Abstract
Although our lab, as well as several others, has demonstrated a role for corticosterone in cocaine self-administration, there are no studies of the central dynamics of this hormone over the course of a behavioral session when rats are self-administering cocaine or receiving passive injections. The assay of corticosterone in microdialysates collected during such sessions allows for determinations of changes in brain corticosterone during drug-taking behavior. By using the combination of microdialysis in terminal fields for the mesocorticolimbic dopaminergic system and the yoked-triad model, one can distinguish between the direct cocaine-induced activation of the hypothalamo-pituitary-adrenal (HPA) axis from the activation of the HPA axis related to drug-taking. In these experiments, we measured corticosterone in microdialysis samples collected from probes aimed at the medial prefrontal cortex, nucleus accumbens and basolateral amygdala in rats self-administering cocaine and receiving identical, passive infusions of cocaine or saline. While corticosterone was increased in all three brain regions in rats receiving cocaine, medial prefrontal cortex corticosterone was increased significantly more in rats receiving non-contingent infusions of the drug compared to rats self-administering cocaine. The results of these experiments demonstrate that control over drug delivery can affect the influence of a hormonal input on the functional characteristics of specific anatomical projections of the central nervous system. These results also provide evidence of the role steroid hormones play in shaping the functional activity of the brain.
Keywords: cocaine, microdialysis, self-administration, yoked, corticosterone, medial prefrontal cortex, nucleus accumbens, basolateral amygdala
Introduction
The abuse of cocaine and other psychomotor stimulants persists as a major health problem in the United States (National Drug Threat Assessment, 2009). Clearly, there is a significant need for new pharmacological treatments to combat the abuse of these drugs; however, no pharmacotherapy has yet demonstrated sufficient efficacy for widespread clinical use (Carroll et al., 1999; Vocci et al., 2005, Vocci and Montoya, 2009). A better understanding of the effects of cocaine on central nervous system neurochemistry will help to identify more effective pharmacologic approaches for treating cocaine dependence.
The role of adrenal steroids in the neurobiology of cocaine has been intensively studied, and the actions of cocaine within the hypothalamo-pituitary-adrenal (HPA) axis have been implicated in its abuse potential (Goeders 2002a,b; Goeders 2004; Brady and Sinha, 2005; Li and Sinha, 2008). Cocaine stimulates the release of corticosterone in rats (Moldow and Fischman 1987) via mechanisms dependent on the secretion of corticotropin-releasing factor (CRF) from the paraventricular nucleus of the hypothalamus (Rivier and Vale 1987; Koob and Kreek 2007; Koob et al., 1990). During the last several years, the ability of stressors (with the subsequent activation of the HPA axis) to alter the acquisition of psychomotor stimulant self-administration has received considerable attention (Goeders 2002a,b; Goeders, 2004). Corticosterone is also involved in ongoing cocaine self-administration: pretreatment with metyrapone, which blocks the 11β-hydroxylation reaction in the production of corticosterone (Haleem et al., 1988), decreases cocaine self-administration in rats, suggesting a role for corticosterone in cocaine-taking behavior (Goeders and Guerin 1996). These data were supported by experiments with ketoconazole, an oral antimycotic agent that also inhibits the 11β-hydroxylation and 18-hydroxylation steps in the synthesis of adrenocorticosteroids (Engelhardt et al. 1985) and may also function as a glucocorticoid receptor antagonist (Loose et al. 1983). Pretreatment with ketoconazole reduced low dose (0.125-0.25 mg/kg/infusion) cocaine self-administration without affecting food-reinforced responding during the same session (Goeders et al., 1998). Ketoconazole also reduces the cue-induced reinstatement of extinguished cocaine seeking in rats (Goeders and Clampitt, 2002), further suggesting a role for corticosterone in cocaine-seeking behavior.
However, while the role of corticosterone (and the HPA axis) in cocaine self-administration has been supported empirically, there are no studies on the central dynamics of this hormone over the course of the behavioral session when rats are self-administering cocaine or receiving passive injections. The assay of corticosterone in microdialysates (Linthorst and Reul, 2008) collected during such sessions allows for determinations of changes in brain corticosterone during drug-taking behavior. By using the combination of microdialysis in terminal fields for the mesocorticolimbic dopaminergic system (Koob et al. 1998) and the yoked-triad model (Smith et al., 1982, 2003; Hemby et al. 1997), one can distinguish between the direct cocaine-induced activation of the HPA axis from the activation of the HPA axis related to drug-taking. Accordingly, the results of these experiments demonstrate how control over drug delivery can affect the influence of a hormonal input on the functional characteristics of specific anatomical projections of the central nervous system. These results also provide evidence of the role steroid hormones play in shaping the functional activity of the brain.
