) or BSAM-2 (1:1:1:1 mixture of AMA1- FVO, AMA1-3D7, MSP142-FVO and MSP142-3D7) at concentrations of 1.56, 3.13, 6.25, 12.5, 25, 50, 100, 200 or 400 μg/mL were prepared with 1600 μg/mL Alhydrogel®. MSP142-C1 (1:1 mixture of MSP142-FVO and MSP142-3D7) standards were prepared with 1600 μg/mL Alhydrogel® at concentrations of 1.95, 3.90, 7.82, 15.63, 31.25, 62.50, 125, 250 or 500 μg/mL. Alhydrogel® stock (2%, or 20,000 μg/mL) diluted with 1x PBS (pH 7.4) was used for the analysis of MSP142-3D7 and MSP142-C1 formulations, or diluted with saline (pH 6.9) for AMA1-C1, AMA1-C2 and BSAM-2 formulations.
Test samples of MSP142-3D7 in PBS were prepared at concentrations of 10, 20, 40, 70, 120 and 160 μg/mL with 1600 μg/mL of Alhydrogel® by first mixing the proteins, then adsorption onto Alhydrogel® in 50 ml centrifuge tubes. The antigen/adjuvant mixtures were then rotated in a rotary spinner (Appropriate Technical Resources, Laurel, MD) at 16–24 rpm for 1 h at room temperature. The formulations were then aliquoted in 1 ml aliquots and stored at 4°C until use. AMA1-C1/Alhydrogel, AMA1-C2/Alhydrogel, or BSAM-2/Alhydrogel were prepared similarly in saline.
40 and 160 μg/mL of clinical grade AMA1-C1/Alhydrogel in saline, and 80 and 320 μg/mL of clinical grade MSP142-C1/Alhydrogel in PBS were prepared by the Pharmaceutical Development Service (PDS), National Institutes of Health. 160 μg/mL of clinical grade AMA1-C2/Alhydrogel, and 80 and 320 μg/mL of clinical grade BSAM-2/Alhydrogel were prepared by the Biopharmaceutical Development Program (BDP), NCI-Frederick.
2.3 Assay conditions
Plate incubation times of 0, 2, 5, 10 and 15 min were tested. The pH and buffer systems tested included 0.9 % sodium chloride (pH 6.9), PBS (4 mM phosphate, 0.16 M sodium chloride, pH 7.4) and 50 mM borate buffer solution (pH 9.5). Four different lots (lots 3777, 3792, 3853 or 4021) of Alhydrogel® were also tested, including two lots (lots 3777 and 3792) that had expired prior to testing.
2.4 Test sample preparation
The pH of one vial from each batch of the formulations (test samples) was measured by a pH meter and the assay buffer (the same buffer used in preparing the formulation) was adjusted to match the pH of the formulation prior to analysis. The entire content of a 1.0 ml vial of formulation was mixed by vortex, transferred to a 1.8 ml eppendorf tube, and then centrifuged at 16,000 g for 2 min. The supernatant volume was measured during transfer to a new tube and the pellet was then re-suspended in the pH adjusted assay buffer in an amount equivalent to the supernatant volume removed. Samples of clinical grade vaccines were prepared similarly.
2.5 OPA assay procedure
The OPA assay was performed using 96-well black flat-bottomed plates. Standards were freshly prepared in 1,600 μg/mL of Alhydrogel® with appropriate buffer. For the study to determine the effect of Alhydrogel® on the standard curve, Alhydrogel® was used at 0, 400, 1600 or 6400 μg/mL. The highest concentration of standard was prepared on 1,600 μg/mL of Alhydrogel® with lower concentrations made by 2-fold serial dilutions of this stock into 1,600 μg/mL of Alhydrogel®. Samples (40 μl/well) were tested in triplicate on each plate by addition of 200 μl of OPA reagent. The plates were incubated in the dark and read at 340 nm excitation 455 nm emission at 0, 2, 5, 10 or 15 min. The results from the 5 min reading were used for data analysis. Controls included buffer alone, OPA reagent alone and placebo (Alhydrogel® without antigen) alone. The readings from placebo were used as background.
