Abstract
Central administration of Neuropeptide S (NPS) in rodents induces arousal and prolonged wakefulness as well as anxiolytic-like effects. NPS has also been implicated in modulation of cognitive functions and energy homeostasis. Here we present a comprehensive phenotypical analysis of mice carrying a targeted mutation in the NPS receptor (NPSR) gene. NPSR knockout mice were found to exhibit reduced exploratory activity when challenged with a novel environment, which might indicate attenuated arousal. We also observed attenuated late peak wheel running activity in NPSR knockout mice, representing reduced activity during the subjective evening. These mice also displayed increased anxiety-like behaviors when compared to their wildtype littermates, although analysis of anxiety behaviors was limited by genetic background influences. Unexpectedly, NPSR knockout mice showed enhanced motor performance skills. No phenotypical differences were detected in the forced-swim test, startle habituation and pre-pulse inhibition paradigms. Together, these data indicate that the endogenous NPS system might be involved in setting or maintaining behavioral arousal thresholds and that the NPS system might have other yet undiscovered physiological functions.
Keywords: arousal, circadian, anxiety, motor performance, neuropeptide
1. Introduction
Neuropeptide S (NPS) is the endogenous ligand of a formerly orphan G protein-coupled receptor and was shown to produce arousal and increased wakefulness [41, 35, 6]. In addition, central administration of NPS can also promote anxiolytic-like effects [41, 18, 40, 16] and has been shown to facilitate extinction of fear memories [16] and modulate expression of contextual fear conditioning [21]. Further studies have also found evidence for a role of NPS in feeding behavior [3, 37, 25, 7].
Transcripts for NPS receptors (NPSR, also known as GPR154 or GPRA) are found to be widely expressed in the rodent brain, including several hypothalamic nuclei, nuclei of the thalamic midline, thalamic paraventricular nucleus, amygdala, subiculum and various cortical regions [42]. In contrast, the NPS precursor protein is expressed only in three discrete brainstem nuclei in the rat brain: the pericoerulear region, lateral parabrachial nucleus and the principle sensory 5 trigeminal nucleus [41, 42].
The NPS receptor was originally identified as a candidate gene for asthma susceptibility [17] and specific haplotypes in the human NPSR locus have been associated with a number of allergic or immunological disorders such as rhinoconjunctivitis [22], respiratory distress syndrome [32] and irritable bowel syndrome [10]. It was therefore surprising that a study using NPSR knockout mice found no evidence for abnormal respiratory or immunological functions [2]. However, one of the asthma-related haplotypes includes a coding single nucleotide polymorphism in human NPSR that significantly changes agonist efficacy of the receptor protein in vitro [33] and this polymorphism was recently found to be associated with panic disorder in male patients [27] or circadian phenotypes, such as average bedtime, in a normal population cohort [11]. Arousal, defined as the level of alertness during the wake state, is one of the major factors controlling average bedtime, i.e. the time when a person usually goes to sleep. Thus converging evidence from our preclinical studies in mice showing that central NPS administration increases arousal and the genetic association of NPSR with bedtime behavior indicate that the NPS system might be involved in regulating behavioral arousal levels.
In the present study we have used NPSR knockout (KO) mice and their wildtype (WT) littermates to study physiological functions of the NPS/NPSR system. By using a battery of behavioral assays we have assessed phenotypical differences between NPSR WT and KO mice with respect to arousal, circadian profiles, anxiety-related behaviors, emotional reactivity, sensory-motor functions, reflex adaptation and motor performance.
2. Materials and Methods
2.1 Animals
NPSR KO mice were generated in a 129S6/SvEvTac background as previously described [2]. Initially, four male and four female NPSR KO mice and an equal number of 129S6/SvEvTac male and female mice were obtained from Taconic Farms Inc. (Germantown, NY) to establish a breeding colony. NPSR KO mice were either maintained by breeding of homozygous animals or were mated with 129S6/SvEvTac mice and heterozygous offspring were then used for further breeding. The breeding scheme followed common guidelines for colony maintenance of transgenic mice to minimize the potential for genetic drift [9]. WT littermates were used as controls in all experiments, except analysis of NPS-induced hyperlocomotion where additional male 129S6/SvEvTac mice (8 weeks old) were purchased from Taconic Farms. All offspring were group-housed (3–5 animals per cage) under controlled conditions (temperature 21 ± 2°C; relative humidity 50–60%; 12-hour light-dark cycle, lights on 6:00 AM) with free access to food and water. Offspring were obtained from stable breeding pairs and genotyped by PCR as described previously [2]. Male mice between 8–15 weeks of age were used for behavioral analysis and were regularly handled prior to experimentation. Experiments involving non-automated observational data collection were performed by an experienced individual who was blind to the genotype condition. All experiments were carried out in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research [24] and were approved by the local Institutional Animal Care and Use Committee.
2.2 Drug Administration
For intracerebroventricular (i.c.v.) drug injections, mice were briefly anesthetized with isoflurane. NPS was synthesized by the Peptide Proteomic Centre, Brain Research Centre, University of British Columbia (Vancouver, BC, Canada). NPS was dissolved in phosphate-buffered saline (PBS, pH 7.4) containing 0.1 % bovine serum albumin and injected into the lateral ventricle (total volume: 2 μl) as described before [41].
