Abstract
Dynamic mechanical processes shape the embryo and organs during development. Little is understood about the basic physics of these processes, what forces are generated, or how tissues resist or guide those forces during morphogenesis. This review offers an outline of some of the basic principles of biomechanics, provides working examples of biomechanical analyses of developing embryos, and reviews the role of structural proteins in establishing and maintaining the mechanical properties of embryonic tissues. Drawing on examples we highlight the importance of investigating mechanics at multiple scales from milliseconds to hours and from individual molecules to whole embryos. Lastly, we pose a series of questions that will need to be addressed if we are to understand the larger integration of molecular and physical mechanical processes during morphogenesis and organogenesis.
Keywords: mechano-transduction, cellular mechanics, biomechanics, gastrulation
1. Introduction - Morphogenesis is all about mechanics
Few would deny that physical mechanical processes are important in morphogenesis. After all, cells and tissues move great distances as the form of the embryo is sculpted and then again when organs take shape within the body. Large scale movements of cells and tissues during embryo development involve force production leading to mechanical deformation. From this perspective a principle role of tissue stiffness and force production is to sculpt form from the multicellular aggregate of the early embryo or to sculpt organs from germ-layers after the initial movements of gastrulation are complete.
However, new roles for mechanics during morphogenesis have come to light from recent studies of cellular mechanics in cultured cells and within developing Drosophila embryos (Wozniak and Chen, 2009, Discher et al., 2005). These studies have revealed that mechanical cues within the micro-environment are capable of generating spatial information for the specification of cell types including triggers for apoptosis, the differentiation of progenitor cells, pathways for specialized cell migration, and progression of cancer. The molecular pathways that sense mechanical properties within the micro-environment and the programmed cellular responses are termed “mechano-transduction.” Thus, as mechano-transduction joins other biophysical and biochemical processes that pattern gene expression and physically shape the embryo we must consider the control of mechanical properties within embryos during morphogenesis.
Like other aspects of embryogenesis, tissue mechanical properties must be under genetic control during oogenesis (by maternal genes) and during embryogenesis (by zygotic genes inherited from both parents). Maternally inherited proteins, RNAs, and organelles along with zygotically expressed genes create new protein complexes that together must regulate the mechanical properties of cells, interactions between cells and their environment, and the mechanical properties of developing tissues. Molecular genetic approaches have been key to understanding the systems-level organization of signal transduction and gene-regulatory networks and the same approaches are beginning to be applied to understand the physical processes and biomechanics of morphogenesis.
Biomechanics has focused on the mechanics of sub-cellular structures and of the mechanical properties of adult tissues but has largely passed over embryonic tissue-scale mechanics. In large part this gap is due to the development of fine tools such as microrheology and atomic-force microscopy which are useful for exploring sub-cellular mechanics and to the use of the strain-gauges, micro-positioners, and rheometers for exploring large structures or material available in relatively large volumes. Only a few devices have been designed to investigate the intermediate mechanics of the cellular microenvironment or small soft tissues obtained from embryos (Table 1). Furthermore, the development of novel tools has driven the measurements without much consideration of the relevance of the measurements to the function of cells and tissues. This begs us to ask what mechanical properties are important to cells and cell biologists.
Table 1.
Devices for measuring mechanical properties of embryos.
| Device type | Where used | Advantages | Limitations |
|---|---|---|---|
 |
chick embryonic heart tissues [1, 2] |
Small tissue fragments can continue developing. |
- geometric constraints on tissues - samples stiffness from both surface and deep cells. - complex effects of pre- stress |
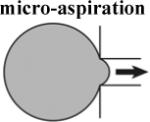 |
whole frog embryos; eggs [3] [4] |
Does not require microsurgery - allows continued development. |
- geometric constraints on tissues - samples stiffness from both surface and deep cells. - unknown effects of pre- stress |
 |
axial tissues of frog; cellular reaggregates from chicken; sea urchin blastula [5], [6], [7] |
Direct measure of stiffness |
- tissues removed from native environment. - chicken embryonic cells need to be dissociated then re-aggregated into sphere |
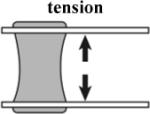 |
measurement of epithelial tensile behavior [8] |
Simple visualization of cell surface Direct measure of stiffness |
- difficult to fashion grips to hold tissue. - tissues removed from native environment. |
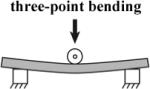 |
late stage notochord [9-11] |
Direct measure of flexural stiffness. |
- only useful for some structures |
Zamir, E.A. and L.A. Taber, Material properties and residual stress in the stage 12 chick heart during cardiac looping. J Biomech Eng, 2004. 126(6): p. 823-30.