2.6 Data analysis
The standard curves were generated with linear regression and the amount of antigen in standards or test samples was calculated from the linear equation. The back calculations were performed by converting the observed readings of the standards to concentration of antigens using the linear regression equations; the percent accuracy was calculated by the following formula: % accuracy = (|calculated concentration − nominal concentration|/nominal concentration) × 100. Inter-assay variation was calculated from the same quality control samples (different aliquot) that were tested in different assays and reported as coefficient of variation (CV), which was the standard deviation divided by the mean. The statistic analysis was performed using one way ANOVA with Newman-Keuls Multiple Comparisons Test (GraphPad Prism).
3. Results
3.1 Optimal reading time
The optimal incubation time was determined by reading the same plate at 0, 2, 5, 10 and 15 min. Overall, the fluorescent signal decreased as incubation time increased. Five minutes after addition of OPA reagent was found to be the optimal reading time because of the overall percent accuracy at this time point was greatest and the mean fluorescent response was the most consistent (Table 1).
Table 1.
Determination of optimal OPA assay incubation time
| Nominal concentration (μg/mL) a | Experimentally determined | Plate Incubation Time (minutes) | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 15 | ||
| 160 | Concentration b | 154.9 ± 5.0 | 160.2 ± 3.5 | 161.3 ± 3.2 | 158.5 ± 1.9 | 156.6 ± 4.4 |
| % Accuracy c | 98.8 ± 3.1 | 98.4 ± 0.2 | 98.6 ± 1.1 | 99.0 ± 1.2 | 97.9 ± 2.7 | |
| 120 | Concentration | 116.4 ± 7.7 | 119.9 ± 3.7 | 122.3 ± 4.4 | 119.4 ± 4.5 | 118.3 ± 7.3 |
| % Accuracy | 95.5 ± 4.3 | 97.8 ± 0.1 | 97.4 ± 2.7 | 97.3 ± 0.7 | 95.7 ± 2.0 | |
| 70 | Concentration | 67.9 ± 0.5 | 70.6 ± 0.6 | 70.9 ± 1.2 | 70.5 ± 0.8 | 71.1 ± 1.6 |
| % Accuracy | 97.1 ± 0.7 | 99.1 ± 0.9 | 98.8 ± 1.7 | 99.2 ± 1.0 | 98.4 ± 2.3 | |
| 40 | Concentration | 36.8 ± 2.8 | 38.3 ± 0.5 | 39.4 ± 1.3 | 38.6 ± 1.4 | 39.6 ± 5.1 |
| % Accuracy | 92.0 ± 7.1 | 95.8 ± 1.4 | 97.7 ± 2.2 | 96.6 ± 3.4 | 91.0 ± 1.4 | |
| 20 | Concentration | 16.3 ± 0.6 | 16.8 ± 0.8 | 17.3 ± 0.2 | 16.7 ± 0.1 | 18.9 ± 3.7 |
| % Accuracy | 81.4 ± 3.0 | 83.9 ± 4.1 | 86.3 ± 1.0 | 83.7 ± 0.3 | 86.8 ± 8.1 | |
| 10 | Concentration | 4.9 ± 2.2 | 5.6 ± 0.3 | 6.0 ± 0.5 | 5.6 ± 0.3 | 8.8 ± 3.7 |
| % Accuracy | 48.6 ± 21.8 | 55.5 ± 3.5 | 60.2 ± 5.3 | 55.6 ± 3.4 | 74.1 ± 16.9 | |
Known amount of MSP142-3D7 was freshly prepared on Alhydrogel®.
Concentrations were back calculated by using linear regression of the standard curve.
Percent accuracy is percent similarity between the amount of protein calculated and the known amount of protein added. Percent accuracy = (|calculated concentration − nominal concentration|/nominal concentration) × 100.