2.3 Behavioral Test Battery
Initially, one group of mice (both genotypes) was tested in a battery of behavioral paradigms in the following order: locomotion/open field, light-dark box, elevated plus-maze, marble burying, rotarod, grip strength, prepulse inhibition, startle habituation, forced swim, wheel running. The sequence of specific tests spaced by a 3-day inter-test interval was adapted from previously published reports [20, 29] showing that this study design would not affect behavioral test performance. In subsequent studies, separate groups of naive mice were tested only once in all four anxiety paradigms in the following order: open field, light-dark box, elevated plus maze, marble burying. Two other groups of naive mice were tested separately for either wheel running behavior or accelerating rotarod in order to increase the number of animals in these paradigms.
2.4 Locomotion
Analysis of locomotor behavior was carried out as described before [41, 28] using an automated activity monitor system (Versamax, Accuscan, Columbus, OH). Number of infrared beam breaks, total distance traveled, vertical activity and episodes of stereotypic behavior were recorded. General activity in NPSR WT and KO mice was recorded over a 90-minute period. Arousal was defined as horizontal and vertical activity during the first 10 minutes of exposure to an unfamiliar arena. Anxiety-like behavior in the open field was measured by recording numbers and total duration of entries into the center of the observation chamber. Center is defined as a 30 × 30 cm imaginary square.
2.5 Wheel Running
Mice were individually housed in cages equipped with a running wheel and had free access to food and water. Initially, mice were entrained to a 12 h light/dark (LD) cycle. After 16 days of wheel running recording in the LD cycle, mice were placed in constant darkness with dim red light (DD) for 2 weeks in order to assess their endogenous circadian period, followed by 3 weeks where a single 1 hour-long light pulse was given each day from 12:00 – 13:00. The free-run period (DD) was analyzed using ClockLab software (Actimetrics, Evanston, IL). During the LD cycle, “early peak” activity is defined as wheel running during the first 6 hours after the lights went off and “late peak” activity is defined as wheel running during the last two hours preceding the onset of the light phase. Early peak activity occurs during the subjective morning of the animal while late peak activity represents activity during the subjective evening. Data are presented as mean wheel revolutions during the last 5 days of each testing period to ensure that animals had adjusted to the light schedules.
2.6 Anxiety-like Behavior
Experimental conditions for elevated plus maze, light dark box, open field and marble burying paradigms have been described previously [41].
2.7 Forced-Swim Test
A potential depressive phenotype was assessed using the forced-swim test as described [31]. Briefly, mice were placed individually into 4-liter Nalgene cylinders (height 28.5 cm, diameter 17.5 cm) containing 15 cm of water at 21 °C, and the time each animal remained immobile during a 6-min test session was recorded. Swimming, climbing and floating data were recorded with ODLog software (Macropod Software, www.macropodsoftware.com). Animals were considered to be immobile when they ceased struggling/swimming and remained floating motionless in the water, making only minimal movements necessary to keep their heads above the water line. The last 4 minutes of each trial were used for data analysis [12, 19].
2.8 Startle Habituation and Prepulse Inhibition (PPI)
The apparatus used for detection of startle reflexes consisted of a clear non-restrictive Plexiglas cylinder resting on a platform inside of a ventilated and illuminated chamber (SR-Lab, San Diego Instruments, San Diego, CA, USA). Background noise (a 65 dB broadband noise) was presented alone before each experiment.
The startle response is an intrinsic reflex exhibited when a sudden, high-intensity acoustic stimulus (120 dB) is presented to a subject. Amplitude of startle responses was measured in Newtons by accelerometers located inside the chambers. Habituation to the startling stimulus was measured for each animal group by calculating the average responses to five consecutive 120 dB stimulus trials from a session that included 25 120-dB stimuli trials separated by 8 s intervals, preceded by a 8-min acclimatization period (65 dB background noise).
Prepulse inhibition (PPI) is defined as a reduction in startle amplitude when a startle-inducing stimulus is preceded by a weaker non-startling warning stimulus. Each PPI test session was preceded by a 10-min acclimatization period with 65-dB background noise. The PPI test session included twelve blocks of trials with a total of 70 trials. Blocks one and twelve consisted of five pulses alone (a 40 ms 120 dB broadband burst). Blocks two to eleven each contained six trials of each type described below that were presented in a pseudo-randomized sequence. Six trial types were presented: a 40 ms broadband 120 dB burst (pulse-alone); four prepulse + pulse trials in which 20 ms-long 69 dB, 73 dB, 77 dB, or 81 dB (4, 8, 12, and 16 dB above background) stimuli preceded the 120 dB pulse by 100 ms (onset to onset); and a no-stimulus trial. Each trial was presented with a false-random intertrial interval (average of 15 s). Startle magnitude was calculated and expressed as the average response to the pulse-alone trials presented during each block of the session. PPI data were calculated and expressed as both percentage and difference scores. Only percentage scores are presented as both measurements showed comparable results. Percent PPI was derived from the equation: [(pulse alone − (prepulse + pulse)) / pulse alone] × 100.
2.9 Motor Performance
Mice were placed on a rotarod (TSE Systems, Bad Homburg, Germany) at an initial speed of 6 rpm. A session consisted of a 5 min interval during which the rotarod accelerated linearly from 6–60 rpm. The latency and rotation speed at which an animal fell off the rod was recorded automatically by an infrared beam located below the rotating rod. Each mouse was given 3 trials per day with intertrial intervals of 1 h. Mice were tested for rotarod performance on three consecutive days.
2.10 Grip Strength, Body Weight and Body Length
Forelimb strength was measured as tension force using a grip strength meter (TSE Systems, Bad Homburg, Germany). Mice were tested 4–6 times with one minute resting intervals between individual measurements and average grip strength (N) for each animal was calculated from three sessions in which the animal properly grasped the handle with both forepaws. Body weight (g) and length (mm) were measured once a week in mice between 2 and 6 weeks of age. Measurements were always taken at the same time and same day of the week. Body length is defined as the distance from nose to root of the tail and was measured with a digital caliper instrument while allowing the animal to hold onto a metal grid.