Materials and Methods
Animals
Experimentally naive male Wistar rats (90-120 days old) were housed in individual cages in a temperature- and humidity-controlled, AAALAC-accredited animal care facility on a reversed 12-hour light, 12-hour dark cycle (lights on at 19:00 hr). The animals had free access to water and food throughout the experiment. All procedures were approved by the LSUHSC-S animal care and use committee and were carried out in accordance with the NIH “Principles of Laboratory Animal Care” (NIH publication No. 85-23).
Guide cannulae implantation
Approximately 7 days before the start of the experiment, the animals were stereotaxically implanted with guide cannulae (CMA/12, CMA/Microdialysis AB, Solna, Sweden) under sodium pentobarbital anesthesia (50 mg/kg, i.p.) with methylatropine nitrate pretreatment (10 mg/kg, i.p.). The cannulae were implanted 2.0 mm above the medial prefrontal cortex (MPC; + 2.5 mm anterior from bregma; ± 0.6 mm from the midline and 1 mm ventral to dura (Paxinos and Watson, 1986), nucleus accumbens (NAC; + 1.4 anterior from bregma, ± 1.5 mm from the midline and 5.4 ventral) or basolateral amygdala (-2.5 anterior from bregma, ± 5.0 mm from the midline, and 5.6 mm ventral). The guide cannulae were secured to the skull using anchor screws and dental acrylic cement.
Implantation of jugular catheters
In the same surgical session, each rat was implanted with a chronic indwelling jugular catheter using previously reported procedures (Goeders and Clampitt 2002; Goeders and Guerin., 2008). The catheter was anchored to tissue in the area and continued subcutaneously to the back where it exited on the head through a 22-gauge guide cannula (Plastics One, Roanoke, VA) assembly that was used for the attachment of a leash. The stainless steel spring leash was attached to the guide cannula assembly and to a leak-proof fluid swivel suspended above the cage. Tubing was connected to the swivel and to a 20-ml syringe in a motor-driven pump located outside the chamber. The swivel and leash assembly were counterbalanced to permit relatively unrestrained movement of the animal.
Behavioral Procedures
Specially constructed, sound-attenuating chambers were used for these experiments. These chambers were designed to hold 3 operant conditioning chambers at the same time so that all three treatment conditions (i.e. self-administration, yoked-cocaine, and yoked-saline) could be tested simultaneously. Each chamber was equipped with an exhaust fan that supplied ventilation and white noise to mask extraneous sounds, a response lever, and a stimulus light located above the response lever. The illumination of the stimulus light above the response lever indicated the availability of cocaine. Responding by one rat controlled drug or saline infusions for all three rats in each triad. The first rat in each triad was allowed to self-administer cocaine (0.25 mg/kg/infusion in 0.2 ml of saline delivered over 5.6 sec) during 2-hour daily sessions under a fixed-ratio 1 (FR1) schedule of reinforcement. The response requirement was subsequently increased to FR2 when responding stabilized (i.e., defined as three consecutive sessions in which the total number of infusions did not vary by more than 15%). Each completion of the ratio requirement resulted in an intravenous infusion of cocaine to the self-administration (SA) rat and an identical, simultaneous infusion to the yoked-cocaine (YC) rat and a simultaneous infusion of saline to the yoked-saline (YS) rat. A 20 s timeout period followed each infusion. Lever presses by the YC and YS rats were counted but produced no programmed consequences. Once responding stabilized under the FR2 schedule, the microdialysis procedure was initiated. An IBM-compatible personal computer and interface system (Med-Associates, Inc., St. Albans, VT) was used to program the procedure and collect the experimental data.
In vivo microdialysis
Microdialysis started at approximately 10:00, the behavioral part of the experiment (self-administration) started at 12:00, and the entire experiment was completed by 16:00. Probes of the concentric design (CMA12, CMA/Microdialysis, N. Chelmsford, MA) were inserted into the guide cannulae approximately 8-12 hr prior to the start of the experiments (i.e., the night before) in order to minimize the damage-induced release of neurotransmitters and metabolites. Probes were perfused with artificial cerebrospinal fluid (aCSF: 1.2 mM CaCl2, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4, 3.4 mM KCl, 140 mM NaCl adjusted to pH 7.2) at a flow rate of 1 μl/min using a CMA/100 microdialysis pump for at least two hours before the start of the behavioral experiments.