Zamir, E.A. and L.A. Taber, On the effects of residual stress in microindentation tests of soft tissue structures. Journal of Biomechanical Engineering-Transactions of the Asme, 2004. 126(2): p. 276-283.
von Dassow, M. and L.A. Davidson, Natural variation in embryo mechanics: gastrulation in Xenopus laevis is highly robust to variation in tissue stiffness. Dev Dyn, 2009. 238: p. 2-18.
Mitchison, J.M. and M.M. Swann, The mechanical properties of the cell surface. I. The cell elastimeter. Journal of Experimental Biology, 1954. 31: p. 443-461.
Zhou, J., H.Y. Kim, and L.A. Davidson, Actomyosin stiffens the vertebrate embryo during critical stages of elongation and neural tube closure. Development, 2009. 136: p. 677-688.
Forgacs, G., et al., Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys J, 1998. 74(5): p. 2227-34.
Davidson, L.A., et al., Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev Biol, 1999. 209(2): p. 221-38.
Wiebe, C. and G.W. Brodland, Tensile properties of embryonic epithelia measured using a novel instrument. J Biomech, 2005. 38(10): p. 2087-94.
Adams, D.S., R. Keller, and M.A. Koehl, The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development, 1990. 110(1): p. 115-30.
Koehl, M.A.R., D.S. Adams, and R.E. Keller, Mechanical development of the notochord in Xenopus early tail-bud embryos., in Biomechanics of active movement and deformation., N. Akkas, Editor. 1990, Springer-Verlag: Berlin Heidelberg. p. 471-485.
Koehl, M.A.R., K.J. Quillin, and C.A. Pell, Mechanical design of fiber-wound hydraulic skeletons: The stiffening and straightening of embryonic notochords. American Zoologist, 2000. 40: p. 28-41.
In this paper we will review key engineering terms, how mechanical properties are measured, the molecular sources of mechanical properties, and what is currently known about the mechanical properties of embryonic tissues. At the conclusion of this paper we will pose a set of five “big” questions and discuss how they may be studied jointly by cell biologists, physicists, and bioengineers. We focus on “passive” mechanical properties because these are often overlooked in favor of mechanisms of force generation. “Passive” mechanical properties and “active” force generation must be equally important in morphogenesis since the tissue deformations that comprise morphogenesis are a function of both the forces involved and the extent to which the tissue resists those forces (see Question # 3 in Section 4). Other reviews have discussed force generation during morphogenesis (Lecuit and Lenne, 2007, Quintin et al., 2008, Keller et al., 2000).
2. Tissue mechanical properties: meaning and measurement
Before discussing the important developmental consequences of mechanics we need to define some terms commonly used in the field of mechanics that may be unfamiliar to cell and developmental biologists (Box 1; figure 1). These terms are often common terms used by biologists, such as “force” or “stress”, but have distinctly different meanings when used by biomechanicians. Standardized terms were developed by the fields of physics, rheology, and biomechanics so that theories could be formulated and results compared regardless of the geometry or origin of the material. Our discussion in box 1 is brief and readers are encouraged to seek an introductory textbook (Vogel, 2003) as well as more detailed reviews or advanced texts (see (Wainwright, 1975, Wainwright et al., 1976, Koehl, 1990, Janmey et al., 2007, Taber, 2004) for more complete discussion.
Box 1.
Morphogenesis involves large three-dimensional deformations and complex three-dimensional stresses. The mechanical properties of biological materials can vary with the amount and rate of applied force and with the direction in which the force is applied. Full mechanical descriptions of these processes can be quite complex so we present here a highly simplified description that still remains helpful for many purposes. Explicit theoretical definitions of elasticity, stress, and strain in three dimensions with biological applications can be found in many excellent texts [1-3].
Strain (ε): A non-dimensional measure of how a material deforms, including changes in shape and size. For small deformations 1-dimensional strain can be defined as:
(1) L is the deformed length and L0 is the initial length of a piece of the deformed material.
Stress (σ): The force (F) exerted on a surface divided by the area (A) of that surface.