2.11 Statistical Analysis
Behavioral data were analyzed by analysis of variance (ANOVA) with genotype and time or treatment and time as variables, respectively, followed by Bonferroni's post-hoc test where appropriate. Comparisons between genotypes for cumulative activity data, anxiety-related behaviors, behavioral despair and grip strength were made by unpaired t-test. P values of < 0.05 were considered significant. All statistical analyses were performed with GraphPad Prism (GraphPad, San Diego, CA).
3. Results
3.1 Locomotion and Behavioral Arousal
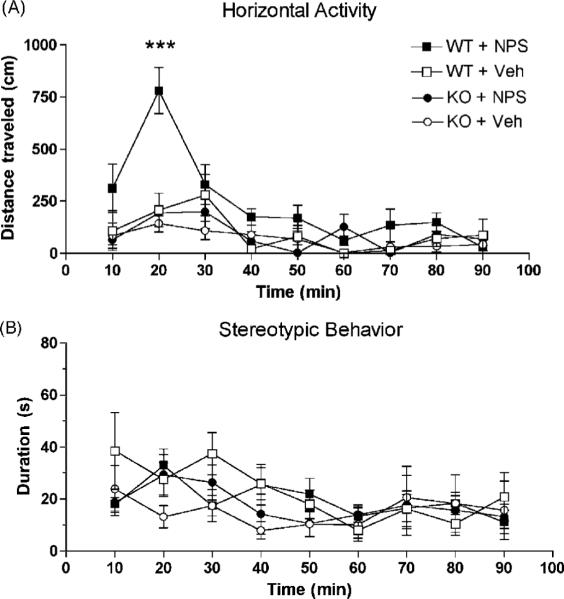
Central administration of 0.1 nmole NPS in 129S6/SvEvTac WT mice caused significant hyperlocomotion lasting about 30 min as compared to vehicle-injected animals [treatment: F1,104 = 11.27, p = 0.0051; time: F8,104 = 9.98, p < 0.0001] (Fig. 1A). In contrast, horizontal activity did not increase in NPSR KO mice injected with 0.1 nmole NPS. Two-way analysis of variance (ANOVA) indicated significant main effects of genotype for NPS-treated animals [F1,112 = 13.31, p = 0.0026] and time [F8,112 = 10.3, p < 0.0001]. These results confirm our previous findings in C57Bl/6 mice [41], suggesting that NPS-induced hyperlocomotion occurs independent of strain. As expected, NPSR KO mice did not show any effect on locomotor behavior after NPS treatment and were indistinguishable from either WT or KO mice treated with vehicle (Fig. 1). These data demonstrate that increases in horizontal activity promoted by activation of central NPS receptors are absent in NPSR KO mice. Vertical activity was virtually absent in all groups of mice, possibly due to the injection procedure or strain influences (data not shown). All mice in this experiment had been habituated to the testing chamber for 60 min prior to the test in order to eliminate novelty-induced exploratory behavior.
Fig. 1.
NPS-induced hyperlocomotion is mediated by NPSR. Central injection of 0.1 nmole NPS causes increased horizontal activity only in NPSR WT mice (A). No changes in stereotypic behavior were recorded in the same animals (B). Animal numbers and treatment groups: WT + NPS n = 9; WT + Vehicle (Veh) n = 7; KO + NPS n = 8; KO + Veh n = 8; *** p< 0.001 in WT + NPS treated mice vs. all other groups, two-way ANOVA with Bonferroni post-hoc test.
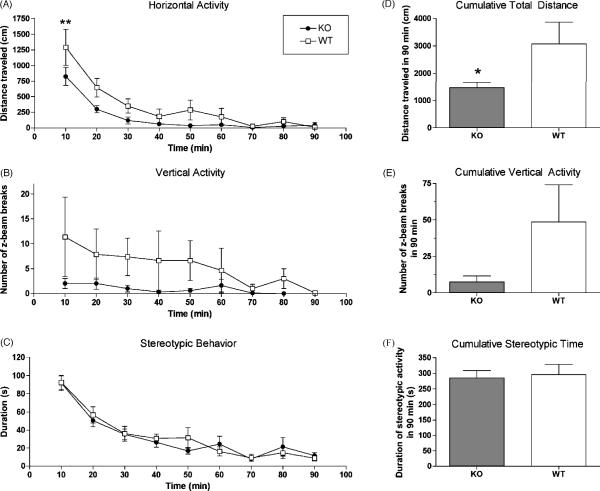
In order to investigate responses of NPSR KO and WT mice under conditions of behavioral arousal, their exploratory behavior in a novel environment were recorded over 90 min. As shown in Fig. 2, when exposed to an unfamiliar observation chamber, NPSR KO mice showed significantly less exploratory activity as compared to their WT littermates (Fig. 2A and D) while stereotypic behavior was similar between the genotypes (Fig. 2C and F). Two-way ANOVA of horizontal activity indicated significant main effects of genotype [F1,144 = 14.09, p = 0.0003] and time [F8,144 = 21.89, p < 0.0001]. Total distance traveled over 90 min was also significantly different between the two genotypes (p < 0.05, t-test; Fig. 2D). Conversely, NPSR KO mice displayed increased resting time compared to WT mice [genotype: F1,144 = 12.48, p = 0.0006; time: F8,144 = 24.61, p < 0.0001; data not shown]. Two-way ANOVA also indicated significant differences in vertical activity between the genotypes [F1,144 = 6.93; p = 0.0013] (Fig. 2B). Analysis of cumulative vertical activity indicated an obvious trend toward reduced rearing movements in NPSR KO mice but mean values did not reach statistical significance (p = 0.09) due to large variability among WT animals (Fig. 2E). Total time spent moving [genotype: F1,144 = 12.48, p = 0.0006; time: F8,144 = 24.61, p < 0.0001; data not shown] and number of horizontal movement bouts [genotype: F1,144 = 15.15, p = 0.0002; time: F8,144 = 23.82, p < 0.0001; data not shown] were also significantly different between the two genotypes over the 90 min observation period whereas ambulation speed (cm/s) did not differ. Together, these data indicate that the reduced exploratory activity of NPSR KO mice is not caused by locomotor deficits but is a result of increased rest time.
