Abstract
NRCAM (Neuronal Cell Adhesion Molecule) has an important role in axonal guidance and the organization of neural circuitry during brain development. Association analyses in human populations have identified NRCAM as a candidate gene for autism susceptibility. In the present study, we evaluated Nrcam-null mice for sociability, social novelty preference, and reversal learning as a model for the social deficits, repetitive behavior, and cognitive rigidity characteristic of autism. Prepulse inhibition of acoustic startle responses was also measured, to reflect sensorimotor-gating deficits in autism spectrum disorders. Assays for anxiety-like behavior in an elevated plus maze and open field, motor coordination, and olfactory ability in a buried food test were conducted to provide control measures for the interpretation of results. Overall, the loss of Nrcam led to behavioral alterations in sociability, acquisition of a spatial task, and reversal learning, dependent on sex. In comparison to male wild type mice, male Nrcam-null mutants had significantly decreased sociability in a three-chambered choice task. Low sociability in the male null mutants was not associated with changes in anxiety-like behavior, activity, or motor coordination. Male, but not female, Nrcam-null mice had small decreases in prepulse inhibition. Nrcam deficiency in female mice led to impaired acquisition of spatial learning in the Morris water maze task. Reversal learning deficits were observed in both male and female Nrcam-null mice. These results provide evidence that NRCAM mediates domains of function relevant to symptoms observed in autism.
Keywords: Adhesion molecule, Autism, Cognition, Sensorimotor gating, Social approach, Spatial learning
1. Introduction
Cell adhesion molecules (CAMs) are transmembrane proteins that allow cells to bind to other cells or to the extracellular matrix. A subset of CAM genes, including NRCAM (Neuronal Cell Adhesion Molecule), encodes neural cell recognition molecules that are critical for the cell-cell interactions underlying brain development. Nrcam has regionally-selective expression in rodent brain, including cerebral cortex, hippocampus, olfactory bulb, striatum, thalamus, and cerebellum [13,14,36]. Significant Nrcam mRNA expression has also been reported in specific neuronal populations, including hippocampal pyramidal cells and cerebellar Purkinje and granule cells [14,36]. These findings suggest that NRCAM may play an important role in multiple domains of function subserved by different brain regions.
Autism is a severe neurodevelopmental syndrome that typically emerges during early childhood. Diagnostic indicators include social deficits, abnormal repetitive behavior, and a resistance to change learned response patterns [3]. Association analyses in human populations have identified NRCAM as a possible candidate gene for autism susceptibility [5,18,37]. NRCAM is located on chromosome 7q, a loci which has been implicated in autism etiology by several linkage studies (see reviews by [1,11]). Bonora et al. [5] conducted a genetic study on several genes on 7q in a European cohort, and identified NRCAM polymorphisms with significant association to autism. These associations were not enhanced when only male subjects were analyzed, indicating that the effects were not sex-selective. The link between NRCAM and autism has recently been confirmed by studies in a Japanese population [18]. One report found altered NRCAM transmission only in a subset of autism subjects characterized by high levels of obsessive-compulsive behavior [37], suggesting that there may be specificity between significant effects and particular endophenotypes of the disorder.
Mice with a deficiency of Nrcam demonstrate alterations in brain that may reflect the changes found in autism, including reduced size of the cerebellar lobes [36]. These mice also exhibit selective changes in anxiety-like behavior and reaction to stress [19]. The following study determined whether the targeted disruption of Nrcam in mice leads to alterations in behavioral domains relevant to symptoms of autism. Nrcam-null mice were evaluated for sociability and social novelty preference in a three-chambered choice task, as a way to model the decreased preference for social proximity and interaction observed in autistic children [3,15,21]. Reversal learning in the Morris water maze task was used to reflect the perseveration of response patterns and reduced cognitive flexibility reported for autism [7,17]. Previous work has shown that mice with genetic alterations associated with autism spectrum disorders can have significant changes in social approach [8,23,25,39] and deficits in reversal learning procedures [4,6,40]. Nrcam-deficient mice were also evaluated for prepulse inhibition of acoustic startle responses, since clinical studies have shown impaired sensorimotor gating in subjects with autism, Asperger syndrome, and fragile X syndrome [10,20,33].
One issue for the interpretation of social and cognitive behavioral tests is that alterations in motor function, sensory ability, activity, or anxiety, can underlie differences found between experimental groups. This may be an especially important consideration for the Nrcam mice, since changes in anxiety-like behavior, grip strength in a motor task, and cerebellar development have been observed in the null mutants [19,36]. The present study used a battery of control measures, including anxiety-like behavior in an elevated plus maze, exploration in an open field, motor coordination on an accelerating rotarod, olfactory function in a buried food test, and visual ability in the water maze, to determine possible factors underlying abnormal behavioral phenotypes.
2. Materials and methods
2.1. Animals
Wild type (Nrcam+/+) and null mutant (Nrcam−/−) mice were on a mixed 129S6/SvEvTac (129S6) × Swiss Webster (CFW) background [36], maintained by heterozygous matings from breeder stock kindly provided by Dr. Martin Grumet (Rutgers University, Piscataway, NJ). Subjects were taken from two separate cohort groups. The first cohort consisted of 28 wild type mice (18 males and 10 females) and 20 null mutant mice (7 males and 13 females). The second cohort included 18 wild type mice (10 males and 8 females) and 19 null mutants (12 males and 7 females). Testing began when animals were 5 to 8 weeks of age. Overall numbers for each of the Nrcam groups are given in Table 1. A separate set of 10 female 129S6/SvEvTac mice (3–4 weeks in age at arrival; Taconic Farms) were tested for social behavior, in order to evaluate effects of background strain.
Table 1.
Control measures in Nrcam +/+ and −/− mice. Data shown are means ± SEM for body weight at the beginning of the study, performance on an elevated plus maze, and olfactory ability in a buried-food test.
| Males | Females | |||
|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | |
| Number of subjects | 28 | 19 | 18 | 20 |
| Body weight (g) | 21 ± 0.4 | 22 ± 0.8 | 18 ± 0.6 | 17±0.5 |
| Elevated plus mazea | ||||
| Percent open arm time | 30 ± 4 | 27 ± 6 | 24 ± 4 | 31 ± 5 |
| Percent open arm entries | 28 ± 3 | 22 ± 5 | 25 ± 4 | 29 ± 4 |
| Total number of entries | 21 ± 1 | 16 ± 2 | 17 ± 2 | 19 ± 3 |
| Olfactory test | ||||
| Latency to find food (sec) | 613 ± 66 | 551 ± 90 | 528 ± 84 | 596 ± 77 |
| Percent of group finding food | 46% | 47% | 56% | 50% |
Data were removed for one female Nrcam−/− mouse that remained on one open arm for the entire 5-min test.