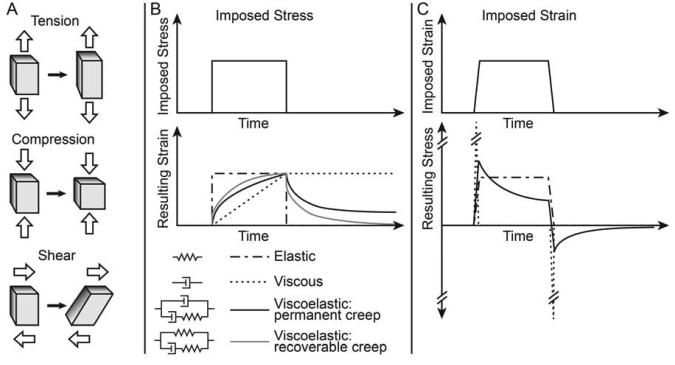
(2) In contrast to pressure, which is also a force per unit area acting isotropically or equally in all directions, stress can have different magnitudes in different directions. When force is applied perpendicular to the surface of a block, the block will be under compressive or tensile stress depending on whether the force is directed inwards or outwards with respect to the block (Fig. 1A); when a force is applied parallel to the surface of the block, it exerts shear stress (Fig. 1A).
Mechanical properties relate the forces applied to an object (stresses) to deformation of the object (strain). Stiffness is a measure of the ability of an object or material to resist deformation. A whole object has a structural stiffness that depends both on the geometry and arrangement of materials within the object, as well as the nature of the applied force (i.e. the direction and location in which it is applied). For example the resistance to bending of a long object (such as a beam or a notochord) is flexural stiffness. Flexural stiffness is dependent on both the composition of the beam and the diameter and length of the beam. Stiffness of a material is typically described by a set of parameters or material properties that reflect its response to applied forces. In order to relate mechanical properties from one structure to another material properties are defined so that they are independent of size or shape.
For simplicity we use “stiffness” generically to include both structural and material properties.
Properties of materials
Elastic materials (e.g. a solid like an idealized spring) deform a finite amount under stress. For elastic materials, strain depends only on applied stress, not time (figure 1B and C). The stiffness of an isotropic elastic material under unconfined, uniaxial compressive or tensile stress can be described by a Young's modulus, “E” (also known as a modulus of elasticity):
(3) If stress is applied in multiple directions the relationship between stress and strain is more complex.
Viscous materials (e.g. a fluid like water) deform continuously under an applied shear stress, and the rate of deformation (strain rate, dε/dt) is a function of the stress (figure 1B and C). The relationship between strain rate and shear stress is characterized by a coefficient of viscosity (η).
(4) Viscoelastic materials show a mixture of behaviors: both strain and strain rate depend on stress (figure 1B and C). A viscoelastic material can be characterized in three ways: 1) by an empirically measured time-dependent Young's modulus (also referred to as relaxation modulus), 2) represented analytically by an equation relating stress, strain and change of strain over time, or 3) by a network model of viscous and elastic elements (figure 1B) [4].
- 1.Taber LA. Nonlinear theory of elasticity. Applications in biomechanics. First ed. World Scientific Publishing Co; Singapore: 2004. [Google Scholar]
- 2.Fung YC. Biomechanics: Mechanical properties of living tissues. Springer-Verlag; Berlin: 1981. p. 433. [Google Scholar]
- 3.Fung YC. Biomechanics: motion, flow, stress, and growth. Springer-Verlag; New York: 1991. p. 569. [Google Scholar]
- 4.Findley WN, Lai JS, Onaran K. Creep and relaxation of nonlinear viscoelastic materials. Dover Publications, Inc; New York: 1989. [Google Scholar]
Figure 1. Basic mechanical terms.
(A) Diagrams illustrating definitions of tension, compression and shear forces on a block of material. Open arrows indicate directions of forces on the block before (left) and after (right) deformation. (B-C) Graphs showing the relationship between stress and strain after application of step changes in stress (B) or after rapid changes in strain (C). The responses of four hypothetical materials are shown: elastic (an ideal spring), viscous (a fluid), and two examples of viscoelastic materials. Also shown in B are simple network models in which the four materials are represented by combinations of springs and dashpots.
Embryonic tissues and their constituent cells are extremely small and delicate requiring the construction of custom devices to measure their rheological properties (see summary in Table 1). Embryonic tissues may deform in direct proportion to the magnitude of applied force (e.g. a linear-response) or may deform non-linearly with force. Additionally, embryonic tissues may resist a force applied along one direction but deform easily if the force is applied along another (e.g. anisotropic response; Moore et al., 1995). Full theoretical descriptions of non-linear anisotropic materials have been developed (Landau and Lifshitz, 1986, Taber, 2004) but so little is known about the basic mechanical properties of embryonic tissues that our review of mechanical properties and methods to measure them will focus on the simple case of isotropic materials that deform linearly to applied forces.
