Abstract
Transplantation experiments have shown that neurologic deficits may be reversed by engrafting fresh tissue or engineered cells within dysfunctional neural circuitry. In experimental and clinical settings, this approach has provided insights into the pathology and treatment of neurologic diseases, primarily movement disorders. The present experiments were designed to investigate whether a similar strategy is feasible as a method to investigate, and perhaps repair, circuitry integral to emotional disorders. We focused on the amygdala, a macrostructure known to be involved in the expression of anxiety- and fear-related behaviors. GABAergic cell-rich suspensions were prepared from E17 rat lateral ganglionic eminence and engrafted bilaterally into the lateral and basolateral amygdaloid nuclei of young adult rats. After six weeks, increased numbers of GABAergic neurons were identified in the vicinity of the graft sites, and electron microscopy provided evidence for functional integration of transplanted cells. Rats with these grafts spent more time in the open arms of the elevated plus maze, consistent with an anxioloytic-like phenotype. These rats were also less sensitive to the unconditioned anxiogenic effects of light on the acoustic startle response, although fear-potentiated startle was not affected, suggesting that the grafts produced an attenuation of unlearned fear but did not affect acquisition of conditioned fear. Our results raise the possibility that distinct components of emotion can be modulated by strategic neural engraftment.
Keywords: lateral ganglionic eminance, basolateral amygdaloid nucleus, lateral amygdaloid nucleus, GABAergic, elevated-plus maze, fear-potentiated startle, unconditioned light response
Introduction
Neural transplantation is an experimental approach by which brain and spinal cord circuitry can be modified and/or neuroactive agents can be supplemented or provided de novo. This methodology continues to grow in sophistication, particularly with advancements in stem cell biology. Engraftment approaches have focused primarily upon the study and treatment of neurologic diseases; however, as the substrates of psychiatric syndromes are characterized, modulating emotional systems through the introduction of cellular components may be a viable strategy for investigating the mechanisms of affect, motivation, and perception. The study of emotional systems in the brain has emerged as an important area of research in neuroscience, and the neural circuits and systems involved in fear and anxiety have been studied extensively. The fear-conditioning paradigm has provided a model that has operationalized the study of emotion and elucidated key structures, such as the amygdala, which has been shown to play a critical role in the development and expression of fear and anxiety states [5, 20, 26].
The amygdala is a macrostructure located in the temporal lobe and is implicated in memory, attention, and the generation of emotions [37]. The amygdala can be divided into subnuclei, or divisions, which may represent more discrete functional units. Stimuli entering the amygdala are processed within parallel circuits which involve an array of intra- and inter-divisional connections and are modulated by or associated with information such as context, memories, and response choices from other brain areas. The generation of amygdala-dependent behavioral responses likely depends on the activity of local-circuit interneurons with intradivisional connectivity [37]. In anxiety states, these local gating mechanisms could be deficient, leading to overactivity of amygdalar responses and the manifestation of fear and anxiety symptoms. It may therefore be possible to increase the gating threshold by supplementing the local circuitry with inhibitory neurons.
The amygdaloid substructures that appear to be most important in the generation of fear responses are the lateral (LA), basolateral (BL), basomedial (BM), and the central (CE) nuclei. In fear conditioning, auditory and somatosensory information is received primarily by the LA [21]. Contextual information, implicating experience and memory, is relayed from the hippocampus to the BL and BM [25]. The LA, BL, and BM together make up the basolateral complex (BLC) acting as an integrated functional unit [11], sending converging information to the CE and the amygdalo-hippocampal areas. These signals are then relayed to the central gray, hypothalamus, and brainstem areas, which mediate the signs and symptoms of fear and anxiety [3, 22].
GABAergic interneurons are believed to play a pivotal role in regulating amygdalar activity. The unusually low firing rate of amygdalar projection neurons [35] suggests that their activity is tightly regulated by GABAergic neurons intercalated within the circuitry. Stimulation of amygdalar afferents elicits an initial excitatory postsynaptic potential (EPSP) in pyramidal neurons that is promptly interrupted by a large-amplitude and prolonged hyperpolarizing potential [35]. Moreover, long-lasting inhibition of neuronal firing has been associated with release of GABA within the BLC [44]. It is hypothesized that dysfunction of the inhibitory gating mechanism within the amygdala may play a role in the pathoetiology of anxiety disorders [38]. Intra-amygdala infusion of a GABAA receptor agonist (muscimol) reduces fear conditioning in rats [41, 53], whereas a GABAA antagonist (bicuculline) potentiates anxiety in animal models [39, 40]. Indeed, the putative mechanism of action for first-line anxiolytic drugs (benzodiazepines) is to potentiate GABA, and a key site of action is likely the amygdala [15, 28].
Modulation by amygdalar activity, however, involves more than just altering receptor function or potentiating inhibitory neurotransmitter. For example, the complex integrated wiring of GABAergic neurons plays an important role in synchronizing the oscillatory activity of pyramidal neurons [9, 33, 34] and therefore probably contributes to the mechanisms of emotional memory. As such, the primary objective of the present study was to not only provide a supplementary source of inhibition into a fear processing center, but to introduce GABAergic neurons that are capable of integrating within local circuitry, perhaps providing inhibitory tone during ongoing cell-to-cell interactions. We engrafted cell suspensions rich in GABAergic neurons within the lateral and basolateral amygdaloid nuclei (LBA) of the BLC and examined their anatomical integration and their behavioral effects using models of anxiety and fear. We hypothesized that engrafted GABAergic neurons may have the potential to attenuate the fear response possibly by increasing inhibitory transmission and/or enhancing local filter mechanisms.
2. Materials and methods
2.1 Subjects
A total of 57 male Sprague-Dawley rats (Charles River Laboratories) weighing 220–240 grams at the time of transplantation were used in this study. Twenty animals received transplants of viable LGE cell suspension (Transplant group) and 15 animals received freeze-thaw killed cell suspension (Control group). An additional 22 animals (Naïve group) were maintained in parallel with the Transplant and Control groups and were used as unoperated controls for the behavioral studies. Animals were handled daily and maintained on a 12-hour light/dark schedule, with food and water provided ad libitum. All procedures were approved by the McLean Hospital IACUC and were in compliance with principles expressed in the National Institutes of Health, United States Public Health Service Guide for the Care and Use of Laboratory Animals.