3.2 Qualifications of the assay
3.2.1 Standard curves and percent accuracy
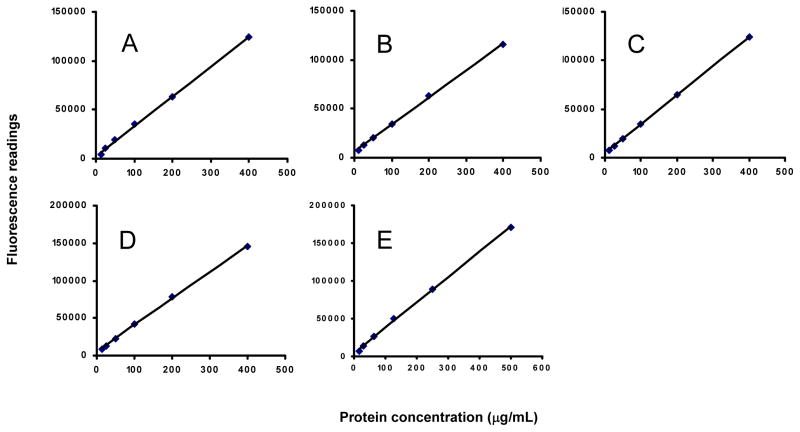
Standard curves were generated between the range of 12.5 to 400 μg/mL with AMA1-C1, AMA1-C2, BSAM2, MSP142-3D7 or the range of 15.63 to 500 μg/mL with MSP142-C1, with a correlation coefficient (R2) greater than 0.99 (Fig. 1). The lower doses (1.56–6.25 μg/mL for all with an exception of 1.95–7.82 μg/mL for MSP142-C1) were also tested, but these data points were eliminated due to poor correlations to the nominal concentration (data not shown).
Fig. 1.
OPA assay standard curves for protein determination of Alhydrogel® formulations. A: MSP142-3D7; B: AMA1-C1; C: AMA1-C2; D: BSAM-2; E: MSP142-C1. All standard curves were generated by dilution of protein in 1600μg/mL Alhydrogel® and the best fit obtained by linear regression with R2 greater than 0.99.
Back calculation and percent accuracy confirmed the reliability of the standard curves and were used to establish detection limits (Table 2). The detection limits were defined as the highest and lowest doses whose percent accuracy was 85% or higher. The OPA assay was consistently accurate for these formulations within the range of 25 to 400 μg/mL for the AMA1-C1, AMA1-C2, BSAM2 and MSP142-3D7 vaccines, or 31.3 to 500 μg/mL for the MSP142-C1 vaccine.
Table 2.
Back calculation and percent accuracy of standard curvesa
| Nominal concentration (μg/mL)b | Experimentally determined a | Proteins used to generate the standard curve |
||||
|---|---|---|---|---|---|---|
| AMA1-C1 | AMA1-C2 | BSAM-2 | MSP142-3D7 | MSP142-C1 | ||
| 400 (500)d | Concentration | 397 ± 5.3 | 398 ± 3.4 | 395.2 ± 0.8 | 394.3 ± 2.7 | 492.7 ± 3.7 |
| % Accuracy | 98.9 ± 1.0 | 99.1 ± 0.5 | 98.8 ± 0.2 | 98.6 ± 0.7 | 98.5 ± 0.7 | |
| 200 (250) | Concentration | 203 ± 9.9 | 204 ± 8.3 | 206.5 ± 2.4 | 206.0 ± 6.8 | 257.4 ± 7.6 |
| % Accuracy | 95.9 ± 2.5 | 96.4 ± 2.6 | 96.7 ± 1.2 | 96.9 ± 3.3 | 97.0 ± 3.0 | |
| 100 (125) | Concentration | 105 ± 6.8 | 101 ± 6.7 | 106.6 ± 2.7 | 109.4 ± 3.9 | 136.2 ± 0.9 |