Mice were housed in ventilated cages, with free access to water and Purina 5058 chow. The housing room had a 12-hr light/dark cycle (lights off at 7:00 p.m.). Genotyping was conducted by PCR from tail tissue samples. All procedures were conducted in strict compliance with the policies on animal welfare of the National Institutes of Health and the University of North Carolina (stated in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animal Resources, National Research Council, 1996 edition).
2.2. Testing procedures
All Nrcam mice were tested with the following assays, in the following order: 1) elevated plus maze, 2) activity in an open field, 3) rotarod, 4) social approach test, 5) acoustic startle test, 6) buried food test for olfactory ability, and 6) Morris water maze test. The separate group of female 129S6 mice was tested for activity in an open field and social approach. Only one procedure was conducted per day. Detailed descriptions of these tests have been previously published [26–28].
2.2.1. Elevated plus-maze test
Mice were given one 5-min trial on a metal plus-maze, which had two closed arms, with walls 20 cm in height, and two open arms. The maze was elevated 50 cm from the floor, and the arms were 30 cm long. Animals were placed on the center section (8 cm × 8 cm), and allowed to freely explore the maze. Arm entries were defined as all four paws entering an arm. Entries and time in each arm were recorded during the trial by a human observer via computer coding. Percent open arm time was calculated as 100 × (time spent on the open arms/(time in the open arms + time in the closed arms)). Percent open arm entries was calculated using the same formula.
2.2.2. Open field
Exploratory activity in a novel environment was assessed for one hour in a photocell-equipped automated open field (40 cm × 40 cm × 30 cm; Versamax system, Accuscan Instruments). Measures were taken of total distance traveled, number of rearing movements, and time spent in the center during the test. Activity chambers were contained inside sound-attenuating boxes, equipped with houselights and fans.
2.2.3. Rotarod performance
Mice were assessed for balance and motor coordination on an accelerating rotarod (Ugo-Basile, Stoelting Co., Wood Dale, Il). Revolutions per minute (rpm) were set at an initial value of 3, with a progressive increase to a maximum of 30 rpm across the 5-min test session. Each animal was given a test session consisting of three trials, with 45 seconds between each trial. Two additional trials were given 48 hours later. Latency to fall, or to rotate off the top of the turning barrel, was measured by the rotarod timer.
2.2.4. Sociability and preference for social novelty
Mice were tested in an automated three-chambered box [27,29]. Dividing walls had retractable doorways allowing access into each chamber. The automated box had photocells embedded in each doorway to allow quantification of entries and duration in each chamber of the social test box. The chambers of the apparatus were cleaned with water and dried with paper towels between each trial. At the end of each test day, the apparatus was sprayed with 70% ethanol and wiped clean with paper towels.
The choice test had three 10-min phases: 1) Habituation. The test mouse was first placed in the middle chamber and allowed to explore, with the doorways into the two side chambers open. 2) Sociability. After the habituation period, the test mouse was enclosed in the center compartment of the social test box, and an unfamiliar mouse (stranger 1; an adult C57BL/6J male) was enclosed in a wire cage (11 cm H, 10.5 bottom diameter, bars spaced 1 cm apart; Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, Ohio) and placed in a side chamber. The location for stranger 1 alternated between the left and right sides of the social test box across subjects. An empty wire cage was placed in the opposite side, to serve as a novel object control. Following placement of stranger 1, the doors were re-opened, and the subject was allowed to explore the entire social test box. Measures were taken of the amount of time spent in each chamber and the number of entries into each chamber by the automated testing system. 3) Preference for social novelty. At the end of the sociability test, each mouse was further tested for preference to spend time with a new stranger. A new unfamiliar mouse (stranger 2; an adult C57BL/6J male from a home cage different from that of stranger 1) was placed in the wire cage that had been empty during the previous session. The test mouse then had a choice between the first, already-investigated mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). The same measures were taken as with the sociability test.
2.2.5. Acoustic startle procedure
The acoustic startle measure was based on the reflexive whole-body flinch, or startle response, following exposure to a sudden noise. Animals were tested with a San Diego Instruments SR-Lab system, using published procedures [9,32]. Briefly, mice were placed in a small Plexiglas cylinder within a larger, sound-attenuating chamber (San Diego Instruments). The cylinder was seated upon a piezoelectric transducer, which allowed vibrations to be quantified and displayed on a computer. The chamber included a houselight, fan, and a loudspeaker for the acoustic stimuli (bursts of white noise). Background sound levels (70 dB) and calibration of the acoustic stimuli were confirmed with a digital sound level meter (San Diego Instruments).
Each test session consisted of 42 trials, presented following a 5-min habituation period. There were 7 different types of trials: the no-stimulus trials, trials with the acoustic startle stimulus (40 ms; 120 dB) alone, and trials in which a prepulse stimulus (20 ms; either 74, 78, 82, 86, or 90 dB) had onset 100 ms before the onset of the startle stimulus. The different trial types were presented in blocks of 7, in randomized order within each block, with an average intertrial interval of 15 sec (range: 10 to 20 sec). Measures were taken of the startle amplitude for each trial, defined as the peak response during a 65-msec sampling window that began with the onset of the startle stimulus. Levels of PPI at each prepulse sound level were calculated as 100 - [(response amplitude for prepulse stimulus and startle stimulus together/response amplitude for startle stimulus alone) × 100].
2.2.6. Olfactory test following food deprivation
Several days before the olfactory test, an unfamiliar food (Froot Loops, Kellogg Co., Battle Creek, MI) was placed overnight in the home cages of the mice, in order to avoid food neophobia on the day of testing. 16–20 hours before the test, all food was removed from the home cage. On the day of the test, each mouse was placed in a large, clean tub cage (46 cm L × 23.5 cm W × 20 cm H), containing 3 cm deep paper chip bedding (Canbrands Product, Moncton NB, Canada), and allowed to explore for 5 min. The animal was removed from the cage, and 1 Froot Loop was buried in the cage bedding, approximately 1 cm below the surface of the litter. The subject mouse was then returned to the cage for a 15-min test. Measures were taken of latency to find the buried food.
2.2.7. Water maze test
A subset of mice from each cohort was tested in the Morris water maze task, based on published methods [24,30]. The water maze consisted of a large circular pool (diameter = 122 cm) partially filled with water (45 cm deep, 24–26°C), located in a room with numerous visual cues. Mice were tested for their ability to find an escape platform (diameter = 12 cm) under three different learning conditions: with a cued visible platform, acquisition in the hidden (submerged) platform test, and reversal learning with the hidden platform moved to the opposite quadrant. In each case, the criterion for learning was an average latency of 15 sec or less to locate the platform across a block of 4 consecutive trials per day. In addition, at the end of the acquisition and reversal learning phases, mice were given 1-min probe trials with the platform removed. In these probe trials, spatial learning could be demonstrated by higher levels of swimming in the quadrant where the platform had been located in the training trials, versus swimming in the other quadrants of the pool.