2.2 Fetal Cell Isolation
On embryonic day 17 (E17), the uterus was harvested from pregnant Sprague-Dawley rats and transported on ice to the cell isolation site. Using sterile technique, the uterus was opened and the fetuses were isolated (crown-rump length 14–16 mm). The fetal brains were suspended in dissection media, and the lateral ganglionic eminence (LGE) was located by anatomical landmarks previously described and isolated from the ventrolateral wall of the lateral ventricle [32]. The LGE tissue from a litter of 8–20 fetuses was pooled and incubated with 0.5% trypsin-EDTA in Hanks’ Balanced Salt Solution. The tissue was then washed and mechanically dispersed by trituration in the presence of DNase (final concentration 50 μg/ml). A single cell suspension was washed and re-suspended in Transplantation Media (TM, 0.9 w/v sodium chloride, 0.35% w/v glucose) at a concentration of 80,000 to 100,000 cells per microliter. A subset of cells (5%) was labeled with red fluorescent microspheres (FMs, 0.04 μm, 5% solids, Molecular Probes, Eugene, OR) diluted 1:100 in Hank’s solution and incubated at 37° for 30 minutes with gentle agitation, then rinsed twice with TM and added back to the main suspension. Viability studies using ethidium bromide and acridine orange method demonstrated >95% viability upon preparation and >90% viability after six hours when maintained at 4 °C. A control transplant suspension was prepared from a portion of the final suspension by killing the cells using five cycles of rapidly freezing and thawing the suspension.
2.3 Transplantation Surgery
Transplant and Control animals were anesthetized with ketamine/xylazine (87/13 mg/kg, intraperitoneally). A micrografting approach [31] was used to deliver precise volumes and accurately target amygdalar subnuclei bilaterally. Two, one-volumes of cell suspension were dispersed within each LBA: 1 μL delivered within the posterior division of the basolateral nucleus (BLp), and a second 1 μL injection delivered just dorsal to the first within the LA region at the following coordinates relative to bregma with the incisor bar set at –3.3: Posterior -2.3, Lateral -5.1, and Ventral -7.6 (BLp) and -6.8 (LA). Upon reaching the appropriate ventral coordinate, the micropipette remained in position for one minute before injection of 1μL of suspension over two minutes. One minute after injection was completed, the microcannula was slowly retracted (over two minutes) to the second vertical coordinate. The injection process was then repeated. Following transplantation, the incision was closed, animals were returned to their cages, and food and water were provided ad libitum. Animals were handled regularly during the interim between surgery and behavioral analysis.
2.4 Behavioral Testing
Six weeks after transplantation, all subjects underwent the following sequence of behavioral testing: open field locomotor activity, the elevated plus maze, unconditioned light response, and fear-potentiated startle (Fig. 1). They were given a two-day respite between behavioral tests. This sequence was graded as to level of stress in order decrease the likelihood that the behavior or effects of one test would carry over to the subsequent test(s) thereby introducing a confound [50]. To control for the effect of time of day on activity levels, for each behavioral testing procedure, animals were alternated or run in small groups, each containing subjects from each of the experimental groups (Transplant, Control, and Naïve).
Figure 1.
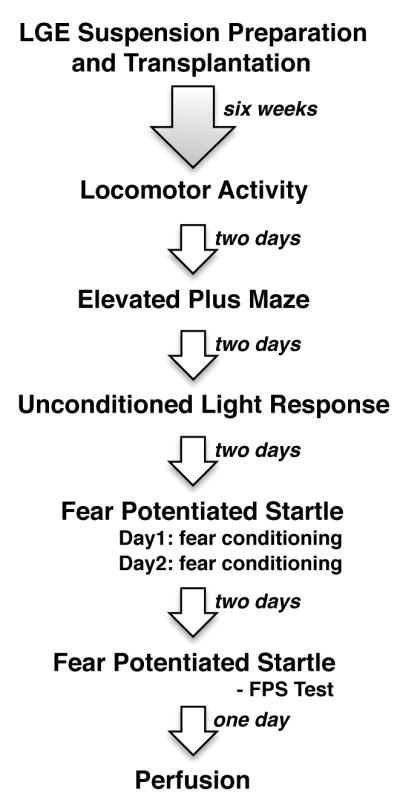
Experimental design illustrating sequence of behavioral tests.
2.4.1 Locomotor Activity
Each subject was habituated to the testing environment for 1 hour and placed in one of six automated Plexiglas activity monitor chambers (43.2×43.2×30.5 cm; interfaced with a computer, Med-Associates, St. Albans, VT) for 60 minutes of testing. The chambers were composed of two horizontal (x and y plane) 16 beam infrared photobeam (I/R) arrays (two cm from the floor) and one vertical (z plane) 16 beam I/R array (12 cm from the floor). All of the activity monitor chambers were located in an isolated sound-attenuated room. Each activity monitor chamber was housed individually in a sound attenuating chamber with a house fan which also provided white noise (~70 db). These chambers measure several different forms of activity; this study focused on total distance traveled, stereotopy counts, and vertical rears both in the center zone and the periphery of the chamber. Transplant, Control, and Naïve animals were compared for each form of activity over the first five minutes, the first 20 minutes, the second 20 minutes, and the final 20 minutes, as well as over the entire hour of testing.
2.4.2 Elevated plus-maze (EPM)
Prior to testing, animals were habituated to the environment by placing their cages in the testing room for one half hour before beginning. The plus maze, consisting of two opposite open arms and two enclosed arms, each 11 cm wide, was elevated 50 cm above the floor. Animals were placed in the closed arm of the EPM facing the closed end and were allowed to move freely for five minutes. The percentage of time each animal spent upon the open arms (with at least both forepaws) over five minutes was recorded and analyzed relative to total testing time (open/total × 100).
