Abstract
A series of alkanediamide-linked bisbenzamidines was synthesized and tested in vitro against a drug-sensitive strain of Trypanosoma brucei brucei, a drug-resistant strain of Trypanosoma brucei rhodesiense and Pneumocystis carinii. Bisbenzamidines linked with longer alkanediamide chains were potent inhibitors of both strains of T. brucei. However, bisbenzamidines linked with shorter alkanediamide chains were the most potent compounds against P. carinii. N,N′-bis[4-(aminoiminomethyl)phenyl] hexanediamide, 4 displayed potent inhibition (IC50 = 2–3 nM) against T. brucei and P. carinii, and was non-cytotoxic in the A549 human lung carcinoma cell line. The inhibitory bioactivity was significantly reduced when the amidine groups in 4 were moved from the para to the meta positions or replaced with amides.
Keywords: Benzamidine, Pentamidine, DNA binding, Human African Trypanosomiasis, Trypanosoma brucei, Pneumocystis carinii
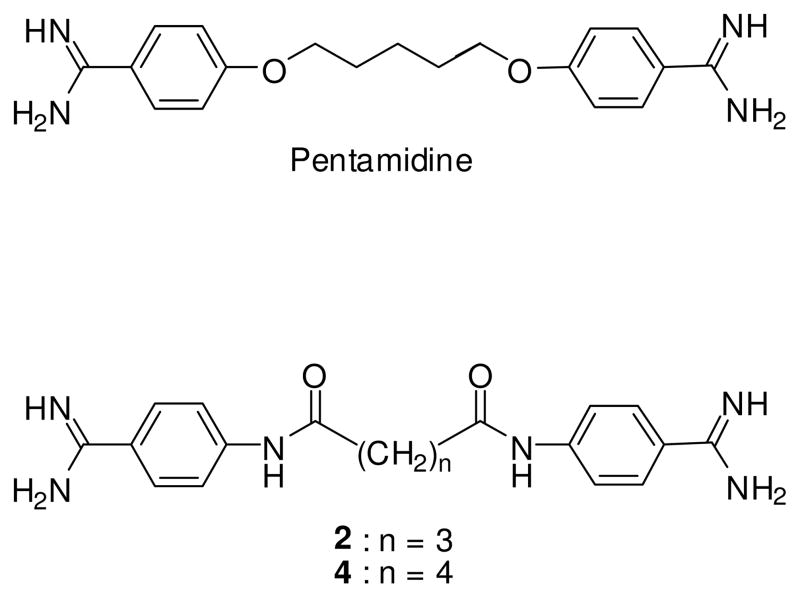
The benzamidine scaffold is an important structural motif found in many bioactive compounds exhibiting antifungal, 1–2 antiparasitic, 3–4 anticoagulant, 5 antitumor6 or antiviral7 properties. The two benzamidine units in the antimicrobial drug, pentamidine, are attached with a flexible pentyldioxy chain (Fig. 1). Pentamidine is clinically used in the treatment of pneumonia caused by the opportunistic fungus, Pneumocystis jirovecii, early stage human African trypanosomiasis (HAT) and antimony-resistant Leishmaniasis. However, its clinical use is hampered by lack of oral bioavailability, and serious adverse effects including hypotension, nephrotoxicity, abdominal pain, pancreatic complications, cardiotoxicity and hypoglycemia which may progress to insulin-dependent diabetes mellitus.4,8 In recent years, a large number of pentamidine analogs have been synthesized with the goals of enhancing its useful therapeutic properties, improving its oral bioavailability, and reducing the undesirable adverse effects.1,3,4,9–13 The design strategy in many of these studies has focused on modification of the central pentyldioxy chain, the two aromatic moieties, or the terminal amidine groups.
Figure 1.
Structures of pentamidine and lead compounds 2 and 4.