Fig. 2.
Attenuated exploratory activity of NPSR KO mice in a novel environment. Horizontal activity (A), vertical activity (B) and stereotypic behavior (C) were recorded for 90 min. Cumulative data for the entire observation period are shown for total distance (D), vertical activity (E) and time spent with stereotypic behaviors (F). Animal numbers: NPSR KO n = 10; NPSR WT n = 8; ** p < 0.01 KO vs. WT mice after two-way ANOVA followed by Bonferroni's post-hoc test (A); * p < 0.05, unpaired t-test (D).
3.2 Circadian Activity
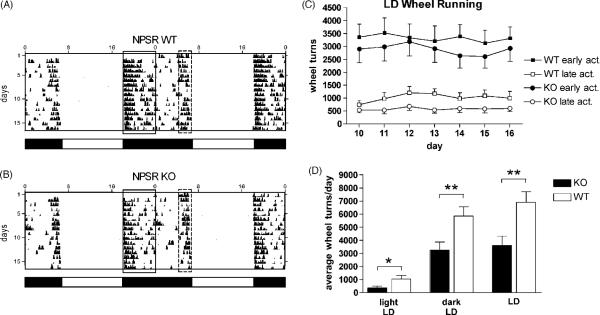
Both NPSR KO and WT mice quickly adapted to dark-phase wheel running under a 12:12 h light/dark (LD) cycle and running activity became stable after about 5–8 days (Fig. 3). Both genotypes displayed the typical pattern of two distinct phases of activity: a prolonged wheel running activity at dark-onset (early peak) and a shorter second phase preceding the beginning of the light phase (late peak) (Fig. 3A and B). After the initial habituation period (days 1–9), both genotypes showed similar levels of early peak wheel running activity, averaging about 3000 wheel turns per dark phase during the weeklong observation (days 10–16) (Fig. 3C). Although NPSR WT mice displayed consistently higher early peak activity than NPSR KO mice, this difference was not statistically significant [genotype: F1,168 = 2.60, p = 0.109; time: F6,168 = 0.15, p = 0.989; two-way ANOVA]. However, late peak activity was significantly reduced in NPSR KO mice as compared to their wildtype littermates (Fig. 3C). Two-way ANOVA revealed a significant effect of genotype [F1,168 = 19.53; p < 0.0001] with no effect of time or interaction. Mean daily late peak wheel running activity during days 10–16 of the LD recording period was also significantly different between the genotypes [NPSR WT: 1024 ± 58.33 mean daily wheel turns; NPSR KO: 581.2 ± 19.84; p < 0.001, t-test].
Fig. 3.
Circadian activity in NPSR WT and KO mice. Representative double-plot actograms from individual NPSR WT (A) and KO (B) mice during the first 15 days of LD cycle are shown. Early peak activity is indicated by a solid box and late peak activity is indicated by a dashed box. Periods of light and dark are indicated by open and closed bars below the actograms and absolute time is displayed in the x-axis on top of the actograms. (C) Analysis of early (early act.) and late (late act.) peak wheel running activity during days 10–16 of the 12:12 h light-dark (LD) period. Animal numbers: NPSR KO n = 13; NPSR WT n =13. (D) Mean number of daily wheel revolutions during the light phase, dark phase and complete LD schedule. Data from five consecutive days were averaged. Animal numbers: NPSR KO n = 13; NPSR WT n = 13. * p < 0.05, ** p < 0.01, unpaired t-test.
Both groups of mice displayed arrythmic activity patterns during two weeks of constant darkness (DD) and it was therefore not possible to calculate phase shifts (data not shown). A one-hour light pulse per day during the final 2 weeks of the experiment partially reinstated early and late peak wheel running activity, but circadian activity remained arrythmic (data not shown). The fragmentation of activity under DD therefore appears to be strain dependent.
Wheel running activity under LD conditions was significantly lower in NPSR KO mice than in their wildtype littermates, both during the light and the dark phase (Fig. 3D). Within genotypes average daily wheel running activity remained similar across the LD, DD and 1-hour light pulse periods and NPSR KO mice displayed consistently lower activity levels than NPSR WT mice (data not shown).
3.3 Anxiety-like Behavior
Since central administration of NPS is able to produce anxiolytic-like effects [41, 18, 40, 16], we investigated behavioral responses of NPSR KO mice and WT littermates in four different paradigms that are commonly used to measure anxiety-like behavior.