2.4.3 Unconditioned light effect on startle (Unconditioned Light Response, ULR)
Rats have a natural aversion to bright light, which can be measured using several assays (e.g. dark-light box, open field) including an unconditioned enhancement of the acoustic startle response. Hence, prior to fear-conditioning, we sought to examine the unconditioned effects of exposure to bright light on startle reactivity as a measure of anxiety-like behavior. The apparatus included four identical startle cages consisting of 19 × 9 × 14-cm Plexiglas and wire-mesh cages attached to a load-cell platform. Both the startle cage and platform were contained within a 69 × 36 × 42-cm ventilated sound-attenuating isolation box with the inside temperature monitored and maintained at approximately 20°C (Med Associates, Georgia, VT). Cage movement resulted in displacement of a transducer in the platform where the resultant voltage was amplified and digitized on a scale of 0–±2000 units by an analog-to-digital converter card interfaced to a PC computer. Startle amplitude was proportional to the amount of cage movement and was defined as the maximum peak-to-peak voltage that occurred during the first 200 ms after onset of the startle stimulus. Constant wide band background noise (60 dB, 10 –20 kHz) and 50-ms startle stimuli (1 – 32 kHz white noise, five-ms rise/decay) were generated by an audio stimulator (Med Associates) and delivered through speakers located seven cm behind the startle cage. Illumination was provided by a white fluorescent bulb (8 W), placed 18 cm behind and at floor level to the test cages. This light produced an illumination level of 700 footlamberts as measured from the middle of the test cage with a Gama Scientific Inc. Telephotometer (Model 2000).
On testing day, rats were startled in the absence or presence of light in the same test session. Animals were placed in the startle chambers and, after five minutes, presented with 30 startle-eliciting noise bursts; 15 startle-stimulus alone test trials and 15 intermixed trials of a 10-s light coterminating with a startle stimulus. Throughout these procedures, noise bursts were presented at an interstimulus interval of 30 sec and at an intensity of 95 dB. The unconditioned effect of light on startle was calculated as percent change in startle amplitude on light plus startle trials versus startle alone trials.
2.4.4 Fear-potentiated startle (FPS)
The apparatus for FPS has been described previously [17], training and testing of animals was conducted in an apparatus identical to that described above. The conditioned stimulus (CS) was light produced by an 8 W fluorescent bulb located 18 cm behind, and at a 45° angle above, the cage. The unconditioned stimulus (US) was a scrambled footshock delivered through the floor bars of each cage by an external shock generator. The calibration, presentation, and sequencing of all stimuli were under the control of the PC computer using specially designed software (Med Associates).
Rats were placed in the startle cages and received a 5-min acclimation period followed by conditioning session during which they received 10 light-shock pairings (3.7-s light coterminating with a 0.5-s, 0.6-mA footshock). The mean intertrial interval was three minutes (range 2–4 min, pseudorandom). Rats received two consecutive days of fear-conditioning followed 48 h later by a test for fear-potentiated startle. On the test day, animals were placed in the startle cages and after a five-minute acclimation they received nine habituating startle stimuli (three stimuli at 95, 100, and 105 dB, 30-s ISI) followed by 18 startle stimuli, six at each of three different intensities (95, 100, 105 dB; 30-s ISI) given in the presence or absence of the light given in a pseudorandom order. Conditioned fear was defined as the difference in startle magnitude in the presence versus the absence of the light and calculated as % FPS: thus, % FPS = [(startle in the presence of the light − startle alone)/startle alone] × 100.
2.5 Histology
On the day following completion of behavioral testing, animals were overdosed with intraperitoneal pentobarbital (200 mg/kg) and perfused using a method for optimizing GABA immunocytochemistry [43]. An 18-gauge catheter was placed in the left ventricle, the descending aorta was occluded, and the right atrium was incised. Each animal was perfused transcardially with 0.01 M phosphate buffered saline (PBS; pH 7.4 at 4 °C) at a rate of 50 ml per minute until the right atrium cleared (100 – 200 ml). PBS was followed by 75 ml 2% paraformaldehyde (PFA) in 0.1 M sodium acetate buffer (pH 6.5 at 4 °C) at a rate of 20 ml/min. An additional 750 ml 2% PFA and 0.1% glutaraldehyde in 0.1 M borate buffer (pH 8.5) was perfused over 45 minutes at a rate of 15 ml/min. The brain was removed intact from the cavarium and emersion-fixed 12 hours in the final fixative solution (2% PFA and 0.1% glutaraldehyde in 0.1M borate buffer). The brains were then transferred to a cryoprotectant solution consisting of 20% glycerol in 0.1 M PBS. Brains were blocked and 40 μm sections were cut on a vibratome. Sections were stored in Tris buffer (pH 7.3 at 4 °C) with 0.1% sodium azide for histological techniques including immunohistochemistry.
To assess transplant placement, representative sections (every sixth) from each animal were stained with the fluorescent nissl, Neurotrace (Invitrogen, Eugene, OR, USA): Tissue sections were mounted on gelatin-coated slides and then flooded with Neurotrace for three minutes. Slides were rinsed with PBS three times and the tissue sections were coverslipped with Gelmount mounting medium (Biomeda Corporation, Foster City, CA, USA). Animals with transplants not clearly within the boundaries of the LBA in both hemispheres (six Transplant and two Control subjects) were eliminated from the study.
For GABA immunocytochemistry, tissue sections containing amygdala were rinsed with 0.1 M PBS, pH 7.4, containing 0.5% TritonX-100 (PBS/Tx) three times for ten minutes each (3 × 10). Endogenous peroxidase activity was quenched in a solution of 3% hydrogen peroxide (H2O2) and 10% methanol in PBS/Tx for 10 minutes. Sections were rinsed 3 × 10 minutes and then placed in a blocking solution (10% normal goat serum [NGS] and 3% bovine serum albumin [BSA] in PBS/Tx) for one hour at room temperature. Subsequent to blocking, sections were incubated in a 1:15,000 dilution of rabbit anti-GABA antibody (Sigma, St. Louis, MO, USA) in PBS/Tx with 2.5% NGS for three nights at 4 °C. Sections were then rinsed 3 × 10 minutes in PBS/Tx, followed by incubation in a 1:200 dilution of biotinylated anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) in PBS/Tx with 1% NGS for one hour at room temperature. Sections were then rinsed 3 × 10 minutes and immersed in a solution of streptavidin-horseradish peroxidase complex (Vector Laboratories) in PBS/Tx for one hour at room temperature. Following 3 × 5 minute rinses in phosphate buffer (PB; pH 7.4), sections were incubated in a 3,3′-diaminobenzidine tetrachloride (DAB) substrate solution with H2O2 and nickel sulfate for seven minutes. Sections were mounted on gelatin-coated slides, air dried, dehydrated through a graded series of ethanol, cleared in xylene, and coverslipped using Cytoseal 60 mounting medium (Richard-Allan Scientific, Kalamazoo, MI).