There is clearly an urgent need to develop more effective and safer drugs for the treatment of Pneumocystis jirovecii pneumonia, HAT and Leishmaniasis. The current chemotherapeutic agents suffer from drawbacks such as severe toxicities, limited efficacy, poor oral bioavailability or emergence of microbial resistance. These infectious diseases threaten millions of people worldwide, and are the major cause of human suffering and economic burden, especially in developing countries where these diseases are more prevalent. In recent years, the cases of patients dually infected with HIV and parasitic infections, such as Leishmaniasis, have been growing worldwide.14 This is of concern since coinfections of HIV with Leishmania exert a synergistic detrimental effect on the host cellular immune response, reduce the effectiveness of therapies, greatly increases the risk of relapse and mortality rate.14
We previously reported on the potent anti-Pneumocystis carinii activity of a series of bisbenzamidines linked by various linkers.6,15 From these studies, bisbenzamidines linked by a pentanediamide 2 or hexanediamide 4 chain (Fig. 1), emerged as exceptionally potent agents with limited cytotoxicity to mammalian cells. Screening of these compounds against various protozoal parasities showed excellent in vitro activity against T. brucei. Consequently, compounds 2 and 4 served as lead compounds for the synthesis of the 11 analogs reported in this paper. These compounds are structurally related to pentamidine, in which the strong electron-donating ether functions of the pentyldioxy chain in pentamidine has been replaced by poor electron-donating amide functions separated by alkanes of increasing chain length (Fig. 1). The effect of moving the terminal bisamidine functions in 4 from the para to the meta positions of the phenyl rings on bioactivity was also investigated.
The synthesized compounds were tested in vitro against a pentamidine-sensitive strain of Trypanosoma brucei brucei LAB 110 EATRO16,17, a drug-resistant strain (melarsoprol, pentamidine, and berenil resistant) of Trypanosoma brucei rhodesiense KETRI 24316,17 and Pneumocystis carinii.18 The cytotoxicity of the compounds was evaluated with the A549 human lung carcinoma cell line.18 The biological data is reported in Table 1.
Table 1.
Structure, cytototoxicity, and in vitro inhibitory bioactivity of compounds 1–13 against Trypanosoma brucei and Pneumocystis carinii
 | |||||||
|---|---|---|---|---|---|---|---|
| IC50 (μM)a |
|||||||
| Cpd. # | n | R | R′ | T.b. bruceib | T.b. rhodesiensec | P. cariniid | Cytotox. A549e |
| 1 | 2 | p-Am | p-Am | 9.0 | 2.19 | 0.442 | >44.2 |
| 2 | 3 | p-Am | p-Am | 0.009 | 0.096 | 0.003f | 2276 |
| 3 | 3 | p-CN | p-CN | 6.50 | 7.90 | NTg | NT |
| 4 | 4 | p-Am | p-Am | 0.003 | 0.002 | 0.002f | 1193 |
| 5 | 4 | p-CO-NH2 | p-CO-NH2 | 2.80 | 1.10 | >100 | NT |
| 6 | 4 | m-Am | m-Am | 0.041 | 0.021 | 33.7 | NT |
| 7 | 4 | m-CO-NH2 | m-CO-NH2 | NT | 1.97 | >100 | >261 |
| 8 | 4 | p-Am | m-Am | 0.012 | 0.007 | 2.27 | >227 |
| 9 | 5 | p-Am | p-Am | 0.002 | 0.002 | 2.37 | >237 |
| 10 | 6 | p-Am | p-Am | 0.003 | 0.001 | 1.95 | 176 |
| 11 | 7 | p-Am | p-Am | 0.400 | 0.240 | 2.44 | 2.87 |
| 12 | 8 | p-Am | p-Am | 0.002 | 0.004 | 4.28 | 249 |
| 13 | 10 | p-Am | p-Am | 0.008 | 0.007 | 3.07 | 18 |
| Pentamidine | 0.002 | 0.002 | 0.50f | 24.0f | |||
Each value is the average of at least two determinations.
The Trypanosoma brucei brucei strain was Lab 110 and the Trypanosoma brucei rhodesiense strain was KETRI 243 (see refs 16 and 17).
Pneumocytis carinii was obtained from rat lungs (see ref 18).
Cytotoxicity with A549 epithelial lung cell monolayers from human carcinoma (see ref 18).
Data obtained from reference 15.
NT, Not tested.
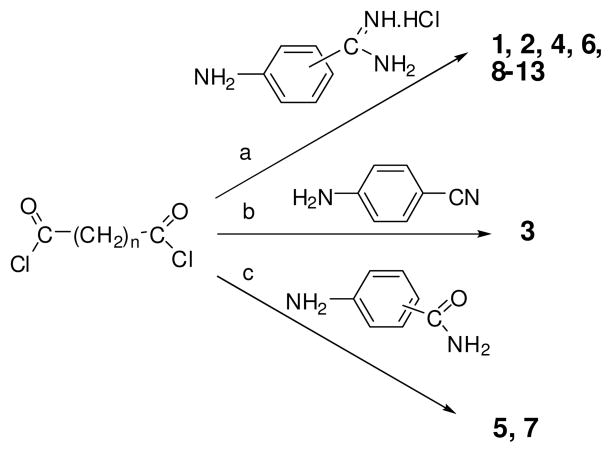
The synthesis of these compounds is accomplished as shown in Scheme 1. Starting with the appropriate diacid chlorides and aminobenzamidine or aminobenzonitrile or aminobenzamide, the resultant final compounds 1–13 were readily obtained in fair yields. The average yields for the bisbenzamidines (1, 2, 4, 6, 8–13) and bisbenzamides (5, 7) were 40% and 90% respectively. The chemical structures of the synthesized compounds were confirmed by their spectral data (1H NMR and FTIR) and their purity was verified by elemental analyses. Representative synthesis and characterization of compounds 2, 3 and 5 are described. 19
Scheme 1.