| % Accuracy | 93.3 ± 4.4 | 95.8 ± 4.9 | 93.4 ± 2.7 | 90.6 ± 3.9 | 91.1 ± 0.7 | |
| 50 (62.5) | Concentration | 55 ± 0.4 | 51.8 ± 4.2 | 51.5 ± 1.0 | 55.1 ± 0.2 | 70.6 ± 1.8 |
| % Accuracy | 90.5 ± 0.8 | 94.3 ± 7.1 | 96.9 ± 2.0 | 89.9 ± 0.4 | 87.0 ± 2.8 | |
| 25 (31.25) | Concentration | 26 ± 1.5 | 25 ± 1.7 | 22.9 ± 0.6 | 24.5 ± 2.3 | 32.3 ± 1.5 |
| % Accuracy | 93.3 ± 1.5 | 95.2 ± 4.3 | 91.7 ± 2.3 | 93.3 ± 5.1 | 95.3 ± 2.4 | |
| 12.5 (15.63) | Concentration | 10.2 ± 2.7 | 11.3 ± 1.7 | 9.0 ± 0.8 | 8.9 ± 0.3 | 12.8 ± 1.7 |
| % Accuracy | 80.2 ± 20.0 | 88.6 ± 12.6 | 72.0 ± 6.7 | 71.5 ± 2.1 | 82.0 ± 11.0 | |
Back calculation was performed by converting the observed readings of the standards to concentrations of antigens using linear regression equations; Percent accuracy = (|calculated concentration − nominal concentration|/nominal concentration) × 100. Results are means ± standard deviation of 3–7 independent OPA assays.
A known amount of indicated protein was freshly prepared on Alhydrogel®.
The nominal concentrations of MSP142-C1 prepared by 2-fold dilutions from 500 μg/mL.
3.2.2 Inter-assay variation
The BSAM-2/Alhydrogel formulations (320 μg/mL) were used as quality control samples throughout the study of the BSAM-2/Alhydrogel vaccine to evaluate the inter-assay variation. The coefficient of variation (CV) was 2.7% for all 9 experiments performed, indicating that the OPA assay is highly reproducible (Table 3).
Table 3.
Inter-assay variation of BSAM-2/Alhydrogel quality control samples with a nominal concentration of 320 μg/mL
| Nominal concentration (μg/mL) | Assay | Experimentally determined Concentration a (μg/mL) |
|---|---|---|
| 320 | 1 | 324 |
| 2 | 317 | |
| 3 | 338 | |
| 4 | 332 | |
| 5 | 347 | |
| 6 | 337 | |
| 7 | 329 | |
| 8 | 342 | |
| 9 | 333 | |
| Mean | 333.2 | |
| SD b | 9.2 | |
| CV c | 2.7 |
Concentrations detected by OPA assay were obtained by calculation using linear regression of the standard curve.
SD, Standard Deviation.
CV, Coefficient of variation = (Standard Deviation/Average) × 100.