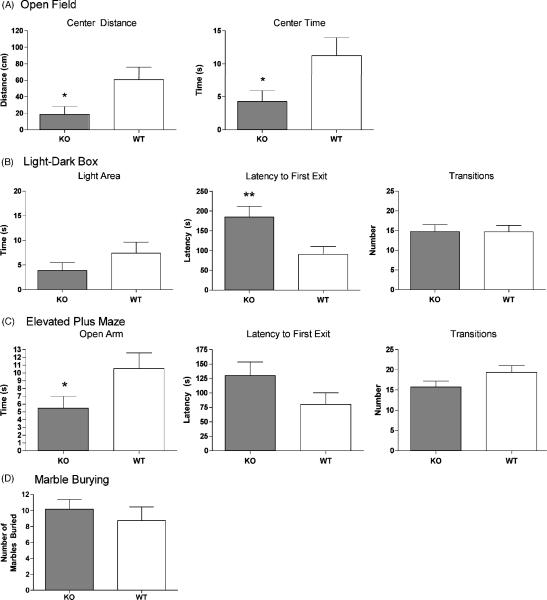
In the open field test, NPSR KO mice spent significantly less time and traveled shorter distances in the imaginary central zone than their WT littermates (Fig 4A). Similarly, in both the elevated plus maze and the light-dark box, NPSR KO mice displayed increased signs of anxiety-like behavior by spending less time in the unprotected (open) areas and showing higher average latencies to emerge from the enclosed parts of the apparatus (Fig. 4B and C). NPSR KO mice spent significantly less time on the open arms of the elevated plus maze and showed significantly longer latencies to emerge from the enclosed dark compartment of the light dark box. General activity, measured as number of total transitions, did not differ between the genotypes in both elevated plus maze and light-dark box, indicating that the reduced exploration of unprotected zones by NPSR KO mice is not confounded by altered levels of general activity in these tests. In the marble burying paradigm, the average number of marbles covered with bedding by NPSR KO mice was slightly higher than the number of marbles buried by NPSR WT mice, albeit not reaching statistical significance (Fig. 4D).
Fig. 4.
Analysis of anxiety-related behaviors in NPSR WT and KO mice. NPSR KO mice display increased levels of anxiety-like behaviors. (A) Open field: NPSR WT n = 23; NPSR KO n = 20; (B) Light-dark box: WT n = 23; KO n = 22; (C) Elevated plus maze: WT n = 23; KO n = 22; (D) Marble burying: WT n = 8; KO n = 11. * p < 0.05, ** p < 0.01, unpaired t-test.
It should be noted, that the absolute values of time spent exploring the unprotected areas in the open field, elevated plus maze and light-dark box tests were extremely low in NPSR WT mice as compared to C57Bl/6 mice that we had used previously under the same conditions and in the same location. C57Bl/6 mice usually spend 10–20% of the total observation time in the unprotected zones [41] while NPSR WT mice, which are on a pure 129S6/SvEvTac background, only spent 2–5% of the total time exploring these areas. Our data confirm previous reports showing that 129S6/SvEvTac mice are known as a highly anxious mouse strain [4]. It is therefore possible, that the high level of innate fear in NPSR WT mice concealed a further increase of anxious behaviors in NPSR KO mice.
3.4 Behavioral Despair
No significant difference between genotypes was observed in the forced-swim test, indicating that absence of NPSR does not produce a depressive phenotype (Fig. 5). Absolute values of time spent swimming, climbing or floating in NPSR KO and WT mice were similar to values obtained from C57Bl/6 [23] and therefore indicate no effect of genetic background.
Fig. 5.
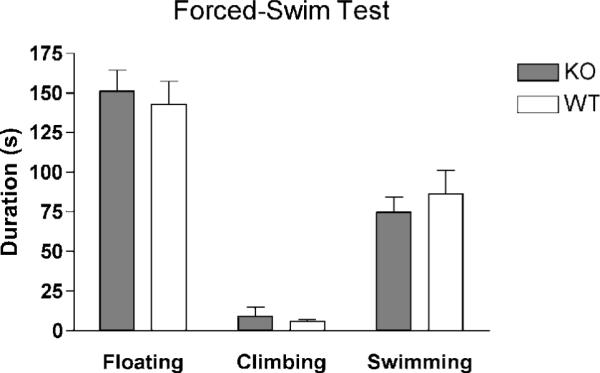
Behavioral despair measured in the forced-swim test. No difference in time spent floating, climbing or swimming was detected between NPSR WT and KO mice. NPSR WT n = 8; NPSR KO n = 11.
3.5 Sensorimotor Gating
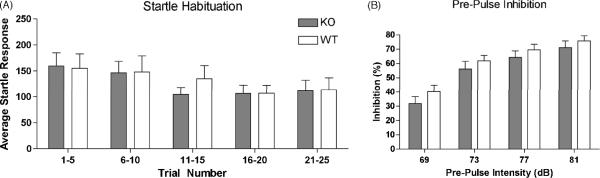
NPSR is expressed in neurons of the cingulate cortex and in limbic regions that have been implicated in neuronal circuits activated by repeated exposure to acoustic startle [38]. Therefore, we tested NPSR KO and WT mice in two paradigms that measure sensorimotor gating functions. In the acoustic startle habituation paradigm, no difference in startle amplitude or in the subsequent progressive reduction in average startle responses after repeated exposure (i.e. habituation) was observed between NPSR KO and WT mice (Fig. 6A). Similarly, both genotypes displayed virtually identical responses in pre-pulse inhibition that is considered the most reliable operational paradigm for the measurement of sensorimotor gating mechanisms (Fig. 6B). Therefore, genetic ablation of NPSR does not appear to interfere with processing of sensorimotor responses.
Fig. 6.
Acoustic startle habituation (A) and sensorimotor gating measured in the pre-pulse inhibition paradigm (B). No difference in startle responses or pre-pulse inhibition was observed between NPSR WT and KO mice. In (A) startle amplitudes from each 5 consecutive trials were averaged. NPSR WT n = 10; NPSR KO n = 10.