In the visible platform test, each animal was given 4 trials per day, across 3 days, to swim to an escape platform cued by a patterned cylinder extending above the surface of the water. For each trial, the mouse was placed in the pool at 1 of 4 possible locations (randomly ordered), and then given 60 sec to find the cued platform. If the mouse found the platform, the trial ended, and the animal was allowed to remain 10 sec on the platform before the next trial began. If the platform was not found, the mouse was placed on the platform for 10 sec, and then given the next trial. Measures were taken of latency to find the platform and swimming velocity, via an automated tracking system (Ethovision, Noldus Information Technology, Wageningen, the Netherlands).
The following week, mice were evaluated for acquisition in the hidden platform test. Using the same procedure as described above, each animal was given 4 trials per day, for up to 9 days, to learn the location of the submerged platform. At the end of the day that the group met the 15-sec criterion for learning, or else on day 9 of testing, mice were given a 1-min probe trial in the pool with the platform removed. Selective quadrant search was evaluated by measuring percent of time spent in each quadrant of the pool. In the week following the acquisition phase, mice were tested for reversal learning, using the same procedure. In this phase, the hidden platform was located in a different quadrant in the pool, diagonal to its previous location. On the day that the criterion for learning was met, or else on day 9 of testing, the platform was removed from the pool, and the group was given a probe trial to evaluate reversal learning.
2.3. Statistical analysis
Data were analyzed using 2-way ANOVAs (analysis of variance) or repeated measures ANOVAs, with the factors genotype and sex. Separate ANOVAs were then conducted for male and female groups to further explore genotype main effects or interactions. Group means were compared using post-hoc Fisher’s PLSD (protected least-significant difference) tests. Social preference was determined using within-genotype repeated measures ANOVAs, with the factor of chamber side (e.g., stranger 1 side or the opposite side). Similarly, quadrant preference in the Morris water maze was determined using within-genotype repeated measures ANOVAs, with the factor quadrant location. For all comparisons, significance was set at p < 0.05.
3. Results
3.1. Control measures
Mice were evaluated for sensory and motor ability, anxiety-like behavior and activity, since impairment in any of these domains of function could alter performance in the three-chambered social approach test. As shown in Table 1, the Nrcam +/+ and −/− mice were similar in body weight. The overall ANOVAs did not reveal any significant effects of genotype or sex on anxiety-like behavior on the elevated plus maze or latencies in the olfactory test. All experimental groups had relatively poor performance in the test for olfactory ability, with only about half of the mice locating the buried food. Failure to complete the olfactory task may have been due to the inability to sense the hidden food or a lack of motivation to obtain the food during the test.
3.1.1. Open field test
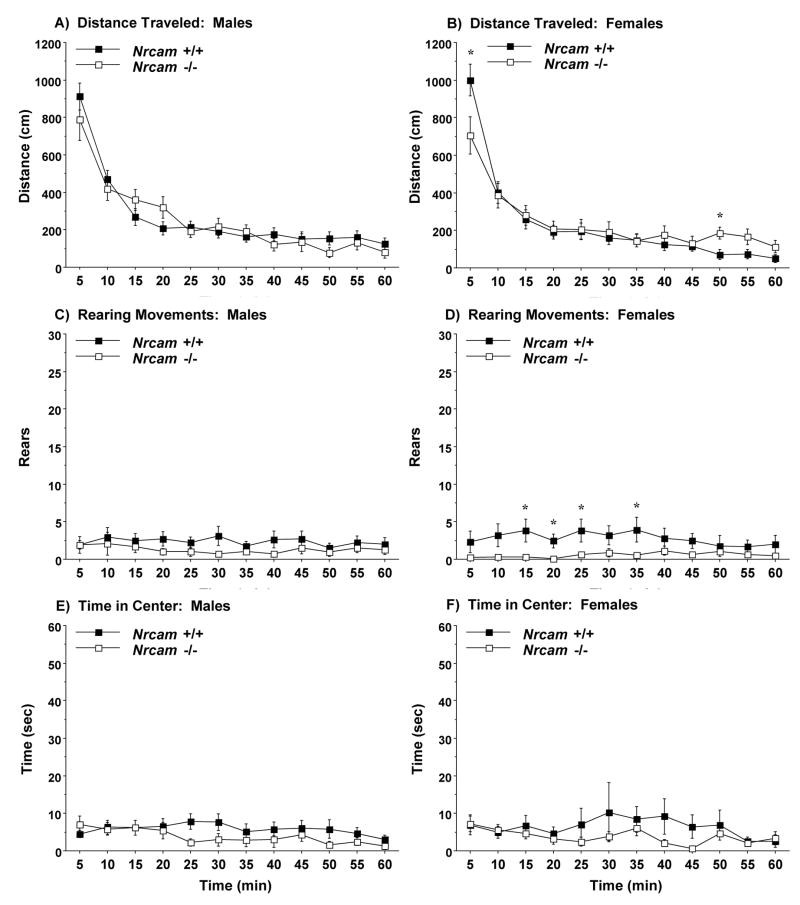
Mice were given a 1-hr session in a novel open field to evaluate activity and exploration. Overall ANOVAs indicated significant effects of genotype, but not sex, on total distance traveled [genotype × time interaction, F(11,891)=2.98, p=0.0007] and rearing movements [main effect of genotype, F(1,81)=5.5, p=0.0215]. Separate repeated measures ANOVAs revealed that the male Nrcam +/+ and −/− mice had similar levels of activity (Fig. 1). In the female groups, the null mutant mice had lower locomotor activity at the beginning of the test, and higher activity near the end of the test [post-hoc comparisons following genotype × time interaction, F(11,396)=2.88, p=0.0012]. The female Nrcam−/− mice also had lower numbers of rearing movements [main effect of genotype, F(1,36)=5.78, p=0.0215]. Overall, all of the groups had markedly low numbers of rearing responses during the open field test. Low rearing counts have previously been reported in 129 substrains and mutant mouse lines maintained on 129 backgrounds [25,27,34,35]. No differences were observed in time spent in the center region by the Nrcam−/− male or female mice. Similar to the measure for rearing, all of the groups had very low center times, suggesting that levels of exploration were generally low in both wild type and null mutant mice.
Fig. 1.
Locomotor activity, rearing movements, and time spent in the center region by Nrcam wild type (+/+) and null mutant (−/−) mice during a 1-hr test in a novel open field. Data are means (± SEM) for each group. *p<0.05.