3.2.3 Effect of different amount of Alhydrogel on standard curves
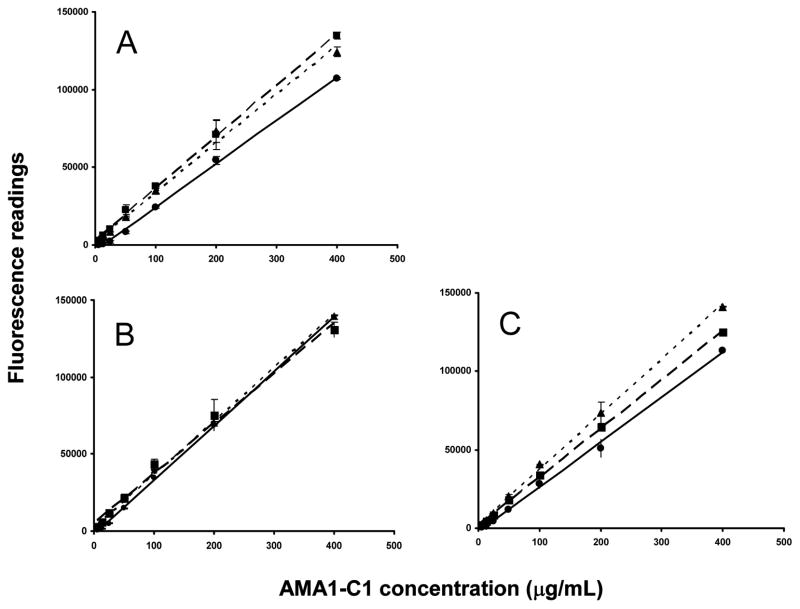
The AMA1-C1 standard samples were prepared in 400 (data not shown), 1600, and 6400 μg/mL Alhydrogel® in three assay solutions (saline, PBS or borate buffer), and the standard curves were generated using linear regression (Fig. 2). The fluorescence readings were higher (p<0.05) in the presence of Alhydrogel® when saline or borate buffer was used compared to PBS. These data suggest that Alhydrogel® in saline or borate buffer may generate a high level of fluorescent signal, and the inclusion of Alhydrogel® in the standard curve is crucial for the accurate measurement of protein content in alum based vaccines.
Fig. 2.
Effect of different buffers and amounts of Alhydrogel® on standard curves. For each curve the assay conditions are as follow: A. saline, pH 6.3; B. PBS, pH 7.4; and C. borate buffer, pH 9.5, and containing ( ) buffer alone (no Alhydrogel®); (
) buffer alone (no Alhydrogel®); ( ) 1600 μg/mL Alhydrogel®; (
) 1600 μg/mL Alhydrogel®; ( ) 6400 μg/mL Alhydrogel®. Each data point represents mean ± standard deviation.
) 6400 μg/mL Alhydrogel®. Each data point represents mean ± standard deviation.
3.2.4 Effect of different pH or buffer systems
Freshly-prepared MSP142-3D7/Alhydrogel formulations at 10, 20, 40, 70, 120 or 160 μg/mL in PBS were analyzed in saline (pH 6.9), PBS (pH 7.4) or borate buffer (pH 9.5) by OPA assay. No significant difference (p<0.05) was observed between PBS (pH 7.4) and saline (pH 6.9) in the range of 10 to 160 μg/mL, and also no significant difference was found between borate and saline when higher concentrations (> 70 μg/mL) of formulations were tested (Table 4). The results also confirmed that the OPA assay was not appropriate for testing formulation doses lower than 20 μg/mL.
Table 4.
The effect of different buffer system on OPA assay protein determination of MSP142-3D7/Alhydrogel formulations
| Nominal concentration (μg/mL) | Experimentally determined | Buffer system |
||
|---|---|---|---|---|
| Saline pH 6.9 | PBS pH 7.4 | Borate buffer pH 9.5 | ||
| 160 | Concentration | 156 ± 3.4 | 161.6 ± 3.2 | 157.8 ± 0.1 |
| % Accuracy | 97.5 ± 2.1 | 98.6 ± 1.4 | 98.6 ± 0.1 | |
| 120 | Concentration | 121.7 ± 0.2 | 123.1 ± 0.4 | 118.7 ± 2.2 |
| % Accuracy | 98.6 ± 0.2 | 97.5 ± 0.3 | 98.7 ± 1.6 | |
| 70 | Concentration | 67.2 ± 0.6 | 69.3 ± 1.6 | 65.1 ± 1.0 |
| % Accuracy | 95.9 ± 0.9 | 98.4 ± 1.4 | 93.0 ± 1.4 | |
| 40 | Concentration | 39.0 ± 0.2 | 38.6 ± 0.9 | 31.4 ± 6.7 |
| % Accuracy | 97.4 ± 0.5 | 96.5 ± 2.1 | 78.6 ± 16.7 | |
| 20 | Concentration | 16.9 ± 0.2 | 16.7 ± 1.4 | 15.0 ± 1.3 |
| % Accuracy | 84.3 ± 1.1 | 83.4 ± 6.8 | 74.9 ± 6.6 | |
| 10 | Concentration | 6.3 ± 0.1 | 6.1 ± 0.3 | 4.1 ± 0.3 |
| % Accuracy | 63.0 ± 1.4 | 61.4 ± 2.8 | 41.4 ± 3.1 | |
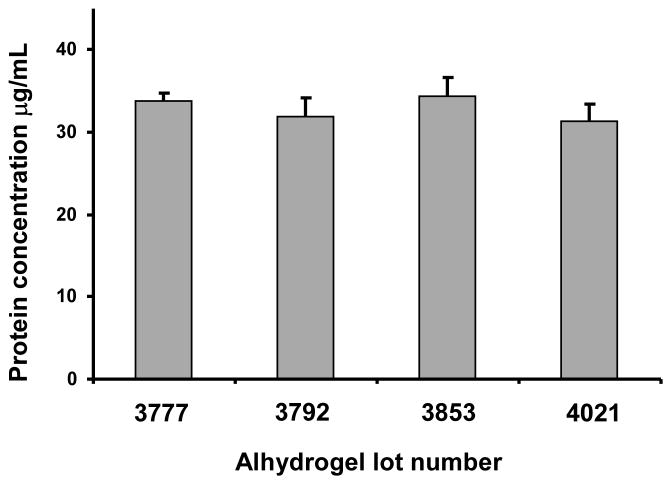