On the day of the experiment, four baseline samples were collected directly into 250 μl microcentrifuge tubes (Fisher Scientific, Pittsburgh, PA). Samples were collected every 20 min throughout the dialysis procedure and were immediately frozen on dry ice and stored at -70°C until analysis.
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Park, N.C.) and was dissolved in bacteriostatic 0.9% NaCl.
ACTH and corticosterone assays
For the determination of adrenocorticotropin hormone (ACTH), blood samples (0.5 ml) were collected via the implanted catheters into chilled tubes containing EDTA prior to the start of the self-administration session and again after the end of the session. ACTH concentrations were determined using the Allegro RIA kit (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). Plasma corticosterone was assayed by RIA using ICN kits (ICN Pharmaceuticals, Costa Mesa, CA, USA). “Free” corticosterone was measured in the microdialysis samples using the same RIA kits as described above, with a modification of the assay procedure (Linthorst et al. 1995). For this assay, microdialysate samples (20 μL) were directly used for the assay without the dilution recommended by the manufacturer. The volume was brought up to 50 μL with assay buffer as indicated in the assay manual, and then all components of the assay (i.e., the antibody and labeled corticosterone) were added in the amounts suggested according to the manufacturer's protocol. Concentrations of corticosterone in the microdialysate samples were expressed as ng/ml of microdialysate. “Free” (i.e., not bound to a protein carrier) corticosterone reflects tissue concentrations of the hormone. “Free” corticosterone concentrations in tissues directly correlate with serum corticosterone concentrations and can be used to characterize the activity of the HPA axis (Linthorst et al. 1995). However, no calculations can be made to estimate serum concentration of corticosterone from tissue concentrations due to the unknown recovery of corticosterone during microdialysis sampling.
Histology
The locations of the probes were verified histologically after the completion of the experiment, and only rats in which more than half of the probe was found in the target area were included in the analysis. Briefly, animals were anesthetized and perfused intracardially with phosphate-buffered saline followed with 10% formalin within 2-3 days after the completion of experiment. The brains were removed and stored in a 10% formalin solution. To verify probe placements, brains were frozen and sectioned in 40-μm sections that were mounted onto slides and stained with neutral red. The slide-mounted tissue sections were examined under the microscope.
Statistical analysis
Repeated measures ANOVA and post hoc tests were used to determine significant differences over time and between groups. Group was the main factor and Time was the repeated measure. Post hoc analyses were conducted as needed using Student's Newman-Keuls test. The null hypothesis was rejected when P<0.05.
Results
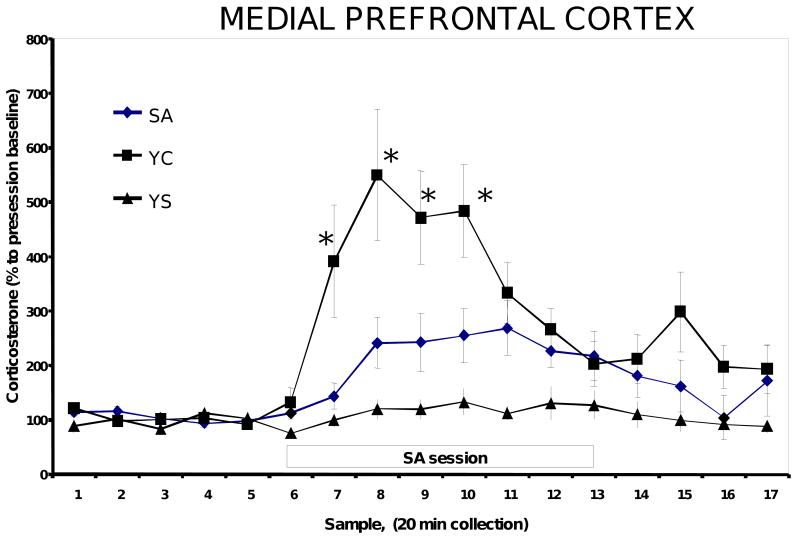
Baseline MPC corticosterone concentrations were low and stable in all groups prior to the start of the self-administration session and remained stable throughout the entire experiment. The actual concentration of corticosterone was approximately 100 ng/ml in the YS rats, suggesting that the procedure itself was not overly stressful (Goeders and Clampitt, 2002; Linthorst et al., 2008; Figure 2). A two-way ANOVA with repeated measures revealed a significant effect of time [(F2, 234)=4.5, P=0.002], treatment [(F2,54)=6.8, P=0.003], and a time × treatment interaction (P<0.001). MPC corticosterone was significantly elevated in microdialysates from the SA rats during the self-administration session (a 269% increase) and was increased 553% above baseline in the YC rats (Figure 2). These differences were statistically significant [(F2, 190)=5.7, P=0.04].