For immunofluorescent detection of GABAergic cells, tissue sections containing amygdala were rinsed in 0.1 M PBS (pH 7.4) 3 × 5 minutes, blocked for one hour at room temperature, and incubated in a 1:1000 dilution of rabbit anti-GABA antibody in PBS/Tx with 2.5% NGS overnight at 4 °C. The next day, sections were rinsed 3 × 5 minutes in PBS, incubated in a 1:200 dilution of biotinylated donkey anti-rabbit antibody (Jackson Laboratories, Bar Harbor, ME, USA) for one hour at room temperature, rinsed 3 × 5 minutes in PBS, and incubated in a 1:500 dilution of Cy2 conjugated streptavidin (Jackson Laboratories) in PBS in the dark for one hour at room temperature. Following 3 × 5 minute rinses, sections were mounted on gelatin-coated slides, allowed to adhere, coverslipped with Gelmount, and stored in darkness at 4 °C.
For electron microscopic detection of GABAergic cells, tissue sections containing amygdala in the vicinity of the graft were photoconverted by applying a DAB solution and exposing the tissue to fluorescent light, a process that transforms the fluorescent microspheres into a dark, electron-dense product visible with electron microscopy. Subsequently, sections were rinsed with PBS, placed in a block solution (10% NDS, 3% BSA, and 0.01% TritonX-100 in PBS) for one hour, and incubated in a 1:500 dilution of rabbit anti-GABA antibody (Sigma) in PBS for 12 hours at 4 °C. Sections were then rinsed with PBS, incubated in a 1:20 dilution of goat anti-rabbit antibody conjugated with colloidal gold particles (20 nm; Ted Pella, Inc., Redding, CA) in PBS with 0.01% TritonX-100, rinsed with PBS, incubated in a 0.1% glutaradehyde for 30 minutes, and rinsed with PBS.
2.6 Microscopy
Engrafted GABAergic neurons were evaluated microscopically using two methods. First, the number of GABA-immunoreactive (-ir) neurons present in the vicinity of the graft site was quantified. However, because such an estimation may include host GABAergic neurons, and since transplanted neurons appeared to be capable of migration, the overall GABA-ir neuron density within the LBA was also quantified. GABAergic neuron counts and densities were analyzed using a Leitz Laborlux light microscope interfaced with a BIOQUANT nova image analysis system (R&M Biometrics, Nashville, TN, USA) via a Hitachi CCD solid state video camera. For graft cell counts, GABA-ir neurons were identified by a blind observer for every sixth section in the vicinity of the graft site, which could be distinguished by the presence of low-grade tissue disturbance and gliosis and the presence of trace amounts of blood product (i.e., heme, see Figure 2). For neuronal density, the cytoarchitectonic borders of the LBA for every sixth section through the amygdala were traced and GABA-ir neurons were counted by a blind observer at a magnification of 25x. The number of labeled cells within each subset was multiplied by six to obtain an estimate of the total number of GABAergic neurons associated with the grafts and within the LBA. Immunofluorescence-stained sections were viewed at low power using a Zeiss Axioscope (Carl Zeiss Inc., Thornwood, New York, USA). Very thin optical sections were analyzed at high power using a Leica TSC NT laser scanning confocal microscope (Leica Microsystems Inc., Bannockburn, Illinois, USA).
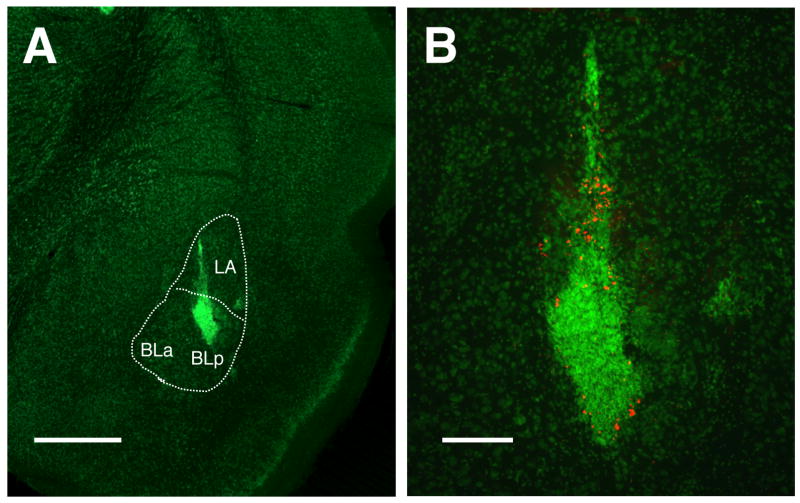
Figure 2.
Transplantation of LGE cell suspension into the LBA. A, fluorescent Nissl stain showing LGE micrograft within the BLp and LA. B, Higher power of “A”; a subset of transplanted cells were labeled with red FMs. Scale bars: A = 1 mm, B = 400 μm.
For ultrastructural analysis, photoconversion was performed as described above, the sections were then postfixed in 1% osmium tetroxide, dehydrated through a graded series of ethanol and propylene oxide, and embedded in Epon epoxy resin. The sections were viewed with a bright field microscope, and areas containing DAB reaction product were isolated through dissection, mounted on Epon blocks, cut on a Reichert-Jung ultramicrotome at a thickness of 30–40 nm, and mounted on copper grids. Sections were not counterstained with lead citrate-uranyl acetate, as this method obscured visualization of photoconverted microspheres. The ultrathin sections were examined and photographed with a JEOL 1200EX electron microscope.
2.7 Statistical Analyses
All behavioral data were analyzed using a one-way analysis of variance (ANOVA) followed by a post-hoc Neuman-Keuls test to evaluate differences between each pair of groups. An unpaired t test was used to compare amygdalar GABAergic neuron density for transplanted and naïve animals.
3. Results
3.1 Intra-amygdalar GABAergic micrografts
Figure 2 illustrates the placement of LGE grafts targeting the LBA. Because the LA is just dorsal to the BLp, injections within both structures could be achieved with a single trajectory of the microcannula (panel A). In panel B red fluorescent microspheres are easily seen indicating the presence of labeled cells.