3.2.5 Effect of different lots of Alhydrogel®
Four different lots of Alhydrogel®, including 2 lots that had expired prior to testing, were investigated for the effect of lot to lot variability on OPA assay performance. AMA1-C1/Alhydrogel prepared in lot 3792 was used as test sample. No significant difference (P<0.05) was found when different lots of Alhydrogel® were used to generate the standard curve, suggesting that lots used to formulate the vaccine do not need to be used to generate the standard curves for this assay. (Fig. 3).
Fig. 3.
Effect of different lots of Alhydrogel® on performance of the OPA assay. A known amount of AMA1-C1/Alhydrogel formulation was analyzed using standard curves generated with 4 different lots of Alhydrogel® (lots 3777, 3792, 3853 or 4021). Each bar represents mean ± standard deviation of 6–9 replicates from 2 or 3 independent experiments. Alhydrogel® lot 3792 was used to prepare the formulation.
3.3 Testing of clinical samples
Clinical vaccines of AMA1-C1/Alhydrogel, AMA1-C2/Alhydrogel, BSAM-2/Alhydrogel and MSP142-C1/Alhydrogel were tested by the OPA assay. The results showed an overall percent accuracy of 92 % or greater, indicating that the OPA assay is accurate and reliable (Table 5).
Table 5.
Analysis of clinical vaccines by OPA assay
| Nominal concentration (μg/mL) | Experimentally determined | Alhydrogel® vaccines tested |
|||
|---|---|---|---|---|---|
| AMA1-C1 | AMA1-C2 | BSAM-2 | MSP142-C1 | ||
| 320 | Concentration | - | - | 338 ± 9.2 | 332 ± 23.3 |
| % Accuracy | - | - | 94.3 ± 2.8 | 93.1 ± 2.1 | |
| 160 | Concentration | 155 ± 12 | 149 ± 0.6 | - | - |
| % Accuracy | 93.9 ± 4.3 | 92.9 ± 0.4 | - | - | |
| 80 | Concentration | - | - | 79 ± 2.6 | 78.5 ± 1.2 |
| % Accuracy | - | - | 97.9 ± 2.6 | 98.1 ± 1.5 | |
| 40 | Concentration | 41 ± 2.2 | - | - | - |
| % Accuracy | 96.3 ± 4.3 | - | - | - | |
4. Discussion
Although aluminum containing adjuvants have been used in human vaccines for many years, there is no generic method available to determine the protein content for the alum-based vaccines. In most cases, the antigens must be desorbed from alum or the adjuvant has to be dissolved in order to evaluate the protein content 22. The conventional procedures are laborious, time-consuming, and the extraction efficacy and the antigen conformations following extraction have been questioned. Our laboratory has recently developed a Direct Alhydrogel Formulation Immunoassay (DAFIA) 23 to determine protein content of Alhydrogel® based vaccines accurately and directly (without prior antigen extraction) with a linear detection range of 0.16–10 μg/mL. However, DAFIA requires the use of antibodies specific to the protein in the vaccine and assay development. The OPA assay, as defined here, is capable of determining the protein content directly without pre-treatment of vaccines or the need for specific antibodies.