Figure 2.
Corticosterone concentrations in microdialysates from the MPC of self-administration (SA), yoked-cocaine (YC) and yoked-saline (YS) rats. N=12-13 in each group. During the collection of samples 7 -13, the first rat in each triad was allowed to self-administer cocaine (0.25 mg/kg/inf in 0.2 ml of saline delivered over 5.6 sec) under a fixed-ratio 2 schedule of reinforcement.
* significantly different from YC (P<0.05).
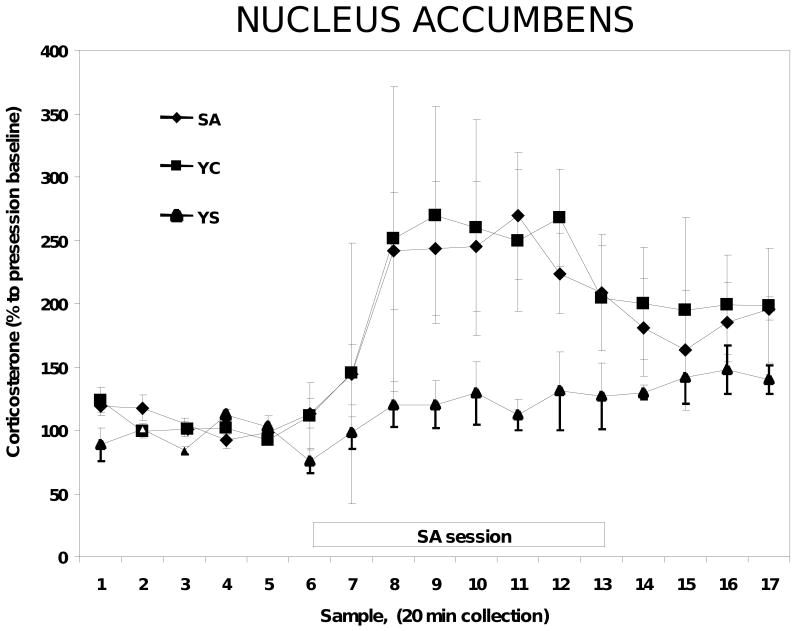
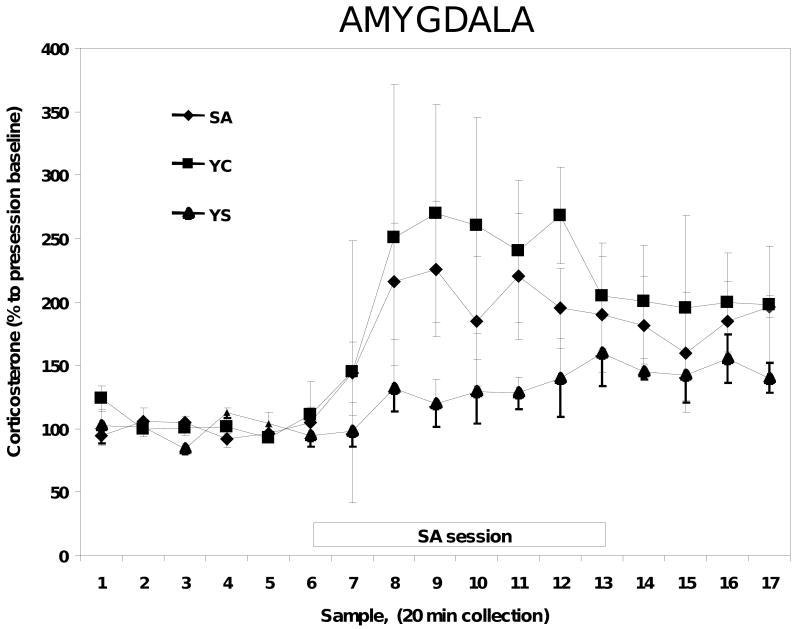
In a separate experiment, microdialysis probes were implanted into the nucleus accumbens and microdialysis samples were collected as above for the corticosterone assay. Exposure to the cocaine session produced a significant increase in nucleus accumbens corticosterone in both the SA and YC animals [(F2, 175)=11.3, P=0.04], while YS animals did not exhibit any significant changes in corticosterone [(F2, 175)=2.3, P=0.36]. However, unlike the MPC, there were no significant differences in corticosterone concentrations in the nucleus accumbens between the YC and SA animals [(F2, 175)=1.76, P=0.12 (Figure 3)]. Similar results were observed in the animals with microdialysis probes implanted into the basolateral nucleus of the amygdala. There were significant increases in corticosterone concentrations in response to the cocaine session in YC and SA rats compared to the YS rats [(F2, 160)=6.3, P=0.02], but once again there were no significant differences between the YC and SA animals [(F2, 165)=1.8, P=0.68; (Figure 4)].