General procedures for the synthesis of compounds 1–13. Reagents and conditions: (a) DMF, pyridine, reflux, 30 min – 2 h; (b) and (c): Dioxane, rt, stirred overnight.
The alkanediamide-linked bisbenzamidines were generally more active against T. brucei than P. carinii with the exception of compounds 1, 2 and 4. Four of the bisbenzamidines, which are linked with a hexanediamide 4, heptanediamide 9, octanediamide 10 or decanediamide 12 demonstrated potent activity (IC50 = 0.001 to 0.004 μM) against both the sensitive and resistant strains of T. brucei. The potency of these compounds approached that of pentamidine (IC50 = 0.002 μM). The most active compounds generally showed similar potency against both strains of T. brucei with the exception of compound 2 which was about 11-fold less active against the resistant strain. The results indicate that bisbenzamidines linked with longer alkanediamide chains were more active as antitrypanosomal agents. However, those compounds with shorter alkanediamide chains, namely 1, 2 and 4 were the most active agents against P. carinii. The potency of compound 1 (IC50 = 0.442 μM) was equivalent to pentamidine (IC50 = 0.50 μM), whereas, compounds 2 (IC50 = 0.003 μM) and 4 (IC50 = 0.002 μM) were 167–250 fold more potent than pentamidine. The remaining compounds were significantly less active than pentamidine. The bioactivity against T. brucei or P. carinii was reduced by at least 10-fold or greater if the bisamidine groups in 4 were moved from the para to the meta positions as in 6 or replaced with amides as in 5.
In addition to high potency against T. brucei or P. carinii, several bisbenzamidines exhibited very low cytoxicity against the A549 cell line, namely, 2, 4, 8, 9, 10 and 12. The selectivity index of these compounds for T. brucei (SIT.b) or P. carinii (SIP.c) can be defined as the ratio of the cytotoxic IC50 to the T. brucei or P. carinii IC50 values. Using this definition, the most selective compounds in this group against P. carinii were compounds 2 (SIP.c = 758,667) and 4 (SIP.c = 596,500) respectively. The selectivity of compounds 2, 4, 8, 9, 10 and 12 for T. brucei ranged from 18,917 to 596,500. The selectivity indexes of pentamidine for P. carinii and T. brucei were 48 and 12,000 respectively. This indicates that several of the compounds in this series are highly selective for the pathogenic cells (P. carinii and T. brucei) than for the mammalian cells, and their selectivity profiles are superior to that of pentamidine. Because of their high potency against P. carinii and low cytotoxicity, compounds 2 and 4 were further evaluated in an immunosuppressed mouse model of Pneumocystosis1. When evaluated at different doses (5, 10, 20 and 40 mg/kg), compound 2 emerged as the most promising anti-Pneumocystis drug. This compound was highly efficacious against the infection at 20 mg/kg and 40 mg/kg doses, with >1,000-fold reductions in organism burden, and resulted in improved survival curves versus those for pentamidine-treated mice at the same doses.
The mechanism of action of bisbenzamidines has been attributed to strong binding to DNA at AT rich sites followed by subsequent inhibition of DNA-dependent enzymes and/or direct inhibition of transcription.8,20–22 In this series of compounds, the introduction of poor electron-donating amide groups in the central linker resulted in significant reduction in DNA binding compared to pentamidine. The binding (ΔTm) of pentamidine to poly (dA-dT) was 21.6 °C whereas the values ranged from 7.5 oC to 14.4 oC for the other bisbenzamidines reported here (data not shown). Although compounds that demonstrated strong potency against P. carinii were strong binders to DNA (ΔTm = 11.4 to 14.4 for compounds 1, 2 and 4), several other poor inhibitors of P. carinii (e.g. 6 and 10) also showed strong binding to DNA (ΔTm = 13.8 °C and 13.2 °C respectively). These results are consistent with our previous reports3,6,17,18,23,24 which indicated that the anti-P. carinii or antitrypanosomal activity of a large number of bisbenzamidines synthesized in our laboratory do not correlate directly with their DNA binding affinity.