3.1.2. Rotarod
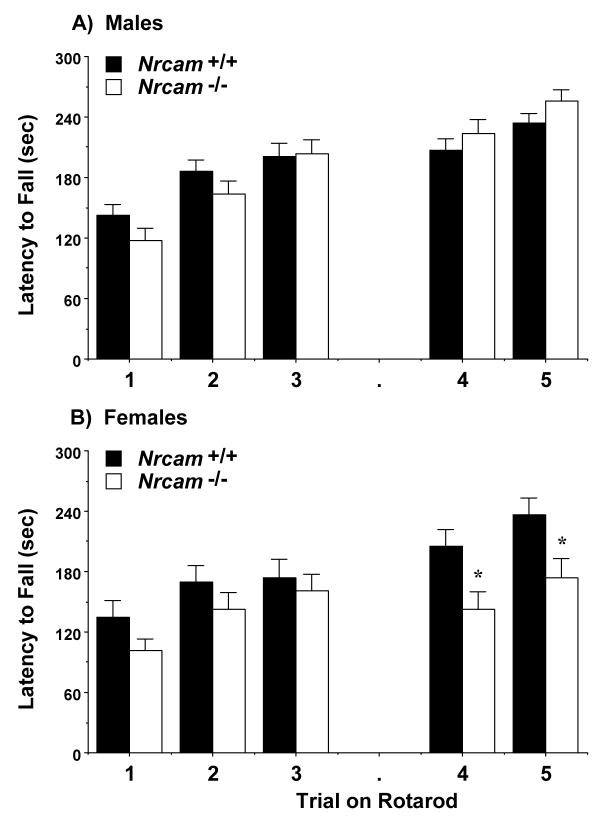
Mice were evaluated for motor coordination on an accelerating rotarod. No significant effects of genotype or sex were observed during the first day of training (Fig. 2). However, during the retest given 48 hr after training, sex-dependent differences emerged between the experimental groups [main effect of sex, F(1,81)=8.62, p=0.0043; and genotype × sex interaction, F(1,81)=8.79, p=0.004]. Separate repeated measures ANOVAs indicated that the female, but not male, Nrcam−/− mice had significantly decreased latencies to fall from the rotating barrel [main effect of genotype, F(1,36)=6.74, p=0.0136]. The results suggest that, in female mice, deficiency of Nrcam interferes with the increase in performance typically observed across trials in motor tasks.
Fig. 2.
Latency to fall from an accelerating rotarod. Trials 4 and 5 were given 48 hr after the first 3 trials. Female Nrcam−/− mice had impaired performance during the retest. Data shown are means (+ SEM) for each group. *p<0.05.
3.2. Sociability and Social Novelty Preference
In the three-chambered assay for sociability, mice were given a choice between spending time in the side with an unfamiliar mouse or in the side with a novel object. The low levels of exploration found in the activity test were also evident in the sociability test. Because 10 of the male mice (6 wild type and 4 null mutant) and 5 of the female mice (2 wild type and 3 null mutant) did not leave the center chamber during the test, data from these animals were removed from the analysis.
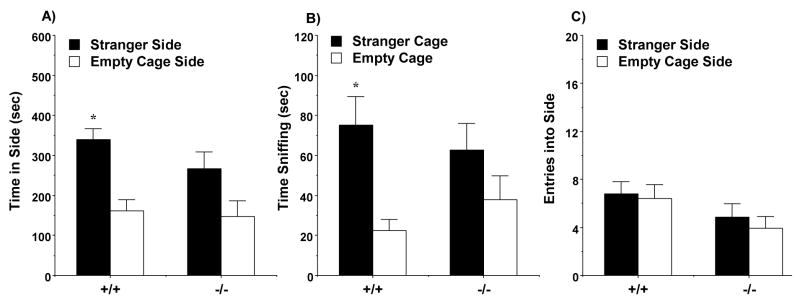
There were no overall effects of genotype or sex on any of the measures in the test for sociability. However, a separate repeated measures ANOVA conducted on data from the male mice revealed a significant main effect of genotype on time spent in the side chambers [F(1,35)=7.47, p=0.0098], and a significant effect of the repeated measure (side of social test box) [F(1,35)=11.15, p=0.002]. A within-genotype ANOVA indicated that the male Nrcam−/− mice did not demonstrate any significant preference for spending more time in the proximity to the unfamiliar stranger mouse versus the empty cage [F(1,14)=2.7, p=0.1225] (Fig. 3). In contrast, the male wild type mice demonstrated significant sociability during the social approach test for measures of time spent in each side [F(1,21)=10.71, p=0.0036] and sniffing at each wire cage [F(1,21)=11.33, p=0.0029]. No differences were observed between the control mice and the null mutant mice for number of entries during the test, suggesting that the genotype effects could not be explained by hypoactivity in the Nrcam−/− group.
Fig. 3.
Lack of sociability in male Nrcam−/− mice during a three-chambered choice test. Measures were taken of A) time spent in each side, B) time spent sniffing each wire cage, and C) number of entries into each side. Data shown are mean (+ SEM) for +/+ (n=22) and −/− (n=15) groups. The results do not include subjects that failed to leave the center chamber (+/+, n=6; −/−, n=4). * p < 0.05, within-genotype repeated-measure comparison, stranger 1 side different from empty cage side.
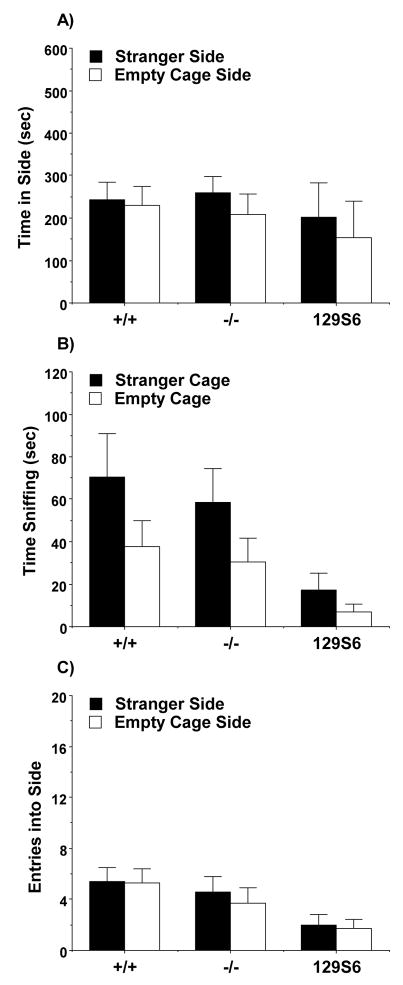
In the female mice, repeated measures ANOVAs did not detect any effects of genotype on time or entries in each side, or time spent sniffing each wire cage. Within-genotype ANOVAs indicated that neither the wild type [F(1,15)=0.03, p=0.8726] nor null mutant mice [F(1,15)=0.43, p=0.521] had significant sociability in the choice task (Fig. 4). A similar lack of side preference was observed in a separate group of female 129S6 inbred mice [F(1,6)=0.11, p=0.7467], which was tested to determine whether the general lack of sociability might be related to the mixed background of the Nrcam lines.