Figure 3.
Corticosterone concentrations in microdialysates from the nucleus accumbens of SA, YC and YS rats. N=8-9 in each group.
Figure 4.
Corticosterone concentration in the microdialysate from the basolateral amygdala of SA, YC and YS rats. N=8-9 in each group.
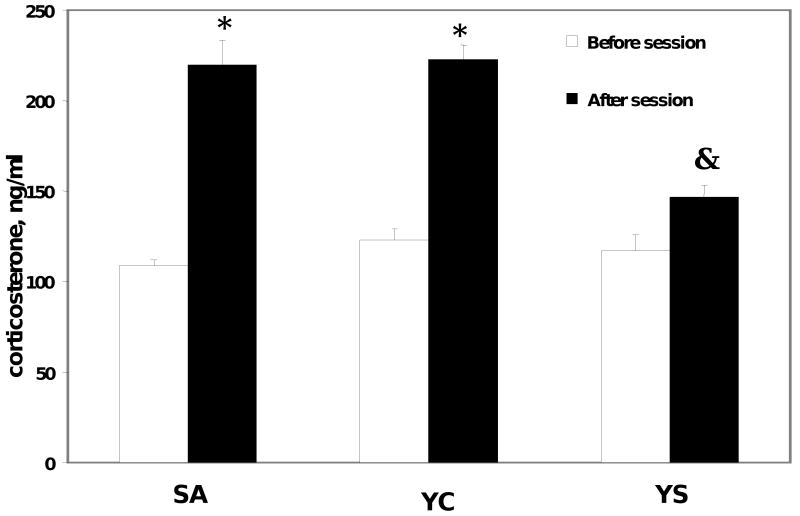
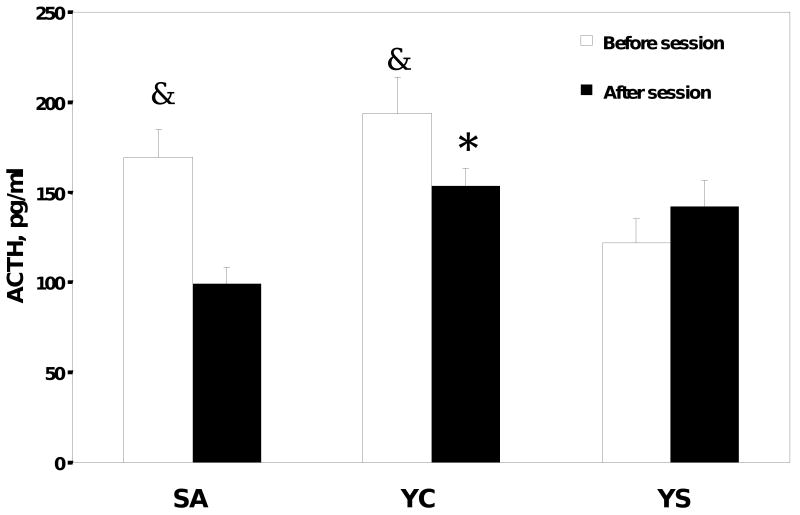
The determination of corticosterone in plasma samples taken before and after the behavioral sessions demonstrated that prior to the start of the behavioral session, plasma corticosterone levels were not different among the groups and were approximately 105±15 ng/ml (Figure 5). However, after the end of the behavioral session, plasma corticosterone was significantly elevated [(F2, 147)=5.8, P=0.034] in the YC and SA rats (210±17 ng/ml), while there were no significant differences in pre- and post-session plasma corticosterone in YS rats. The measurement of ACTH in pre-session and post-session samples revealed a somewhat different picture. The concentrations of ACTH in the YC and SA rats were significantly higher than in the YS group [(F2, 146)=9.2, P=0.01] prior to the start of the session (Figure 6), reaching 170-190 pg/ml (compared to 120 pg/ml in the YS rats). However, there were no differences in ACTH concentrations among the groups at the end of the behavioral session. This increase in ACTH before the session may indicate an anticipatory response in rats trained to receive cocaine.