3.6 Motor Performance
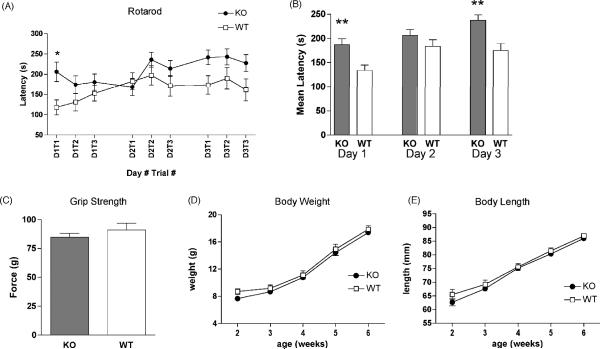
Motor performance and coordination were assessed in the accelerating rotarod test. Twoway ANOVA indicated a significant effect of time [F8,168 = 4.49; p < 0.0001] but only marginal effects of genotype [F1,168 = 4.20; p = 0.053] without interaction. In the first trial, NPSR KO mice performed significantly better than their wildtype littermates [p < 0.05, Bonferroni post-hoc test] (Fig. 7A). Average latencies until falling off the rotating rod remained higher in NPSR KO mice vs. WT littermates throughout the three day testing period (Fig. 7B) and NPSR KO mice apparently reached maximum performance levels on the third day as no further improvement was observed across the individual trials. Analysis of average daily latencies (Fig. 7B) by two-way ANOVA revealed significant effects of genotype [F1,201 = 19.98; p < 0.0001] and time [F2,201 = 7.20; p = 0.001] without interaction. Mean latencies on day 1 and 3 of the trial were significantly higher in NPSR KO mice than in their WT littermates. No difference in forelimb grip strength between NPSR KO mice and WT animals was detected (Fig. 7C), indicating that no inherent physical advantage was responsible for the observed differences in performance. Furthermore, no differences in body weight or body length between the two genotypes were observed in adolescent animals between 2 and 6 weeks of age (Fig. 7D and E), excluding basic physical parameters that could affect motor performance.
Fig. 7.
Motor performance and physical attributes of NPSR WT and KO mice. (A) Mean latencies of NPSR WT and KO mice to fall off an accelerating rotarod from individual trials during the three-day training period, * p < 0.05 after Bonferroni's post-hoc comparison. (B) Mean daily rotarod latencies on each training day, ** p < 0.01, Bonferroni's post-hoc test. (C) Forelimb grip strength in NPSR KO and WT mice does not differ. (D and E) Both genotypes display no difference in body weight or body length between 2 – 6 weeks of age. Animal numbers in A and B: NPSR WT n = 10; NPSR KO n = 13. Animal numbers in C: NPSR WT n = 8; NPSR KO n = 11. Animal numbers in D and E: NPSR WT n = 14; NPSR KO n = 26.
4. Discussion
This study presents data from a comprehensive analysis of behavioral phenotypes produced by the targeted inactivation of the NPS receptor gene in mice. Previous studies had indicated that NPS might be involved in behavioral arousal, sleep-wake regulation, modulation of anxiety and stress responses, as well as feeding behavior and cognitive functions [26, 34]. Genetic studies in humans are suggesting involvement of the NPS system in panic disorder (a form of anxiety disorder) [27] and circadian behavior [11]. All of the above knowledge about potential physiological functions of the NPS system was either obtained by administration of the agonist NPS itself or is based on indirect statistical association of genetic data. Observations from a mouse model with permanent genetic ablation of NPSR can therefore confirm, complement or extend our views of physiological processes that are modulated by NPS.
Two sets of data in our current study suggest that a functional NPS system is required for expression or maintenance of behavioral arousal: First, mice lacking NPSR show reduced exploratory activity in a novel environment (Fig. 2). Second, NPSR KO mice display lower late peak wheel running activity during the subjective evening at the end of the dark phase (Fig. 3). While the first experiment analyzed acute behavioral arousal triggered by an environmental stimulus, the second observation suggests a role for NPS in setting arousal thresholds that control an individual's incentive level to initiate daily activities. Biphasic activity patterns in rodents have been interpreted as the combined activity of two coupled oscillators, one that mediates the early, or subjective morning, response to light (in the case of nocturnal rodents this corresponds to the onset of darkness), and the other controlling the late, or subjective evening, peak of activity [30]. This bimodality is also preserved under conditions of constant light or darkness, but with altered onset, duration and amplitude of peak activities, indicating that both are encoded by intrinsic mechanisms [1]. In wheel running experiments under LD conditions, early peak activity is at least partially triggered by the obvious environmental stimulus, i.e. the onset of the dark phase. In contrast, late peak activity, i.e. initiation of wheel running before the beginning of the light phase, is assumed to depend on the activity of the internal clock. Since there is no sensory input to trigger the onset or determine the duration of late peak wheel running activity, it can be assumed that inherent arousal levels might have a strong influence on this voluntary behavior. Therefore, the observation that NPSR KO mice show significantly reduced late peak wheel running activity may support the hypothesis that activation of NPSR is involved in setting behavioral arousal thresholds during periods of activity. In addition, NPSR-mediated neurotransmission also appears to be required during periods of elevated behavioral arousal that are produced by challenges such as a novel environment (Fig. 2). Similar phenotypes with diminished late peak or total wheel running activity have been described in knockout mouse models of vasoactive intestinal peptide [8], VPAC2 receptor [14], and histamine H3 receptor [39], and it will be interesting to determine whether NPS is functionally interacting with any of these systems.