Fig. 4.
Lack of sociability in female mice during a three-chambered choice test. Measures were taken of A) time spent in each side, B) time spent sniffing each wire cage, and C) number of entries into each side. Data shown are mean (+ SEM) for +/+ (n=16) and −/− (n=17) groups, and a separate set of female 129S6/SvEvTac (129S6) mice (n=7). The results do not include subjects that failed to leave the center chamber (+/+, n=2; −/−, n=3; 129S6, n=3). Data for time and entries in each side for one −/− subject were lost due to equipment failure.
Following the sociability assay, mice were given a test for social novelty preference, with a choice between the original stranger 1 mouse, and a new stranger mouse. None of the groups in the present study demonstrated a significant preference for spending more time in the proximity of the new stranger than with the first stranger (data not shown). No significant effects of the repeated measure (side of social test box) were observed in the males [F(1,35)=2.57, p=0.1178] or females [F(1,31)=0.6, p=0.444], or in the separate set of 129S6 female mice [F(1,6)=2.74, p=0.1488]. Within-genotype analyses confirmed a lack of social novelty preference in male and female wild type and null mutant mice for measures of time spent in each side of the social test box and time spent sniffing each wire cage.
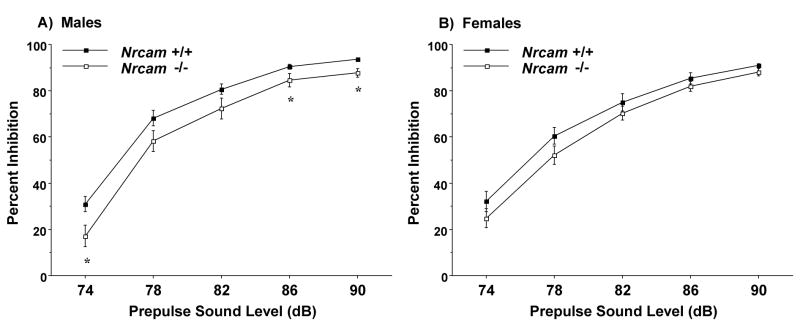
3.3. Prepulse inhibition of acoustic startle responses
A significant effect of sex, but not genotype, was observed for measures of startle amplitude across prepulse sound levels, with a 120 decibel startle stimulus [sex × decibel interaction, F(6,486)=2.69, p=0.0139]. However, a significant main effect of genotype [F(1,81)=8.62, p=0.0043], as well as a significant sex × decibel interaction [F(4,324)=3.53, p=0.0077] emerged for the measure of prepulse inhibition (Fig. 5). Separate repeated measures ANOVAs indicated that the male Nrcam−/− mice had small, but significant, reductions in prepulse inhibition, in comparison to the wild type controls [main effect of genotype, F(1,45)=7.09, p=0.0107]. No differences were found in the female groups.
Fig. 5.
Reduced prepulse inhibition of acoustic startle responses in male Nrcam−/− mice. Data shown are means (+ SEM) for each group. * p < 0.05.
3.4. Morris water maze
3.4.1. Control measures for maze performance
Deficits in motor ability or vision can preclude evaluation in the water maze. Therefore, the Nrcam mice were first tested for performance in a visual cue procedure (Table 2). In this test, mice learned to locate a visible escape platform. An overall ANOVA indicated a three-way interaction between genotype, sex, and day of testing [F(2,136)=3.4, p=0.036]. In the male groups, a repeated measures ANOVA indicated a significant main effect of genotype on latency to reach the platform [F(1,37)=4.89, p=0.0332]. However, post-hoc comparisons showed that the difference between the controls and null mutant mice was only seen on the first day of testing. In fact, by the last day of testing, both male and female groups had highly proficient performance in the visual cue task. One female wild type mouse did not meet learning criterion in the visual cue task, and was dropped from the study.
Table 2.
Visual cue test and swimming ability in the Morris water maze.
| Males | Females | |||
|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | |
| Number of subjects | 20 | 19 | 15a | 18 |
| Latency to cued platform | ||||
| Day 1 | 16 ± 2 | 23 ± 2* | 23 ± 3 | 19 ± 2 |
| Day 2 | 8 ± 1 | 9 ± 1 | 10 ± 3 | 9 ± 2 |
| Day 3 | 5 ± 1 | 7 ± 1 | 10 ± 4 | 7 ± 1 |
| Swimming speed (cm/sec) on Day 1 of testing | ||||
| Visual cue | 20 ± 1 | 18 ± 1 | 19 ± 1 | 18 ± 1 |
| Acquisition | 21 ± 1 | 20 ± 1 | 21 ± 1 | 19 ± 1 |
| Reversal | 20 ± 1 | 17 ± 1 | 22 ± 1 | 17 ± 1* |
One female +/+ mouse failed to meet the learning criterion (average latency less than 15 sec) for the visual cue task. This subject was not tested for acquisition or reversal learning.
p<0.05, comparison with wild type (+/+) group.
Swimming speed is an index of motor ability in the water maze. A genotype × day interaction [F(2,136)=3.67, p=0.0281] was observed for velocity across the first days of visual cue, acquisition, and reversal learning. However, no genotype effects were found within the male groups. In the female groups, the Nrcam−/− mice had slower swim speeds on the first day of reversal learning [post-hoc test following repeated measures ANOVA, genotype × day interaction, F(2,60)=3.55, p=0.0348]. Overall, the findings show that the loss of Nrcam did not lead to any consistent deficits in swimming ability.