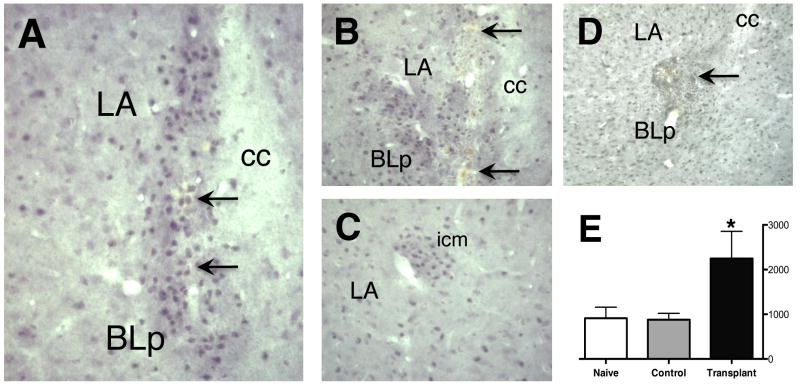
These low-power photomicrographs of fluorescent Nissl-stained sections show a localized concentration of cells at the core of the graft. However, increased numbers of GABA-ir neurons were also found within the parenchyma surrounding the central region of the injection site (Fig. 3A & B). Furthermore, although a single injection targeted the BLp and another the LA, the cells from these injections tended to merge into a single transplant area. The density of GABAergic neurons in these areas was greater than that of the Control and Naïve amydala parenchyma, and the areas of the grafts were much greater than that observed for endogenous GABAergic intercalated cell masses (icm, see Fig. 3C). The numbers of GABAergic neurons within these graft areas ranged from 3,648 to 5,502 (mean, 4,212). However, because engrafted GABA-ir neurons could not always be distinguished from endogenous neurons, overall GABA neuron density (cells/mm2) was analyzed throughout the LBA to more accurately determine increased numbers of GABAergic neurons, taking into account migration of implanted cells away from the immediate area of the graft. While stereological cell counts were not used in these analyses, the difference in numbers between Transplant subjects and Naïve and Control subjects outweighed any expected counting bias. A one-way ANOVA and post-hoc analysis for GABA-ir cell density showed a difference in GABAergic neuronal density (F(2,19) = 5.018, P = 0.0194) with that of Transplant subjects being significantly greater than both Control and Naïve subjects (Fig. 2E).
Figure 3.
GABA immunoreactivity illustrates large numbers of engrafted GABAergic neurons within the LBA (A, B). Graft sites are confirmed by the presence of fluorescent microspheres (see Fig. 3) and by the presence of blood by-products from the injection process (arrows). C, grafts are easily distinguished from endogenous intercalated cell masses (icm), which are more organized, typically comprised of fewer cells, and are devoid of fluorescent microspheres and evidence of trauma from the delivery cannula. D, a control graft of freeze-thaw killed LGE suspension shows disruption and scarring of the BLC and few GABA-ir neurons at the injection site. E, Bioquant analysis demonstrated a significant increase in overall GABA neuron density within the LBA (cells/mm2) for Transplant animals (*p < 0.05).
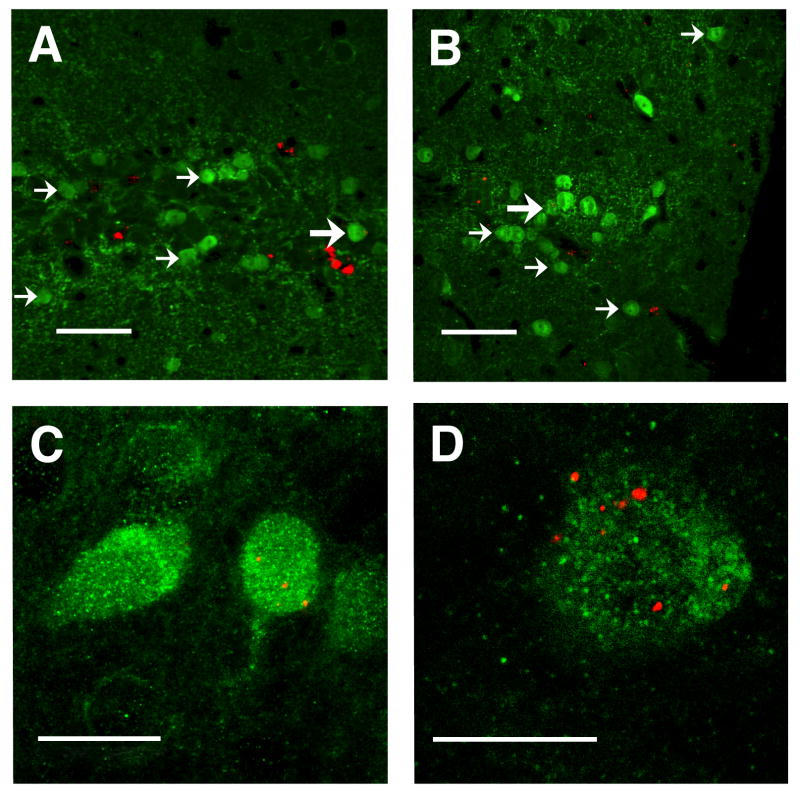
Confocal microscopic analysis identified large numbers of GABA-immunofluorescent (-if) neurons in the vicinity of the graft. Figure 4A & B illustrate with thin optical sections populations of engrafted GABAergic neurons, identified by the presence of FMs within many of the cells. At higher power, the neuronal morphology of transplanted, FM-containing neurons is detectable (Fig. 4C). The co-localization of FMs within a GABA-if neuron is illustrated in Figure 4D with a 0.5 μm optical section.
Figure 4.
Confocal analysis of transplanted GABAergic neurons. A & B, clusters of GABA-ir neurons (small arrows) are seen within the BLC, some of which (large arrows) contain red FMs. Note that non-GABAergic cells containing red FMs are also seen (arrow heads); section thickness: 4 μm. C, high power confocal image of GABA-ir neuron, one containing red FMs. D, confocal micrograph of a 0.5 μm optical section through GABA-ir cell (green) labeled with red FMs. Scale bars: A & B = 50 μm, C = 20 μm, D = 10 μm
3.2 Ultrastructural observations
Photoconversion of FMs produces an electron-dense reaction product readily identifiable with EM. Tissue sections were also immunoreacted for GABA using a secondary antibody conjugated to 20 nm colloidal gold particles, thus allowing co-visualization of FM-containing and GABA-ir elements (Fig. 5). The reaction product resulting from photoconversion of microspheres is roughly circular and approximately 40 μm (or more) in diameter. Because aggregates of micropheres are often encapsulated within the cell, one frequently observes a large area of dense staining that may be surrounded by a halo of diffuse reaction product. This FM photoconversion product is easily distinguished from that of colloidal gold particles, which are near-perfectly circular with a diameter of 20 μm and they are more electron-dense.