Figure 5.
Plasma corticosterone concentration of self-administration (SA), yoked-cocaine (YC) and yoked-saline (YS) rats. The average number of injections during the session was 24 ± 5. Blood samples were collected 15 min before the session and immediately after. Plasma was separated from the red blood cells in 1 minute after blood collection. N=7-8 in each group.
* Significantly different from the pre-session sample, P<0.01,
& Significantly different from SA and YC groups, P<0.05.
Figure 6.
Plasma ACTH in self-administration (SA, n=14), yoked-cocaine (YC, n=19) and yoked-saline (YS, n=14) rats. The average number of injections during the session was 28 ± 5. Blood samples were collected 15 min before the session and immediately after. Plasma was separated from the red blood cells in 1 minute after blood collection.
* Significantly different from SA after the session
& Significantly different from YS prior to the session
Discussion
Over the last several years, our laboratory, as well as a number of others, has explored the complex relationship between stress and the subsequent activation of the HPA axis in psychomotor-stimulant reinforcement (Goeders, 2002a; b; Majewska, 2002; Winhusen and Somoza, 2001; Sarnyai et al., 2001). We have shown that corticosterone is involved in the acquisition and maintenance of cocaine self-administration as well as the cue-induced reinstatement of extinguished cocaine seeking in rats (Goeders 1997; 2002a; b; 2003). In our aforementioned work, we measured plasma corticosterone responses to cocaine and following various pretreatment conditions. However, it is not clear if plasma corticosterone concentrations actually reflect tissue concentrations of the hormone due to the presence of the blood-brain barrier and the presence of specific proteins (e.g., P-glycoproteins) that actively transport corticosterone out of the brain (Droste et al., 2008). Corticosterone in the extracellular space is reported to reflect free, biologically active corticosterone since the extracellular fluid does not contain corticosterone-binding globulin and other plasma proteins, suggesting that the direct measurement of brain corticosterone provides a better estimate of corticosterone available to bind to adrenocorticosteroid receptors in the brain than analyses of plasma concentrations (Linthorst et al., 1994; Linthorst et al., 2008; Droste at al., 2008). Furthermore, it is well established that in the absence of active efflux mechanisms, brain corticosterone also matches plasma concentrations of the hormone, indicating that brain corticosterone reflects temporal changes in plasma corticosterone. It has been previously reported that it is possible to measure corticosterone concentrations in the extracellular fluid in the brains of freely-moving rats using in vivo microdialysis (Linthorst et al., 1994), suggesting this as a viable procedure for measuring tissue concentrations of corticosterone in the brain. Therefore, we used in vivo microdialysis to measure free concentrations of corticosterone in the medial prefrontal cortex, nucleus accumbens, and basolateral amygdala in rats self-administering cocaine and receiving simultaneous, yoked infusions of cocaine or saline.
The yoked triad self-administration model has been used to study the neurobiology of drug addiction in rats for over 25 years (Smith et al., 1982). When using this model, there is general agreement that rats receiving passive infusions of the drug (i.e., the yoked-drug animals) reflect the general neurobiological effects attributable to the drug itself, while self-administrating animals reveal the neurobiological effects of the drug as well as the effects of drug-seeking and/or drug reinforcement (Smith et al., 1982; Hemby et al., 1997; Smith et al., 2003). In the current study, concentrations of corticosterone measured using microdialysis were increased in response to cocaine, attesting to an activation of the HPA axis by the drug. It is important to note, however, that the pattern of changes in corticosterone in the three distinct brain areas that we studied was very different. In the medial prefrontal cortex, we observed a significant increase in corticosterone in YC animals compared to the SA rats (i.e., 553% vs. 269%). This increase is not attributable simply to a cocaine-induced stimulation of the HPA axis, since in that case we would expect to see similar concentrations in both groups of rats. In contrast, the increases in corticosterone in the basolateral amygdala and the nucleus accumbens were very similar in SA and YC rats and probably reflect the general activation of the HPA axis in response to the cocaine infusions, especially since saline infusions had no effect on corticosterone in YS rats in any brain region studied. These data suggest that corticosterone in the medial prefrontal cortex, but not in the nucleus accumbens or basolateral amygdala, is involved in cocaine reinforcement, although it is not readily apparent why medial prefrontal cortex corticosterone was increased more in the YC rats compared to the SA rats.