In this context it should also be noted that a functional polymorphism in human NPSR has been associated with delayed average bedtime [11]. Homozygous carriers of the NPSR Ile107 genotype (this form of the receptor is 5–10 times more sensitive to agonist activation than the other polymorphic variant NPSR Asn107; [33]) go to sleep about 30 minutes later than individuals homozygous for NPSR Asn107. Our present data indicate that the NPS system might be involved in the control of arousal thresholds in mice during their subjective evening. If the NPS system serves a similar function in humans, a more sensitive NPSR variant would result in enhanced tonic arousal during the subjective evening and thus likely delay the moment when the individual starts to feel sleepy. Obviously, more refined experiments will be necessary to determine the precise role of NPSR in the circadian control and modulation of behavioral arousal.
Since central administration of NPS is able to produce significant anxiolytic-like effects, we also investigated phenotypical changes in NPSR KO mice in various tests of anxiety-like behavior. NPSR KO mice were indeed found to display increased levels of anxiety-like behaviors in the open field, elevated plus maze and light dark box test (Fig. 4) after analyzing large cohorts of animals. Defensive behaviors were difficult to assess because strain-dependent “floor effects” were concealing further increases in anxiety-like defensive behaviors in the NPSR KO animals. NPSR KO and WT mice were generated on a pure 129S6/SvEvTac genetic background and this strain is known as a “highly anxious” line of mice [4, 5, 36, 13]. Backcrossing onto a less anxious strain of mice, such as C57Bl/6, will certainly be necessary to further study anxiety-related phenotypes in NPSR KO mice. Our experiments also illustrate the severe limitations of the 129S6/SvEvTac background for analysis of anxiety-like behaviors in mutant mouse models where an increase of anxiety phenotypes is predicted. Based on the anatomical distribution of NPSR mRNA expression, we also analyzed phenotypical differences in sensorimotor gating (startle habituation and PPI) or depressive-like behavior (forced-swim test) but found no evidence for a functional role of NPSR in these behavioral paradigms.
An unexpected finding of the present study was the observation that NPSR KO mice appear to have improved motor coordination in the accelerating rotarod test when compared to their wildtype littermates. Forelimb grip strength, body weight and body length appeared equal between both groups of mice, excluding differences in physical strength to be involved. NPSR KO mice displayed significantly better performance during the first trial of the first day and their latencies to fall off the rod increased only slightly over the three-day trial period. These observations indicate that initial motor performance in NPSR KO mice is very high and little motor learning might have occurred in the subsequent trials. It is therefore not possible to conclude from the present data if - besides superior motor coordination skills – also motor skill learning is enhanced in NPSR KO mice. NPSR mRNA is strongly expressed in the M2 motor cortex and future experiments using local administrations of NPSR agonists and antagonists in this region might help to locate the anatomical substrate for NPSR-mediated modulation of motor coordination or motor skill learning, respectively.
Overall, phenotypical differences between NPSR KO and WT mice were rather mild, indicating that either the absence of NPS signaling has been largely compensated by recruitment of other neuronal mechanisms or that endogenous activation of NPSR in normal animals produces only subtle effects. In summary, we have analyzed behavioral phenotypes in NPSR KO mice and found evidence for a substantial role of the NPS system in regulating behavioral arousal and anxiety-like behaviors. Together with our previous findings showing enhanced arousal after central NPS administration, our current data suggest that the NPS system could be a new member in the ensemble of brain arousal systems that include more established transmitters such as noradrenaline, histamine, glutamate, acetylcholine, dopamine and serotonin as well as neuropeptides such as the orexins/hypocretins [15].
Acknowledgements
The authors would like to thank Drs. Qun-Yong Zhou, Jia-Da Li and Oswald Steward (UC Irvine) for generously sharing equipment and expertise, and Dr. Ralph E. Mistlberger (Simon Fraser University, Burnaby, BC, Canada) for helpful discussions. We are especially grateful to Dr. Beverly H. Koller (University of North Carolina at Chapel Hill; supported by a grant from the National Heart, Lung and Blood Institute; HL-080697) for making the NPSR knockout mice available. DMD was supported in part by a training grant from the National Institute on Drug Abuse. SDC was supported by a postdoctoral fellowship award from the Canadian Institutes of Health Research. This work was supported in part by a grant from the National Institute of Mental Health (MH-71313 to RKR).