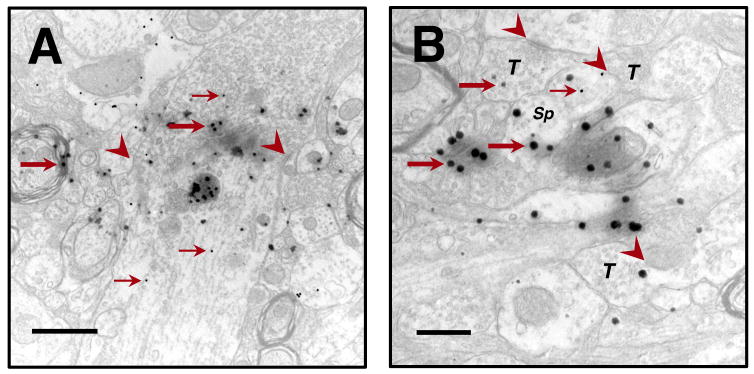
Figure 5.
Photoconversion of FMs was used to identify transplanted cells on the ultrastructural level. FMs (large arrows) can be co-localized with colloidal gold particles (small arrows) identifying GABAergic elements. A, normal-appearing GABAergic FM-containing dendrite well-integrated within the surrounding cytoarchetecture illustrating cell-to-cell connections with synaptic specializations (arrowheads). B, many terminals (T), with well-defined synaptic vesicles, contain FMs and demonstrate synaptic specializations. A GABA-ir, FM-containing spine (Sp) appears to be engaged synaptically with a neighboring terminal. Note the seamless integration of FM-containing elements with surrounding, unlabeled, presumably host structures. Scale bars: A = 1 μm, B = 500 nm.
Transplanted cells having normal-appearing organelles were observed integrated seamlessly within the host parenchyma. Cellular elements possessing both photoconverted FMs and colloidal gold particles were readily identified. Synaptic specializations were also identified suggesting functional integration. In addition, FM-containing elements were seen devoid of colloidal gold particles suggesting either suboptimal sensitivity of the immunoreaction and/or that a proportion of grafted cells were non-GABAergic. Also seen in the graft region were GABAergic elements that were not labeled with FMs. Because only a subset of transplanted cells was labeled, whether these examples represent transplanted or endogenous neurons cannot be determined.
3.3 Behavior
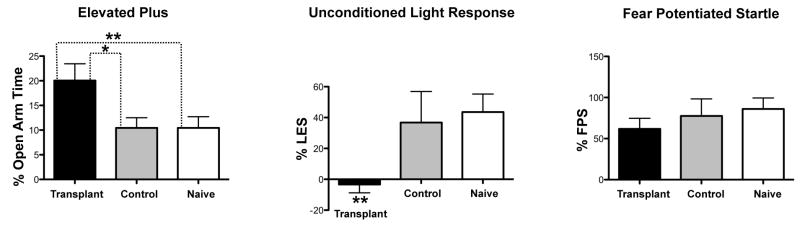
Six weeks after transplantation, grafted and control animals (as well as naive animals) underwent a series of behavioral tests: open field, the elevated plus maze, unconditioned light response, and fear-potentiated startle. We found no differences between groups for any of the behaviors assessed in the open field activity chambers indicating that locomotor activity was unaffected by the transplant. However, a one-way ANOVA for the percentage time spent on the open arms of the EPM revealed a significant effect of the grafting procedure (F(2,52) = 4.2, P=0.0204). Individual comparisons showed that the percentage open arm time for Transplant subjects was significantly greater than that of Control and Naïve subjects (Fig. 6A).
Figure 6.
Effects of grafts on contextual and conditioned fear. A, transplanted animals spend significantly more time in the open arms of the elevated plus maze. B, transplanted animals demonstrated a significant reduction in light-enhanced startle. C, transplanted animals responded in a similar manner to fear potentiated startle (a model of fear) as animals in both Control and Naïve groups (*p < 0.05; **p < 0.01).
Although there was no effect of transplantation on baseline startle (data not shown), there was a significant graft effect on the unconditioned effect of light on startle (F(2,37) = 5.098, P=0.011); the transplant group showed less enhancement of startle by bright light compared to the Control and Naïve groups (Fig. 6B). In contrast, the grafted animals did not differ significantly in fear-potentiated startle tests when compared to non-grafted animals (Fig. 6C). This suggests that the transplant procedure did not disturb the functional integrity of amygdalar circuitry, and animals retained the capacity to acquire fear conditioning.
4. Discussion
The potential for engraftment of specific neural cells for the restoration of brain function has been demonstrated for various models of neurologic diseases. Models of movement disorders, in particular, have been a primary area of focus because of the urgent need for improved treatments for these devastating diseases, but also because the pathoetiology of these disorders is better understood, and behavioral improvements after transplantation can be easily assessed and quantified. Although affective neural networks are integrated with movement networks, analysis of graft effects on emotion is only beginning to be investigated. Our laboratory recently demonstrated in animal models that grafts of genetically-customized monoaminergic neurons can have an antidepressant effect and thus may be capable of modulating corticolimbic circuitry [2]. The present experiments demonstrate that a similar approach is feasible to study the neural circuitry associated with fear and anxiety. GABAergic neuron-rich cell suspensions produced from embryonic LGE were engrafted into the LBA, and transplanted subjects demonstrated attenuation of innate fear as assessed with the EPM and unconditioned effect of light on startle. Increased numbers of GABA-ir neurons were identified within the LBA of transplanted animals, particularly in the vicinity of the graft site. Furthermore, on the ultrastructural level, transplanted GABAergic neurons appeared to be functionally integrated within the host parenchyma.
For these initial studies, fresh embryonic LGE from the same species and strain (as opposed to engineered cell lines) was chosen for transplantation because this tissue is known to be capable of survival and integration after transplantation and the resulting grafts are enriched with GABAergic neurons [1, 14, 24, 49]. Furthermore, autografts are less susceptible to immunorejection, enabling long-term survival without the need for immunosuppression. The disadvantage of fresh tissue grafts is that they typically are heterogeneous, comprised of various cell types, making it difficult to determine with certainty the cell type(s) responsible for the observed effects. While the enriched population of GABAergic neurons contained within LGE suspensions suggests that the anxiolytic effects observed here were due to a GABA-mediated increase of inhibitory tone, similar experiments using purified GABAergic cell lines [10] are considered the next step of investigation and are presently underway.