One plausible explanation for the differences observed in medial prefrontal cortex corticosterone between the SA and YC rats is that there is a narrow concentration range whereby cocaine-induced increases in corticosterone are reinforcing, but only up to a certain level. If corticosterone exceeds this range, this may lead to an exaggerated stress-like response and a decrease in reinforcement, or possibly even aversion. In support of this possibility is the finding that the passive or yoked administration of cocaine is associated with higher mortality rates in rats (Dworkin et al, 1995), suggesting that the non-contingent injection of cocaine is indeed a potent stressor. Of the numerous molecules involved in stress response pathways, the P-glycoprotein multdrug resistance 1 (MDR1) efflux transporter is found in many tissues, including brain, and acts to remove various drugs and endogenous substances (including corticosterone) from the interior of cells to the extracellular space (Carson et al, 2002; Uhr et al, 2002; Vaalburg et al, 2005). The MDR1a subtype is found in vascular endothelial cells and is active at the blood-brain barrier, whereas the MDR1b subtype is found mainly in astrocytes and microglia. We recently tested the hypothesis that MDR1 genes respond differentially to self-administered vs. yoked cocaine administration. We found that levels of the MDR1a, but not MDR1b transcripts were higher in each of the SA animals compared to its respective YC control (unpublished observations). These preliminary data suggest that the MDR1a gene may contribute to the reduced toxicity in SA relative to YC rats and may also be involved in the differences we observed in medial prefrontal cortex corticosterone. Obviously, additional research will be necessary to uncover the mechanisms mediating this effect. Finally, ACTH concentrations were elevated in animals self-administering cocaine and in animals passively infused with the drug compared to animals receiving passive infusions of saline just prior to the start of the behavioral session. These increases in ACTH in animals receiving cocaine may result from the anticipation or expectation of the cocaine session and from the conditioned effects of the cocaine (Goeders and Clampitt, 2002) on the HPA axis since similar increases were not seen in rats passively infused with saline.
In summary, the results of these experiments demonstrate how control over drug delivery can affect the influence of a hormonal input on the functional characteristics of specific anatomical projections of the neural system. Self-administered cocaine increased corticosterone differently than passive infusions of the drug, but only in the medial prefrontal cortex, suggesting a potential role for corticosterone in this brain region in cocaine reinforcement. Accordingly, these results also provide evidence of the role that is played by steroid hormones in shaping the functional activity of the brain, in this case with respect to cocaine reinforcement.
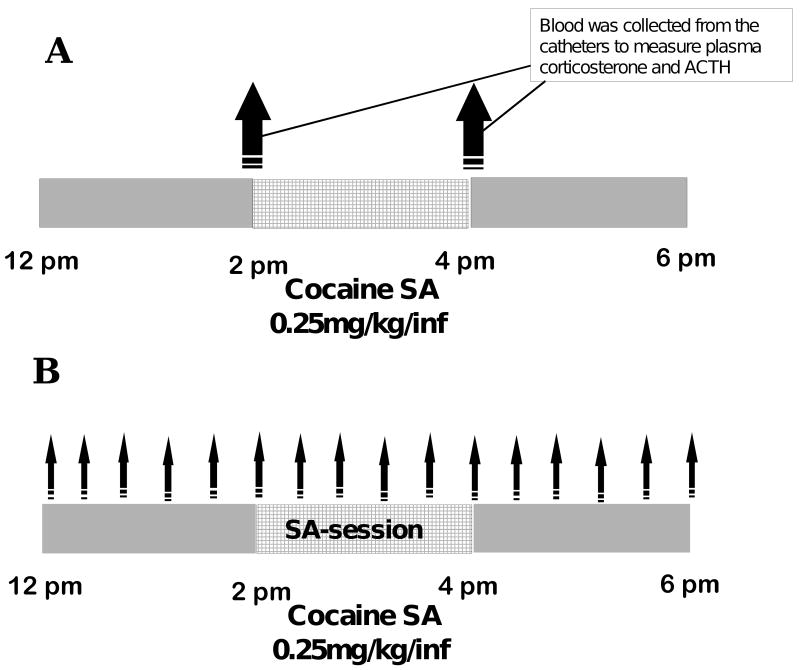
Figure 1.
Experimental design
Panel A. Blood samples were collected for the determination of plasma corticosterone and ACTH prior to and after the self-administration session.
Panel B. Microdialysis samples were collected prior to, during and after the self-administration session every 20 minutes, as indicated by the arrows.