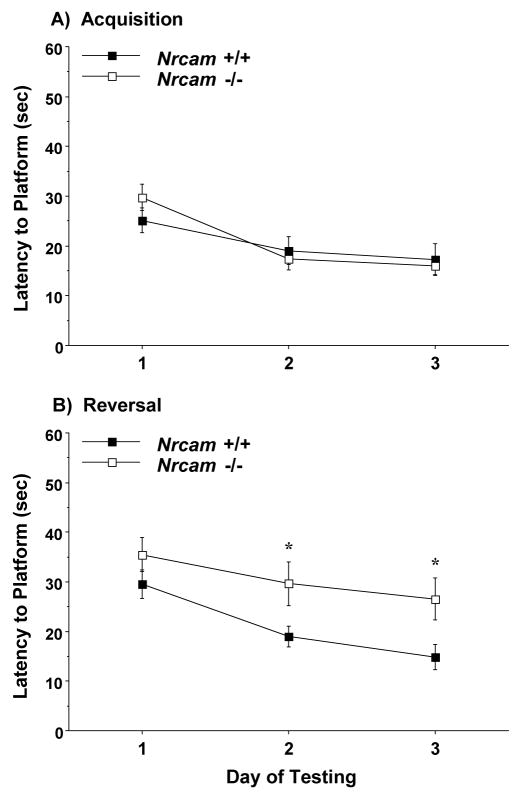
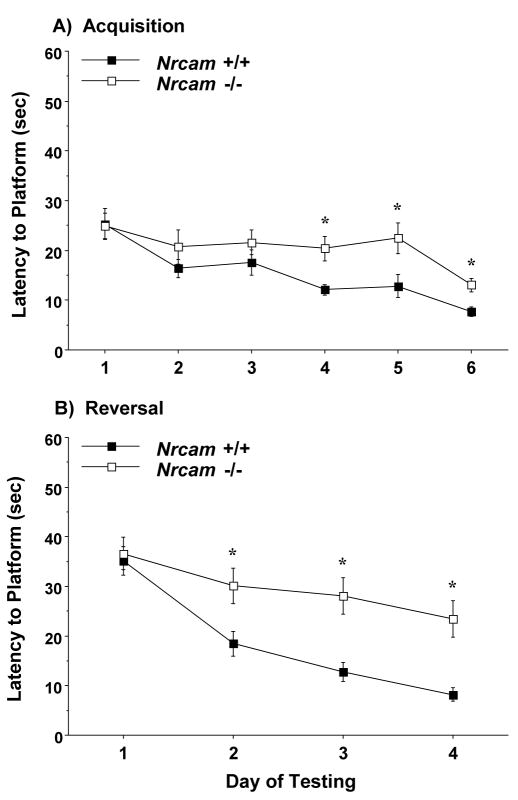
3.4.2. Acquisition and reversal
Following the visual cue test, mice were trained to swim to a hidden, submerged escape platform. During the acquisition phase, the male wild type and Nrcam-null mice had similar levels of performance (Fig. 6), suggesting that loss of Nrcam does not impair spatial learning in male mice. However, during reversal, the male Nrcam−/− group had significantly longer latencies to reach the escape platform on the last two days of testing [main effect of genotype, F(1,37)=7.0, p=0.0119]. In the female groups (shown in Fig. 7), the Nrcam−/− mice were impaired in both the initial task acquisition [main effect of genotype, F(1,30)=5.02, p=0.0327], and in reversal learning [main effect of genotype, F(1,30)=11.85, p=0.0017, and genotype × day interaction, F(3,90)=3.27, p=0.0248].
Fig. 6.
Escape latencies in the Morris water maze for male mice. The Nrcam−/− group showed deficits in reversal learning (B), but not acquisition (A). Data shown are mean (± SEM) of 4 trials per day. *p<0.05.
Fig. 7.
Escape latencies in the Morris water maze for female mice. The Nrcam−/− group showed deficits in both acquisition (A) and reversal learning (B). Data shown are mean (± SEM) of four trials per day. *p<0.05.
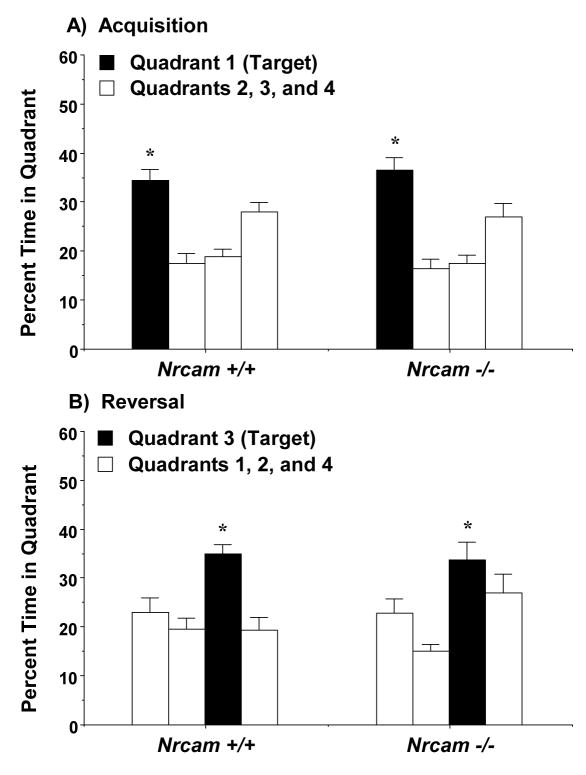
3.4.3. Quadrant selectivity in the probe trials
Mice were given 1-min probe trials at the end of the acquisition and reversal phases. An overall ANOVA revealed a significant interaction between genotype, sex, and test phase [F(1,67)=4.47, p=0.0382]. A four-way interaction between genotype, sex, test phase, and quadrant location approached significance [F(3,201)=2.56, p=0.0565].
Repeated measures ANOVAs indicated that both the male and female mice demonstrated significant quadrant preference, dependent on phase of testing [test phase × quadrant interaction, males, F(3,111)=19.49, p<0.0001, and females, F(3,90)=17.96, p<0.0001]. No significant genotype effects were observed in the male groups (Fig. 8). Both the Nrcam +/+ and −/− male mice showed selective quadrant preference following acquisition [within-genotype comparisons, wild type, F(3,57)=13.05, p<0.0001; null mutant, F(3,54)=12.42, p<0.0001], and following the reversal learning phase [wild type, F(3,57)=6.87, p=0.0005; null mutant, F(3,54)=4.86, p=0.0046].
Fig. 8.
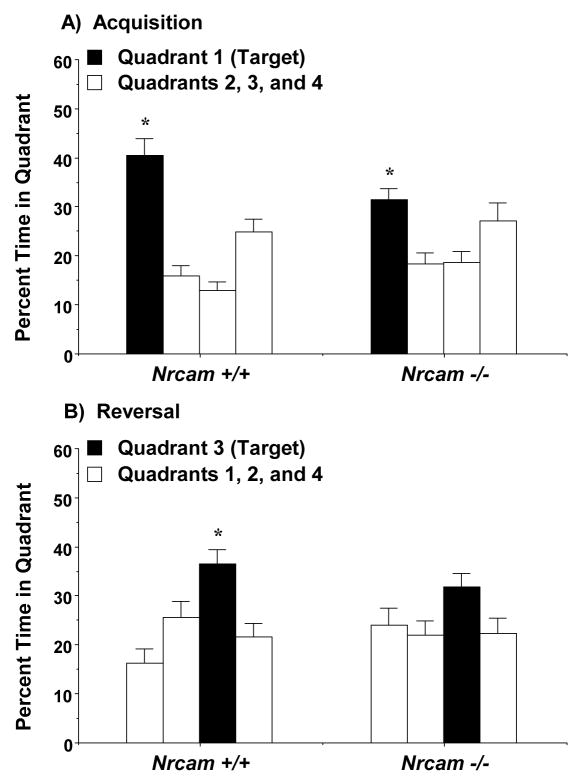
Selective quadrant search on the Morris water maze in male Nrcam mice. Each mouse was given a 1-min probe trial with the escape platform removed. Target indicates the quadrant where the platform was located during each phase. No significant effects of genotype were found in the male groups. * p<0.05, within-group repeated measures ANOVA.