Early work has demonstrated that fetal striatal cells grafted into the lesioned striatum can survive, integrate within the host, and release GABA [42, 51, 52]. Subsequently, Loscher et al. showed that GABAergic-rich LGE cell suspension grafts are capable of increasing afterdischarge threshold and decreasing seizure duration and severity in kindled epilepsy [24]. It appears that just as GABAergic grafts attenuated epileptiform activity in a similar manner as infusion of GABA agonists into seizure foci [6, 23], so too have engrafted GABAergic neurons attenuated contextual fear in a similar manner as infusion of GABA agonist within the amygdala [13, 29, 41].
Because various models of anxiety conceivably may have differences in their mechanisms and circuitries, two different models were chosen for these studies. The EPM is considered a model of innate fear and “anxiety” [36], exploiting rodents’ natural avoidance of height, illuminated environments, and open space, and using as its primary measurement time spent by animals on the open versus closed arms of the apparatus. However, this measure can be misinterpreted if subjects are predisposed to hyperactivity by the experimental condition. Evaluation of hyperactivity using open field activity chambers revealed no differences between groups in distance traveled, stereotopy, or vertical rear behaviors; therefore, increased open arm time was not likely due to locomotor hyperactivity. The ULR also exploits rodents’ discomfort and hypervigilence in illuminated settings and is related to “light-enhanced startle” [45], a model of anxiety in which an animal’s startle response is augmented if the animal receives a startle-eliciting noise in an environment that is brightly lit as opposed to darkened. Although the grafted animals showed an anxiolytic-like phenotype compared to controls in both models of anxiety, they retained a normal FPS response. Together these data suggest a preferential influence upon the neural processing associated with anxiety-like behaviors rather than fear per se [46]
While there is a close association between fear and anxiety, recent studies suggest they may involve separate but overlapping neural systems and processes. Fear is associated with phasic activation of amygdaloid circuitry whereas anxiety may involve sustained, or tonic, activation [19]. The CE can be thought of as the weigh station for processing fear, into which parallel information processing converges, and from which the fear response is triggered. The bed nucleus of the stria terminalis (BNST) appears to be central to processing anxiety [18, 47] Lesions of the CE block FPS as well as conditioned freezing, while lesions to the BNST do not [46]. Conversely, lesioning or chemical inactivation of the BNST does not block FPS or conditioned freezing but can decrease responses in unconditioned models of anxiety such as light-enhanced startle and CRF enhanced startle [4, 48].
The fact that GABA neuron-rich grafts primarily influence anxiety processing but have little effect on conditioned fear raises the possibility that the engrafted neurons reinforced inhibitory circuitry and increased the level of local inhibitory tone, thus diminishing the sustained activation that may be responsible for the experience of anxiety (or unconditioned fear). Because the BNST is a major target of basolateral amygdala (BL) [7], we speculate that supplemented inhibition by virtue of the graft may reduce tonic BL-BNST drive and manifest itself as an anxiolytic-like behavioral phenotype. In the presence of a salient cue associated with an aversive event, however, this inhibitory tone may be overridden by strong inputs to the BL, thus allowing the subject to respond in a normal and adaptive fashion (i.e. escape; freezing), such as that seen in the FPS paradigm.
It is possible that the behavioral effects seen in these experiments may have been due to damage to the LBA (despite the minimally invasive micrografting method used to deliver cells), thus resulting in loss of function. Indeed, attenuation of freezing behavior to both contextual and auditory conditional stimuli has been demonstrated with cytotoxic lesions to the lateral and the central amygdaloid nuclei [8, 12]. Furthermore, Wilensky et al. teach that the essential aspects of the plasticity underlying Pavlovian fear memory occur within the LBA [53]. The present transplant paradigm however preserved a normal FPS response, demonstrating that the animals maintained the ability to learn, and suggesting that the LBA remained functionally intact. Nevertheless, it remains possible that the behavioral effects were due to lesions of the LBA – the extent of the graft affecting innate anxiety, but not being severe enough to alter learned fear. If this were true, however, similar results would be expected from the control animals, which clearly showed comparable disruption of the LBA, but were virtually indistinguishable from their naïve counterparts in their behavioral responses.
The cytoarchetecture of the amygdala is complex, and GABAergic neurons within the amygdala are morphologically and neurochemically heterogeneous. Numerous subpopulations have been identified including those containing the calcium-binding proteins parvalbumin (PV), calbindin (CB), and calretinin (CR), and those containing vasoactive intestinal protein (VIP), somatostatin, neuropeptide Y, and cholecystokinin [16, 27]. Studies are in progress to further characterize the subtypes of GABAergic neurons that may be influential in such grafting paradigms. The nature of their integration and connectivity must also be elucidated; for example, VIP and CB interneurons form synaptic contacts with each another, and they both form pericellular baskets surrounding pyramidal neurons [30]. This interaction may be responsible for the generation of synchronous activity in populations of pyramidal neurons.
Neuronal grafting may provide an alternative to pharmacologic and lesion studies for investigating emotional neural networks. By implementing living cells capable of integrating into functioning neural systems and participating in feedforward and feedback mechanisms used to regulate network function, the present experiments provide further evidence for the role of the LBA in processing anxiety, and they extend the notion that contextual and auditory fear conditioning is differentially modulated within the amygdala. Such engraftment studies may represent a feasible method for the study of molecular, neurochemical, cellular, and electrophysiological functions of the corticolimbic system that enables new insights into the pathoetiology and treatment of mental illness.