The results clearly show that the OPA assay is highly accurate (87–100%) and simple to perform with a protein detection range of 25–400 μg/mL for Alhydrogel® vaccines (except for MSP142-C1, which has a linear detection range of 31.25–500 μg/mL). In general, the detection range of 25–400 μg/mL should satisfy the need of the current vaccine development. We are interested in testing vaccine formulations with antigen contents ranging from 40 to 320 μg/mL which are well within detection range of the OPA assay developed in the present study. When lower doses (below 25 μg/mL) of vaccine formulations are to be tested, however, more sensitive methods such as DAFIA specific to the antigens of interest may be developed and used (reference 23).
In addition, the OPA assay has several important advantages. First, the biochemical properties of antigens, such as surface charge and glycosylation do not seem to affect the accuracy of OPA assay. This was confirmed by the use of recombinant proteins in the present study, including AMA1-FVO and AMA1-3D7 which are O-linked glycosylated proteins with up to 6 hexose residues per antigen molecule. The isoelectric point (PI) and theoretical charge at pH 7.0 for AMA1-FVO and 3D7 are 5.65, −18.67 and 5.66, −17.84, respectively. The MSP142-FVO and MSP142-3D7 are non-glycosylated, non-lipidated proteins with the PI and theoretical charge at pH 7.0 of 6.34, −3.77 and 6.08, −5.79, respectively. The OPA results of our studies were not affected by these protein properties. Secondly, unlike most commercially available protein assays that can only determine protein content in an aqueous solution, this assay can directly quantify protein content on solid particles, suggesting that the existence of solid particles in solution does not prevent readout of fluorescent signal. Thirdly, the operation of the OPA assay is simple and will eliminate the protein extraction process, therefore preventing potential variations resulting from the extraction procedure. Lastly, the OPA assay is not affected by different lots of Alhydrogel® and therefore, this assay should be capable of evaluating aged vaccines with the use of newer lots of Alhydrogel® when the Alhydrogel® lot for the aged vaccines is no longer available. Finally, variation in pH (between pH 6.9 to 7.4) did not affect the assay results, indicating that the assay may be simplified as long as the pH values of the assay reagents are within this range. Overall, the OPA assay represents a new method that may have broad applications in the quality control of vaccine research and development areas where insoluble adjuvants may be used in formulations.
In summary, the OPA assay has been adapted for the determination of protein content on Alhydrogel® vaccines in our laboratory. This assay is accurate, sensitive, reproducible and simple to perform. This assay can be used as a routine assay for protein content testing in alum-based vaccines in vaccine quality control.