Acknowledgments
This work was supported in part by USPHS grants DA06013 and DA13647 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Carson SW, Ousmanou AD, Hoyler SL. Emerging significance of P-glycoprotein in understanding drug disposition and drug interactions in psychopharmacology. Psychopharmacol Bull. 2002;36:67–81. [PubMed] [Google Scholar]
- Droste SK, de Groote L, Lightman SL, Reul JM, Linthorst AC. The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: An in vivo microdialysis study in Wistar rats. J Neuroendocrinol. 2009;21:132–140. doi: 10.1111/j.1365-2826.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Engelhardt D, Dorr G, Jaspers C, Knorr D. Ketoconazole blocks cortisol secretion in man by inhibition of adrenal 11 beta-hydroxylase. Klin Wochenschr. 1985;63:607–612. doi: 10.1007/BF01733014. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002a;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002b;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology. 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res. 1996;722:145–152. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- Goeders NE. A Neuroendocrine Role in Cocaine Reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The Impact of Stress on Addiction. European Neuropsychopharmacology. 2003;3:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress, Motivation and Drug Addiction. Current Directions in Psychological Science. 2004;13:33–35. [Google Scholar]
- Goeders NE, Guerin GF. Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav. 2008;91:181–189. doi: 10.1016/j.pbb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem DJ, Kennett G, Curzon G. Adaptation of female rats to stress: shift to male pattern by inhibition of corticosterone synthesis. Brain Res. 1988;458:339–347. doi: 10.1016/0006-8993(88)90476-3. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Cole BJ, Swerdlow NR, Le Moal M, Britton KT. Stress, performance, and arousal: focus on CRF. NIDA Res Monogr. 1990;97:163–176. [PubMed] [Google Scholar]
- Koob GF, Sannam PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC, van Giersbergen PL, Gras M, Versteeg DH, de Jong W. The nigrostriatal dopamine system: role in the development of hypertension in spontaneously hypertensive rats. Brain Res. 1994;639:261–268. doi: 10.1016/0006-8993(94)91739-6. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Muller-Preuss P, Holsboer F, Reul JM. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci. 1995;15:2920–2934. doi: 10.1523/JNEUROSCI.15-04-02920.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC, Reul JM. Stress and the brain: solving the puzzle using microdialysis. Pharmacol Biochem Behav. 2008;90:163–173. doi: 10.1016/j.pbb.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Reul JM. Water temperature determines neurochemical and behavioural responses to forced swim stress: an in vivo microdialysis and biotelemetry study in rats. Stress. 2008;11:88–100. doi: 10.1080/10253890701533231. [DOI] [PubMed] [Google Scholar]
- Loose DS, Stover EP, Feldman D. Ketoconazole binds to glucocorticoid receptors and exhibits glucocorticoid antagonist activity in cultured cells. J Clin Invest. 1983;72:404–408. doi: 10.1172/JCI110982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. HPA axis and stimulant dependence: an enigmatic relationship. Psychoneuroendocrinology. 2002;27:5–12. doi: 10.1016/s0306-4530(01)00033-6. [DOI] [PubMed] [Google Scholar]
- Moldow RL, Fischman AJ. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides. 1987;8:819–822. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- National Drug Threat Assessment. 2009 http://www.usdoj.gov/ndic/pubs31/31379/cocaine.htm#Overview.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Smith JE, Co C, Freeman ME, Lane JD. Brain neurotransmitter turnover correlated with morphine-seeking behavior of rats. Pharmacol Biochem Behav. 1982;16:509–519. doi: 10.1016/0091-3057(82)90460-9. [DOI] [PubMed] [Google Scholar]
- Smith JE, Koves TR, Co C. Brain neurotransmitter turnover rates during rat intravenous cocaine self-administration. Neuroscience. 2003;117:461–475. doi: 10.1016/s0306-4522(02)00819-9. [DOI] [PubMed] [Google Scholar]
- Uhr M, Holsboer F, Müller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol. 2002;14:753–759. doi: 10.1046/j.1365-2826.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- Vaalburg W, Hendrikse NH, Elsinga PH, Bart J, van Waarde A. P-glycoprotein activity and biological response. Toxicol Appl Pharmacol. 2005;207(2 Suppl):257–260. doi: 10.1016/j.taap.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22:263–268. doi: 10.1097/YCO.0b013e32832a3b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Somoza E. The HPA axis in cocaine use: implications for pharmacotherapy. J Addict Dis. 2001;20:105–119. doi: 10.1300/J069v20n03_09. [DOI] [PubMed] [Google Scholar]