In conclusion, we have synthesized and evaluated the structure-activity relationships of a series of alkanediamide-linked bisbenzamidines as potential new agents against T. brucei and P. carinii. The high selectivity indexes of these compounds against both pathogens warrant further investigations especially in animal models of trypanosomiasis. We will report the findings of such investigations in the near future.
Acknowledgments
This work was supported by grant 2S06GM08008 from the National Institutes of Health. The authors thank Aixa Rodriguez, Steven DeChiricho and Eric Sorrentino for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Cushion MT, Walzer PD, Ashbaugh A, Rebholz S, Brubaker R, Vanden Eynde JJ, Mayence A, Huang TL. Antimicrob Agents Chemother. 2006;50:2337. doi: 10.1128/AAC.00126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsuyama J, Nomura N, Hashimoto K, Yamada E, Nishikawa H, Kaeriyama M, Kimura A, Todo Y, Narita H. Antimicrob Agents Chemother. 2008;52:1318. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang TL, Bacchi CJ, Kode NR, Zhang Q, Wang G, Yartlet N, Rattendi D, Londono I, Mazumder L, Vanden Eynde JJ, Mayence A, Donkor IO. Int J Antimicrob Agents. 2007;30:555. doi: 10.1016/j.ijantimicag.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Bakunova SM, Bakunov SA, Patrick DA, Suresh Kumar EVK, Ohemeng KA, Bridges AS, Wenzler T, Barszcz T, Jones SG, Werbovetz KA, Brun R, Tidwell RR. J Med Chem. 2009;52:2016. doi: 10.1021/jm801547t. [DOI] [PubMed] [Google Scholar]
- 5.Phillips G, Guilford WJ, Buckman BO, Davey DD, Eagen KA, Koovakkat S, Liang A, McCarrick M, Mohan R, Ng HP, Pinkerton M, Subramanyam B, Ho E, Trinh L, Whitlow M, Wu S, Xu W, Morrissey MM. J Med Chem. 2002;45:2484. doi: 10.1021/jm0200660. [DOI] [PubMed] [Google Scholar]
- 6.Vanden Eynde JJ, Mayence A, Johnson MT, Huang TL, Collins MS, Rebholz S, Walzer PD, Cushion MT, Donkor IO. Med Chem Res. 2005;14:143. [Google Scholar]
- 7.De Clercq E, Dann O. J Med Chem. 1980;23:787. doi: 10.1021/jm00181a016. [DOI] [PubMed] [Google Scholar]
- 8.Soeiro MNC, De Souza EM, Stephens CE, Boykin DW. Expert Opin Invest Drugs. 2005;14:957. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]
- 9.Huang TL, Vanden Eynde JJ, Mayence A, Donkor IO, Khan SI, Tekwani BL. J Pharm Pharmacol. 2006;58:1033. doi: 10.1211/jpp.58.8.0003. [DOI] [PubMed] [Google Scholar]
- 10.Bakunova SM, Bakunov SA, Patrick DA, Wenzler T, Barszcz T, Werbovetz KA, Brun R, Tidwell RR. J Med Chem. 2008;51:6927. doi: 10.1021/jm800918v. [DOI] [PubMed] [Google Scholar]
- 11.Mayence A, Pietka A, Collins MS, Cushion MT, Tekwani BL, Huang TL, Vanden Eynde JJ. Bioorg Med Chem Letts. 2008;18:2658. doi: 10.1016/j.bmcl.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Rahmathullah SM, Tidwell RR, Jones SK, Hall JE, Boykin DW. Eur J Med Chem. 2008;43:174. doi: 10.1016/j.ejmech.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Arafa RK, Ismail MA, Munde M, Wilson WD, Wenzler T, Brun R, Boykin DW. Eur J Med Chem. 2008;43:2901. doi: 10.1016/j.ejmech.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet J-P, Gradoni L, Ter Horst R, Lopez-Velez R, Moreno J. Clinical Micro Rev. 2008;21:334. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanden Eynde JJ, Mayence A, Huang TL, Collins MS, Rebholz S, Walzer PD, Cushion MT. Bioorg Med Chem Letts. 2004;14:4545. doi: 10.1016/j.bmcl.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Bacchi CJ, Nathan HC, Livingston T, Valladares G, Saric M, Sayer PD, Njogu AR, Clarkson AB. Antimicrob Agents Chemother. 1990;34:1183. doi: 10.1128/aac.34.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donkor IO, Huang TL, Tao B, Rattendi D, Lane S, Vargas M, Goldberg B, Bacchi C. J Med Chem. 2003;46:1041. doi: 10.1021/jm020375q. [DOI] [PubMed] [Google Scholar]