Acknowledgments
We thank David Narum and Richard Shimp, Jr. for providing antigens. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002;19(6):715–28. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 2.Peek LJ, Martin TT, Elk Nation C, Pegram SA, Middaugh CR. Effects of stabilizers on the destabilization of proteins upon adsorption to aluminum salt adjuvants. J Pharm Sci. 2007;96(3):547–57. doi: 10.1002/jps.20762. [DOI] [PubMed] [Google Scholar]
- 3.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines--US perspective. Vaccine. 2002;31(20 Suppl 3):S18–23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 5.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6(5):685–98. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 6.Glenney AT, Pope CG, Waddington H, Wallace U. XXIII—the antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;29:38–39. [Google Scholar]
- 7.Katz J. Desorption of porcine parvovirus from aluminum hydroxide adjuvant with subsequent viral immunoassay or hemagglutination assay. Vet Res Commun. 1987;11(1):83–92. doi: 10.1007/BF00361329. [DOI] [PubMed] [Google Scholar]
- 8.Katz JB, Hanson SK, Patterson PA, Stoll IR. In vitro assessment of viral antigen content in inactivated aluminum hydroxide adjuvanted vaccines. J Virol Methods. 1989;25(1):101–8. doi: 10.1016/0166-0934(89)90104-3. [DOI] [PubMed] [Google Scholar]
- 9.Shore PA, Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exptl Therap. 1959;127:182–6. [PubMed] [Google Scholar]
- 10.Cohn VH, Jr, Shore PA. A microfluorometric method for the determination of agmatine. Anal Biochem. 1961;2 (3):237–241. doi: 10.1016/s0003-2697(61)80006-7. [DOI] [PubMed] [Google Scholar]
- 11.Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971;43(7):880–2. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- 12.Benson JR, Hare PE. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci. 1975;72(2):619–22. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avarez-Coque MCG, Hernández MJM, Camañas RMV, Fernández CM. Studies on the formation and stability of isoindoles derived from amino acids, o-phthalaldehyde and N-acetyl—cysteine. Anal Biochem. 1989;180:172–176. doi: 10.1016/0003-2697(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Leea, Dennis G. Drescher Fluorometric amino-acid analysis with o-phthaldialdehyde (OPA) Int J Biochem. 1978;9(7):457–67. doi: 10.1016/0020-711x(78)90075-7. [DOI] [PubMed] [Google Scholar]
- 15.Molnár-Perl I. Derivatization and chromatographic behavior of the o-phthaldialdehyde amino acid derivatives obtained with various SH-group-containing additives. J Chromatogr A. 2001;913(1–2):283–302. doi: 10.1016/s0021-9673(00)01200-0. [DOI] [PubMed] [Google Scholar]
- 16.Church FC, Porter DH, Catignani GL, Swaisgood HE. An o-phthalaldehyde spectrophotometric assay for proteinases. Anal Biochem. 1985;146(2):343–8. doi: 10.1016/0003-2697(85)90549-4. [DOI] [PubMed] [Google Scholar]
- 17.Hapuarachchi S, Janaway GA, Aspinwall CA. Capillary electrophoresis with a UV light-emitting diode source for chemical monitoring of native and derivatized fluorescent compounds. Electrophoresis. 2006;27(20):4052–9. doi: 10.1002/elps.200600232. [DOI] [PubMed] [Google Scholar]
- 18.Go K, Horikawa Y, Garcia R, Villarreal FJ. Fluorescent method for detection of cleaved collagens using O-phthaldialdehyde (OPA) J Biochem Biophys Methods. 2008;70(6):878–82. doi: 10.1016/j.jbbm.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank MP, Powers RW. Simple and rapid quantitative high-performance liquid chromatographic analysis of plasma amino acids. J Chromatogr B. 2007;852(1–2):646–9. doi: 10.1016/j.jchromb.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimp RL, Jr, et al. Production and characterization of clinical grade Escherichia coli derived Plasmodium falciparum 42 kDa merozoite surface protein 1 (MSP1(42)) in the absence of an affinity tag. Protein Expr Purif. 2006;50(1):58–67. doi: 10.1016/j.pep.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy MC, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–60. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metz Bernard, et al. Quality-control issues and approaches in vaccine development. Expert Review of Vaccines. 2009;8(2):227–238. doi: 10.1586/14760584.8.2.227. [DOI] [PubMed] [Google Scholar]
- 23.Zhu D, et al. Development of A Direct Alhydrogel Formulation Immunoassay (DAFIA) J Immunol Methods. 2009;344(1):73–8. doi: 10.1016/j.jim.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]