Acknowledgments
This work was funded by the NIH grants 5K08MH75978-2 (MGC) and MH63266 (WAC) as well as the Rappaport Foundation. We would like to thank Rachael Donalds for assistance in data analysis, Dr. Douglas Jacoby and Judson Ratliff for assistance in preparing cell suspensions, and Linda Hassinger for electron microscopy technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borlongan CV, Koutouzis TK, Poulos SG, Saporta S, Sanberg PR. Bilateral fetal striatal grafts in the 3-nitropropionic acid-induced hypoactive model of Huntington’s disease. Cell Transplant. 1998;7:131–5. doi: 10.1177/096368979800700208. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MG, Donalds RA, Carlezon WA, Jr, Hong S, Kim DS, Kim DW, et al. Antidepressant effect of stem cell-derived monoaminergic grafts. Neuroreport. 2007;18:1663–7. doi: 10.1097/WNR.0b013e3282f0eb1c. [DOI] [PubMed] [Google Scholar]
- 3.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 4.Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–31. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–56. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 6.De Sarro G, Meldrum BS, Reavill C. Anticonvulsant action of 2-amino-7-phosphonoheptanoic acid in the substantia nigra. Eur J Pharmacol. 1984;106:175–9. doi: 10.1016/0014-2999(84)90692-7. [DOI] [PubMed] [Google Scholar]
- 7.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 8.Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–34. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 9.Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol. 1997;75:479–87. [PubMed] [Google Scholar]
- 10.Gernert M, Thompson KW, Loscher W, Tobin AJ. Genetically engineered GABA-producing cells demonstrate anticonvulsant effects and long-term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol. 2002;176:183–92. doi: 10.1006/exnr.2002.7914. [DOI] [PubMed] [Google Scholar]
- 11.Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & memory (Cold Spring Harbor, NY) 2001;8:148–55. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2008;8:148–55. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajizadeh Moghaddam A, Roohbakhsh A, Rostami P, Heidary-Davishani A, Zarrindast MR. GABA and histamine interaction in the basolateral amygdala of rats in the plus-maze test of anxiety-like behaviors. Pharmacology. 2008;82:59–66. doi: 10.1159/000131110. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby DB, Lindberg C, Cunningham MG, Ratliff J, Dinsmore J. Long-term survival of fetal porcine lateral ganglionic eminance cells in the hippocampus of rats. J Neurosci Res. 1999;56:581–94. doi: 10.1002/(SICI)1097-4547(19990615)56:6<581::AID-JNR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Kajimura N, Nishikawa M, Uchiyama M, Kato M, Watanabe T, Nakajima T, et al. Deactivation by benzodiazepine of the basal forebrain and amygdala in normal humans during sleep: a placebo-controlled [15O]H2O PET study. Am J Psychiatry. 2004;161:748–51. doi: 10.1176/appi.ajp.161.4.748. [DOI] [PubMed] [Google Scholar]
- 16.Kemppainen S, Pitkanen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–67. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–45. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 18.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–59. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 19.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–59. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 20.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeDoux JE. The amygdala and emotion: A view through fear. In: Aggleton JP, editor. The Amygdala. Oxford: Oxford University Press; 2000. pp. 289–310. [Google Scholar]
- 23.Loscher W, Ebert U. Basic mechanisms of seizure propagation: targets for rational drug design and rational polypharmacy. Epilepsy Res Suppl. 1996;11:17–43. [PubMed] [Google Scholar]
- 24.Loscher W, Ebert U, Lehmann H, Rosenthal C, Nikkhah G. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res. 1998;51:196–209. doi: 10.1002/(SICI)1097-4547(19980115)51:2<196::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–64. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–6. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 27.McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- 28.McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473:137–46. doi: 10.1002/cne.20101. [DOI] [PubMed] [Google Scholar]
- 29.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–91. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 30.Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–36. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- 31.Nikkhah G, Olsson M, Eberhard J, Bentlage C, Cunningham MG, Bjorklund A. A microtransplantation approach for cell suspension grafting in the rat Parkinson model: a detailed account of the methodology. Neuroscience. 1994;63:57–72. doi: 10.1016/0306-4522(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 32.Pakzaban PDT, Burns LH, Isacson O. Increased proportion of acetylcholinesterase-rich zones and improved morphological integration in host striatum of fetal grafts derived from the lateral but not the medial ganglionic eminance. Experimental Brain Research. 1993;97:13–22. doi: 10.1007/BF00228813. [DOI] [PubMed] [Google Scholar]
- 33.Pape HC, Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Ann N Y Acad Sci. 2003;985:92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x. [DOI] [PubMed] [Google Scholar]
- 34.Pare D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–50. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pare D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- 36.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 37.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 38.Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–72. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- 39.Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. Eur J Pharmacol. 2000;394:265–73. doi: 10.1016/s0014-2999(00)00128-x. [DOI] [PubMed] [Google Scholar]
- 40.Sanders SK, Morzorati SL, Shekhar A. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res. 1995;699:250–9. doi: 10.1016/0006-8993(95)00915-d. [DOI] [PubMed] [Google Scholar]
- 41.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–6. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 42.Sirinathsinghji DJ, Dunnett SB, Isacson O, Clarke DJ, Kendrick K, Bjorklund A. Striatal grafts in rats with unilateral neostriatal lesions--II. In vivo monitoring of GABA release in globus pallidus and substantia nigra. Neuroscience. 1988;24:803–11. doi: 10.1016/0306-4522(88)90068-1. [DOI] [PubMed] [Google Scholar]
- 43.Sloviter RS, Ali-Akbarian L, Horvath KD, Menkens KA. Substance P receptor expression by inhibitory interneurons of the rat hippocampus: enhanced detection using improved immunocytochemical methods for the preservation and colocalization of GABA and other neuronal markers. J Comp Neurol. 2001;430:283–305. [PubMed] [Google Scholar]
- 44.Takagi M, Yamamoto C. The long-lasting inhibition recorded in vitro from the lateral nucleus of the amygdala. Brain Res. 1981;206:474–8. doi: 10.1016/0006-8993(81)90550-3. [DOI] [PubMed] [Google Scholar]
- 45.Walker DL, Cassella JV, Lee Y, De Lima TCL, Davis M. Opposing Roles of the Amygdala and Dorsolateral Periaqueductal Gray in Fear-potentiated Startle. Neuroscience and Biobehavioral Reviews. 1997;21:743–53. doi: 10.1016/s0149-7634(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 46.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 48.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 49.Watts C, Brasted PJ, Dunnett SB. The morphology, integration, and functional efficacy of striatal grafts differ between cell suspensions and tissue pieces. Cell Transplant. 2000;9:395–407. doi: 10.1177/096368970000900310. [DOI] [PubMed] [Google Scholar]
- 50.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Wictorin K, Isacson O, Fischer W, Nothias F, Peschanski M, Bjorklund A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum--I. Subcortical afferents. Neuroscience. 1988;27:547–62. doi: 10.1016/0306-4522(88)90288-6. [DOI] [PubMed] [Google Scholar]
- 52.Wictorin K, Simerly RB, Isacson O, Swanson LW, Bjorklund A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum--III. Efferent projecting graft neurons and their relation to host afferents within the grafts. Neuroscience. 1989;30:313–30. doi: 10.1016/0306-4522(89)90256-x. [DOI] [PubMed] [Google Scholar]
- 53.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]