In the female groups (Fig. 9), a repeated measures ANOVA revealed a 3-way interaction between the factors genotype, test phase, and quadrant [F(3,90)=3.03, p=0.0334]. As with the male mice, the female Nrcam +/+ and −/− mice demonstrated significant quadrant preference following acquisition [within-genotype comparisons, wild type, F(3,39)=17.65, p<0.0001; null mutant, F(3,51)=4.33, p=0.0086]. When the platform was switched to a new location during reversal learning, the wild type females formed a significant preference for the new target quadrant [F(3,39)=6.17, p=0.0016]. In contrast, the Nrcam−/− females failed to spend more time in the new target quadrant, versus the other quadrants [F(3,51)=1.68, p=0.1837].
Fig. 9.
Selective quadrant search on the Morris water maze in female Nrcam mice. Each mouse was given a 1-min probe trial with the escape platform removed. Target indicates the quadrant where the platform was located during each phase. The Nrcam−/− mice failed to show significant preference for the target quadrant during reversal learning. * p<0.05, within-group repeated measures ANOVA.
4. Discussion
The present study found that mice with targeted disruption of Nrcam had behavioral changes relevant to symptoms of autism. Male Nrcam-null mice lacked significant sociability in a three-chambered choice task, in contrast to wild type controls. Both male and female Nrcam−/− mice had significant deficits in reversal learning in the Morris water maze. Male, but not female, mutant mice had small significant reductions in prepulse inhibition of acoustic startle responses. The behavioral changes in the male Nrcam-null mice did not include alterations in performance on tests for motor ability, activity, or anxiety-like behavior. A different pattern of behavioral alterations was observed in the female mice, including decreased rearing movements in the open field, impaired coordination on the accelerating rotarod, and deficits in acquisition of spatial learning. These findings extend and complement the behavioral characterization of Nrcam−/− mice conducted by Matzel et al. [19].
Male Nrcam-null mice failed to demonstrate significant sociability with either the measure of time spent in each side chamber, or time spent sniffing each wire cage. Impaired sociability has also been found in other mouse lines with alterations in autism candidate genes, such as Slc6a4, Nlgn3, Gabrb3, and Pten [8,16,25,31,39]. Similarly, reduced social preference has been reported in genetic mouse models for neurodevelopmental disorders associated with autism, including Mecp2 mutant mice, which serve as a model of Rett syndrome, and Fmr1-null mice, a model for fragile X syndrome [23,25]. Olfactory information is a critical component of mouse social behavior, and it is possible that the lack of sociability in the Nrcam-null mice was associated with changes in olfactory ability. In line with this premise, Nrcam is widely expressed in olfactory bulb, and Nrcam-null mice have alterations in olfactory nerve projections [13]. In the present study, both the Nrcam +/+ and −/− mice showed similar, albeit poor, performance in a buried food test for olfaction. Nrcam-null mice have also been reported to have normal performance in an odor discrimination task [19]. Overall, the lack of sociability in the null mutant males is not associated with altered performance in tests of olfactory function.
Neither the female wild type nor null mutant mice demonstrated significant sociability in the present study. The Nrcam mouse line is maintained on a 129S6/SvEvTac (129S6) × Swiss Webster (CFW) background. We tested a separate set of female 129S6 mice, and found a similar lack of sociability. It is possible that increased social preference might be observed in the females if the stranger mice were also female, rather than the adult males used in the present study. The lack of sociability in the female wild type group confounded the detection of deficits in the null mutant females. Further, none of the male or female groups, including the female 129S6 mice, showed significant social novelty preference when given a choice between investigating a new stranger mouse versus the more-familiar first stranger. Male mice from one 129 substrain, 129S1/SVImJ, are also characterized by a lack of social novelty preference [27], while male mice from a different substrain, 129/SvJ (renamed 129X1/SvJ), have significant preference for social novelty [8]. Selecting a 129 substrain with high levels of social approach in a mixed background for a mutation may facilitate the detection of significant social deficits, as observed in Gabrb3-deficient mice [8].
CFW, the second component of the background for the Nrcam lines, is an outbred strain which retains genetic variation not found within inbred mouse strains. CFW mice are able to learn an olfactory discrimination task, suggesting that this strain does not have overt olfactory deficits [22]. Swiss Webster mice are generally more active than 129 substrain mice [34,35], and have been characterized as having intermediate levels of anxiety-like behavior, in comparison to several inbred strains [12]. Agmo et al. [2] used CFW mice in a study on preference for social versus sexual stimuli. The investigators presented ovarectomized CFW females with a choice between an intact or a castrated male CFW mouse, each enclosed in a wire cage. Following treatment with ovarian hormones, the female mice showed higher rates of sniffing directed toward the intact male. Although sociability was not directly evaluated in this study, the results indicate that female CFW mice can discriminate between, and have preference for, different social partners.
While the contribution of the CFW background to the social behavior phenotypes in the Nrcam line remains to be determined, these past findings suggest that Swiss Webster is not characterized by low exploration, high anxiety-like behavior, or avoidance of male stranger mice in a choice procedure. One important caveat is that the genetic diversity in the outbred CFW strain could increase variability in the Nrcam mouse line, due to differential background effects on maternal behavior, susceptibility to environmental stressors, and other factors. The present study used littermate control mice as a comparison group for the null mutant group; however, a more rigorous regimen of using only sex-matched littermate pairs could further control for possible confounding effects of the mixed background.
In addition to social impairment, core symptoms of autism include abnormal repetitive behavior [3]. This domain encompasses perseverative and stereotyped responses, a rigid adherence to schedules, and a resistance to change. Repetitive behavior in autistic adults correlates with deficits in cognitive flexibility and impaired response inhibition [17]. Children with autism have been found to perform more poorly than typically developing children on a spatial-reversal task that requires behavioral flexibility and the inhibition of learned responses [7]. A previous study has shown that Nrcam-null mutant mice have deficits in the inhibition of a learned response in a passive avoidance task [19]. In the present study, both male and female Nrcam−/− mice demonstrated impaired reversal learning in the Morris water maze. This task required mice to learn a new platform location, and to inhibit the tendency to swim to the original platform location. The deficits in reversal learning were particularly pronounced in the female mutant group during the subsequent probe test for quadrant selectivity. The female Nrcam−/− mice also had significant impairment in the acquisition phase of the water maze test. The male null mutant mice did not have deficits in acquisition, similar to previous findings [19].