- 18.Cushion MT, Walzer PD, Ashbaugh A, Collins MS, Rebholz S, Vanden Eynde JJ, Mayence A, Huang TL. Antimicrob Agents Chemother. 2004;48:4209. doi: 10.1128/AAC.48.11.4209-4216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The compounds reported in this study were synthesized in the laboratory of Professor Tien Huang at Xavier University of Louisiana (New Orleans, LA). N,N′-bis[4-(aminoiminomethyl)phenyl] pentanediamide, dihydrochloride salt, (2) was synthesized by heating under reflux for 30 min a mixture of 4-aminobenzamidine monohydrochloride (2,08 g, 10 mmol), glutaryl chloride (0.64 mL, 5 mmol), and pyridine (8 mL, 100 mmol) in N,N-dimethyl formamide (40 mL). The precipitate was filtered and thoroughly washed successively with water and acetone. Yield: 50 %. M.p.: >300 °C (decomp.). 1H NMR (DMSO d6) : 10.5 (s, 2H); 9.1 (br s, 8H); 7.8 (dd, 8H); 2.5 (t, 4H); 1.9 (q, 2H) ppm. IR: 3094; 1923; 1659; 1604; 1351 cm-1. Anal. Calc. for C19H22N6O2. 2 HCl (438.13): C, 51.94; H, 5.51; N, 19.13. Found: C, 51.79; H, 5.31; N, 18.89. (M-H-W Laboratories, Phoenix, AZ).N,N′-bis(4-cyanophenyl)pentanediamide, (3) was synthesized by stirring at room temperature overnight a mixture of 4-aminobenzonitrile (9.25 g, 80 mmol), and glutaryl chloride (3.38 g, 20 mmol) in dioxane (200 mL). The precipitate was filtered and washed successively with water, ethanol and ether. Yield: 68 %. M.p.: 238–239 °C. 1H NMR (DMSO d6) : 10.3 (s, 2H); 7.7 (2d, 8H, J = 8Hz); 2.4 (t, 4H); 1.9 (q, 2H) ppm. IR: 3264; 2221; 1667; 1592; 1506; 1442 cm−1. Anal. Calc. for C19H16N4O2 (332.36): C, 68.66; H, 4.85; N, 16.86. Found: C, 68.83; H, 5.00; N, 16.69. (M-H-W Laboratories, Phoenix, AZ).N,N′-bis[4-(aminocarbonyl)phenyl]hexanediamide, (5) was synthesized by stirring at room temperature overnight a mixture of 4-aminobenzamide (5.44 g, 40 mmol), and adipoyl chloride (1.83 g, 10 mmol) in dioxane (100 mL). The precipitate was filtered and washed successively with water and ethanol. Yield: 90 %. M.p.: >300 °C. 1H NMR (DMSO d6) : 10.1 (s, 2H); 7.8 (d, 4H, J = 7 Hz); 7.8 (s, 2H); 7.6 (d, 4H, J = 8 Hz); 7.2 (s, 2H); 2.4 (br m, 4H); 1.6 (m, 4H) ppm. IR: 3371; 3317; 3171; 1672; 1651; 1620; 1512 cm−1. Anal. Calc. for C20H22N4O4 (382.41): C, 62.82; H, 5.80; N, 14.65. Found: C, 62.75; H, 5.76; N, 14.53. (M-H-W Laboratories, Phoenix, AZ).
- 20.Beerman TA, McHugh MM, Sigmund R, Lown JW, Rao KE, Bathini Y. Biochem Biophys Acta. 1992;1131:53. doi: 10.1016/0167-4781(92)90098-k. [DOI] [PubMed] [Google Scholar]
- 21.Hildebrandt E, Boykin DW, Kumar A, Tidwell RR, Dykstra CC. J Eukaryotic Microbiol. 1998;45:112. doi: 10.1111/j.1550-7408.1998.tb05078.x. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald DJ, Anderson JN. J Biol Chem. 1999;274:27128. doi: 10.1074/jbc.274.38.27128. [DOI] [PubMed] [Google Scholar]
- 23.Tao B, Huang TL, Zhang Q, Jackson L, Queener SF, Donkor IO. Eur J Med Chem. 1999;34:531. [Google Scholar]
- 24.Huang TL, Tao B, Quarshie Y, Queener SF, Donkor IO. Bioorg Med Chem Lett. 2001;11:2679. doi: 10.1016/s0960-894x(01)00541-8. [DOI] [PubMed] [Google Scholar]