Abbreviations
- NPS
neuropeptide S
- NPSR
neuropeptide S receptor
- PBS
phosphate-buffered saline
- LD
12:12 h light-dark cycle
- DD
constant darkness
- KO
knockout
- WT
wildtype
- i.c.v
intracerebroventricular
- PPI
pre-pulse inhibition
- ANOVA
analysis of variance
References
- [1].Abe H, Honma S, Honma K, Suzuki T, Ebihara S. Functional diversities of two activity components of circadian rhythm in genetical splitting mice (CS strain) J Comp Physiol [A] 1999;184:243–251. doi: 10.1007/s003590050322. [DOI] [PubMed] [Google Scholar]
- [2].Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN, Bernier V, Stocco R, Therien AG, Koller BH. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1005–1017. doi: 10.1152/ajplung.00174.2006. [DOI] [PubMed] [Google Scholar]
- [3].Beck B, Fernette B, Stricker-Krongrad A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem Biophys Res Commun. 2005;332:859–865. doi: 10.1016/j.bbrc.2005.05.029. [DOI] [PubMed] [Google Scholar]
- [4].Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- [5].Bouwknecht JA, van der Gugten J, Groenink L, Olivier B, Paylor RE. Behavioral and physiological mouse models for anxiety: effects of flesinoxan in 129S6/SvEvTac and C57BL/6J mice. Eur J Pharmacol. 2004;494:45–53. doi: 10.1016/j.ejphar.2004.04.037. [DOI] [PubMed] [Google Scholar]
- [6].Camarda V, Rizzi A, Ruzza C, Zucchini S, Marzola G, Marzola E, Guerrini R, Salvadori S, Reinscheid RK, Regoli D, Calo' G. In vitro and in vivo pharmacological characterization of the neuropeptide S receptor antagonist [DCys(tBu)5]NPS. J Pharmacol Exp Ther. 2009;328:549–555. doi: 10.1124/jpet.108.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cline MA, Godlove DC, Nandar W, Bowden CN, Prall BC. Anorexigenic effects of central neuropeptide S involve the hypothalamus in chicks (Gallus gallus) Comp Biochem Physiol A Mol Integr Physiol. 2007;148:657–663. doi: 10.1016/j.cbpa.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [8].Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- [9].Crawley JN. Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss; New York: 1999. What's wrong with my mouse? p. 9. [Google Scholar]
- [10].D'Amato M, Bruce S, Bresso F, Zucchelli M, Ezer S, Pulkkinen V, Lindgren C, Astegiano M, Rizzetto M, Gionchetti P, Riegler G, Sostegni R, Daperno M, D'Alfonso S, Momigliano-Richiardi P, Torkvist L, Puolakkainen P, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Kontula K, Lofberg R, Pettersson S, Kere J. Neuropeptide S receptor 1 gene polymorphism is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2007;133:808–817. doi: 10.1053/j.gastro.2007.06.012. [DOI] [PubMed] [Google Scholar]
- [11].Gottlieb DJ, O'Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Med Genet. 2007;8(Suppl 1):S9. doi: 10.1186/1471-2350-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- [13].Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- [14].Hughes AT, Piggins HD. Behavioral responses of Vipr2−/− mice to light. J Biol Rhythms. 2008;23:211–219. doi: 10.1177/0748730408316290. [DOI] [PubMed] [Google Scholar]
- [15].Jones BE. Arousal systems. Front Biosci. 2003;8:s438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- [16].Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, Mäkelä S, Rehn M, Pirskanen A, Rautanen A, Zucchelli M, Gullstén H, Leino M, Alenius H, Petäys T, Haahtela T, Laitinen A, Laprise C, Hudson TJ, Laitinen LA, Kere J. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- [18].Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- [19].Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- [20].McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–17. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- [21].Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PLoS ONE. 2008;3:e2695. doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Melén E, Bruce S, Doekes G, Kabesch M, Laitinen T, Lauener R, Lindgren CM, Riedler J, Scheynius A, van Hage-Hamsten M, Kere J, Pershagen G, Wickman M, Nyberg F, PARSIFAL Genetics Study Group Haplotypes of G protein-coupled receptor 154 are associated with childhood allergy and asthma. Am J Respir Crit Care Med. 2005;171:1089–1095. doi: 10.1164/rccm.200410-1317OC. [DOI] [PubMed] [Google Scholar]
- [23].Montkowski A, Poettig M, Mederer A, Holsboer F. Behavioural performance in three substrains of mouse strain 129. Brain Res. 1997;762:12–18. doi: 10.1016/s0006-8993(97)00370-3. [DOI] [PubMed] [Google Scholar]
- [24].National Research Council . Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington DC: 2003. [PubMed] [Google Scholar]
- [25].Niimi M. Centrally administered neuropeptide S activates orexin-containing neurons in the hypothalamus and stimulates feeding in rats. Endocrine. 2006;30:75–79. doi: 10.1385/ENDO:30:1:75. [DOI] [PubMed] [Google Scholar]
- [26].Okamura N, Reinscheid RK. Neuropeptide S: a novel modulator of stress and arousal. Stress. 2007;10:221–226. doi: 10.1080/10253890701248673. [DOI] [PubMed] [Google Scholar]
- [27].Okamura N, Hashimoto K, Iyo M, Shimizu E, Dempfle A, Friedel S, Reinscheid RK. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1444–1448. doi: 10.1016/j.pnpbp.2007.06.026. [DOI] [PubMed] [Google Scholar]
- [28].Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydrooxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [30].Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol [A] 1976;106:291–331. [Google Scholar]
- [31].Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- [32].Pulkkinen V, Haataja R, Hannelius U, Helve O, Pitkänen OM, Karikoski R, Rehn M, Marttila R, Lindgren CM, Hästbacka J, Andersson S, Kere J, Hallman M, Laitinen T. G protein-coupled receptor for asthma susceptibility associates with respiratory distress syndrome. Ann Med. 2006;38:357–366. doi: 10.1080/07853890600756453. [DOI] [PubMed] [Google Scholar]
- [33].Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide S receptor variants. J Pharmacol Exp Ther. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- [34].Reinscheid RK. Neuropeptide S: anatomy, pharmacology, genetics and physiological functions. Results Probl Cell Differ. 2008;46:145–158. doi: 10.1007/400_2007_051. [DOI] [PubMed] [Google Scholar]
- [35].Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol Behav. 2002;77:301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- [37].Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, Ghatei MA, Bloom SR. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- [38].Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- [39].Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, Watanabe T, Liu C, Chen J, Barbier AJ, Turek FW, Fung-Leung WP, Lovenberg TW. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol. 2002;62:389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- [40].Vitale G, Filaferro M, Ruggieri V, Pennella S, Frigeri C, Rizzi A, Guerrini R, Calò G. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides. 2008;29:2286–2291. doi: 10.1016/j.peptides.2008.08.014. [DOI] [PubMed] [Google Scholar]
- [41].Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [42].Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]