In addition to autism susceptibility, NRCAM has been linked to vulnerability for substance abuse and addiction [14]. Nrcam-null mice tested for conditioned place preference have marked reductions in preference for a location associated with morphine, cocaine, or amphetamine exposure, without alterations in stimulant drug effects on locomotion [14]. The results suggest that NRCAM plays an important role in mechanisms for reward and motivation. In the present study, male Nrcam−/− mice showed changes in the rewarding value of social proximity, as seen in the lack of sociability. Altered motivation for social stimuli may also underlie social deficits in autism. For example, a recent study using functional brain imaging found a correlation between abnormal social interaction and altered activation of neural circuitry linked to reward in adults with autism [38].
Overall, our findings provide evidence that NRCAM is important in domains of function relevant to human neuropsychiatric disorders. The behavioral alterations observed in the male Nrcam-null mice, including a loss of sociability, deficits in reversal learning, and reduced prepulse inhibition, recapitulate aspects of the autism phenotype. The impaired acquisition in the Morris water maze, observed in the female Nrcam−/− mice, provides evidence that NRCAM mediates spatial learning, congruent with the substantial expression of Nrcam mRNA in rodent hippocampus [13,14]. However, these conclusions are based on single behavioral assays for social behavior and learning, and warrant confirmation through use of additional testing paradigms. Further investigations on the role of NRCAM in early brain development may elucidate the neural circuitry and cortical organization underlying alterations in social behavior, reward and motivation, and cognitive function found in autism and other neurodevelopmental disorders.
Acknowledgments
Nrcam mice were obtained from breeder stock generously provided by Dr. Martin Grumet, Rutgers University, Departments of Cell Biology and Neuroscience, W.M. Keck Center for Collaborative Neuroscience, Piscataway, NJ. We would like to thank Dr. Leann H. Brennaman for her assistance with the project. Support for this research was provided by grants from Autism Speaks (PFM), the National Institute for Child Health and Human Development P30 HD03110 (Dr. Joseph Piven), and the National Institutes for Health STAART U54 MH66418 (Dr. Terry Magnuson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agmo A, Choleris E, Kavaliers M, Pfaff DW, Ogawa S. Social and sexual incentive properties of estrogen receptor alpha, estrogen receptor beta, or oxytocin knockout mice. Genes Brain Behav. 2008;7:70–77. doi: 10.1111/j.1601-183X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 4.Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeven AT, Oostra BA, Reyniers E, de Boulle K, D’Hooge R, Cras P, van Velzen D, Nagels G, Martin J-J, de Deyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 5.Bonora E, Lamb JA, Barnby G, Sykes N, Moberly T, Beyer KS, Klauck SM, Poustka F, Bacchelli E, Blasi F, Maestrini E, Battaglia A, Haracopos D, Pedersen L, Isager T, Eriksen G, Viskum B, Sorensen EU, Brondum-Nielsen K, Cotterill R, Engeland H, Jonge M, Kemner C, Steggehuis K, Scherpenisse M, Rutter M, Bolton PF, Parr JR, Poustka A, Bailey AJ, Monaco AP. Mutation screening and association analysis of six candidate genes for autism on chromosome 7q. Eur J Hum Genet. 2005;13:198–207. doi: 10.1038/sj.ejhg.5201315. [DOI] [PubMed] [Google Scholar]
- 6.Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- 7.Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. J Genet Psychol. 2003;164:29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- 8.DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulawa SC, Geyer MA. Psychopharmacology of prepulse inhibition in mice. Chin J Physiol. 1996;39:139–146. [PubMed] [Google Scholar]
- 10.Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 11.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 12.Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 13.Heyden A, Angenstein F, Sallaz M, Seidenbecher C, Montag D. Abnormal axonal guidance and brain anatomy in mouse mutants for the cell recognition molecules close homolog of L1 and NgCAM-related cell adhesion molecule. Neuroscience. 2008;155:221–233. doi: 10.1016/j.neuroscience.2008.04.080. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro H, Liu QR, Gong JP, Hall FS, Ujike H, Morales M, Sakurai T, Grumet M, Uhl GR. NrCAM in addiction vulnerability: positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31:572–584. doi: 10.1038/sj.npp.1300855. [DOI] [PubMed] [Google Scholar]
- 15.Jahr E, Eikeseth S, Eldevik S, Aase H. Frequency and latency of social interaction in an inclusive kindergarten setting: A comparison between typical children and children with autism. Autism. 2007;11:349–363. doi: 10.1177/1362361307078134. [DOI] [PubMed] [Google Scholar]
- 16.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- 18.Marui T, Funatogawa I, Koishi S, Yamamoto K, Matsumoto H, Hashimoto O, Nanba E, Nishida H, Sugiyama T, Kasai K, Watanabe K, Kano Y, Kato N. Association of the neuronal cell adhesion molecule (NRCAM) gene variants with autism. Int J Neuropsychopharmacol. 2009;12:1–10. doi: 10.1017/S1461145708009127. [DOI] [PubMed] [Google Scholar]
- 19.Matzel LD, Babiarz J, Townsend DA, Grossman HC, Grumet M. Neuronal cell adhesion molecule deletion induces a cognitive and behavioral phenotype reflective of impulsivity. Genes Brain Behav. 2008;7:470–480. doi: 10.1111/j.1601-183X.2007.00382.x. [DOI] [PubMed] [Google Scholar]
- 20.McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- 21.McGee GG, Feldman RS, Morrier MJ. Benchmarks of social treatment for children with autism. J Autism Dev Disord. 1997;27:353–364. doi: 10.1023/a:1025849220209. [DOI] [PubMed] [Google Scholar]
- 22.Mihalick SM. Perinatal exposure to diethylstilbestrol improves olfactory discrimination learning in male and female Swiss-Webster mice. Neurobiol Learn Mem. 2003;80:55–62. doi: 10.1016/s1074-7427(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 23.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 24.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social Approach in Genetically-Engineered Mouse Lines Relevant to Autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 29.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 30.Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 31.Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci U S A. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 33.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol Behav. 2002;77:301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers RJ, Davies B, Shore R. Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus-maze: importance and implications of behavioural baseline. Genes Brain Behav. 2002;1:242–251. doi: 10.1034/j.1601-183x.2002.10406.x. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai T, Lustig M, Babiarz J, Furley AJ, Tait S, Brophy PJ, Brown SA, Brown LY, Mason CA, Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J Cell Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai T, Ramoz N, Reichert JG, Corwin TE, Kryzak L, Smith CJ, Silverman JM, Hollander E, Buxbaum JD. Association analysis of the NrCAM gene in autism and in subsets of families with severe obsessive-compulsive or self-stimulatory behaviors. Psychiatr Genet. 2006;16:251–257. doi: 10.1097/01.ypg.0000242196.81891.c9. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. Br J Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- 39.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Dam D, D’Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, Oostra BA, Kooy RF, De Deyn PP. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res. 2000;117:127–136. doi: 10.1016/s0166-4328(00)00296-5. [DOI] [PubMed] [Google Scholar]