In practice, the coupling between mechanical stress and strain is empirically determined by experiments on how materials and structures change shape or deform in response to applied forces (figure 1B and C). In sections 2.1-2.2 below, we discuss a variety of methods for measuring tissue stiffness as well as the advantages and limitations of each method. In the strictest sense “stiffness” refers to the elastic response of a body to an applied force and depends on the geometry of the body, its material properties, and how it is held in place, e.g. boundary conditions. We could alternatively use the term “modulus” to relate the elastic response of a single material. However, since we discuss both materials and structures in this review we will use the term “stiffness” generically to describe the response of a structure or material to an applied force
As pointed out more than 30 years ago, biomechanical properties of tissues may originate from structures or events at three vastly different length-scales (Wainwright, 1975): 1) the properties of individual molecules, their cross-linking, or entanglements, 2) how proteins are organized into subcellular structures or local complexes, and 3) the organization of cells and extracellular matrix. Connectivity between cells and extracellular matrix and how the cell sheets are attached to each other or anchored (e.g. formation of pressurized hydrostats or reinforced columnar structures; for other examples, Wainwright, 1988) can significantly change the way an embryo responds to internally produced or externally applied forces. Thus, structures at these three scales can dictate stiffness of a whole embryonic tissue consisting of distinct architectural elements themselves assembled from multiple smaller subunits or composite blocks of different single materials.
Techniques used to study embryonic tissues depend critically on the mechanical regime of interest. For instance, to measure the stiffness of extensible ECM proteins like fibronectin single fibers need to be gently extended over only a few nanometers; applying micrometer-scale deformations would rip proteins apart. In another case, the mechanical properties of the whole notochord could only be revealed by experiments with much larger forces and deformations since it is a large structure. There are also technical “gaps” in measuring the stiffness within some intermediate ranges of biomaterial size and stiffness. These gaps occur when the tissues are small, the forces are low, and the required applied strains are high.
Another important question is whether the mechanical property being measured is relevant to the biological process being studied. For instance, the mechanical response of a tissue to stress applied at frequencies higher than 10 Hz (i.e. how the tissue responds to forces applied at 10 oscillations per second) may not be relevant to questions involving morphogenesis. Likewise, the long term plastic response of ECM to an applied force (i.e. how ECM slowly stretches and remodels topologically to relieve internal stresses) may be relevant to slow tissue growth during limb development but not be relevant to individual neural crest cells using the ECM as a substrate for traction.
The time-scale of the biological phenomena should dictate the time-scale of the mechanical measurement. In our lab's studies of morphogenesis we are interested in events that occur on three distinct time-scales. The longest scale covers large-scale tissue movements such as convergent extension. These movements occur over hours as the tissue changes shape by 10 to 20% per hour. Longer time-scales are present in slower developing organisms such as gastrulation in mouse or humans or during organogenesis. For instance, cell and tissue movements that shape the highly branched mammary gland can take several days (Ewald et al., 2008). Slow movements such as these may be driven through the action of cycles of actomyosin contractility and lamellipodial extension and retraction that occur on the time-scale of minutes. Traction forces and the response of tissues to those forces need to be measured on the same time-scale. At the shortest time-scale, cycling of molecular motors in the adhesive “clutch” responsible for cell traction (Chan and Odde, 2008) or rapid cellular responses during mechano-transduction (Na et al., 2008) can occur in seconds or milliseconds. Thus, measurements and the tools to apply forces must match both the length-scale and time-scale of the biomechanical events under study.
In the sections below we will review methods for measuring mechanical properties, the contribution of molecular and cellular materials such as the F-actin cytoskeleton and how ECM and multicellular structures such as the early notochord contribute to mechanical properties of embryos. While many methods have been developed to study mechanics on the sub-cellular and cellular scale (for examples see Van Citters et al., 2006, or Ananthakrishnan et al., 2006) we will limit our review to approaches to measure embryonic mechanics at the tissue-scale.
2.1 How are mechanical properties measured?
Experimental measurements of these complex mechanical properties require methods to mechanically perturb embryonic tissues and observe their response. Thus, experiments require imaging techniques to visualize and record deformations as well as force transducers to record the stresses within perturbed tissues. In order to extract values of the stiffness from these measurements existing theoretical models are used or new quantitative theories developed. For instance, measuring embryonic tissue stiffness with micro-indentation or atomic force microscopy (AFM) requires a model for the contact between the indenter and the tissue. Commonly, the Hertzian model is used but requires experimental validation or estimation of potential errors (Lin et al., 2007). Experimental approaches that substantially violate these assumptions require development of more sophisticated models often based on numerical simulation of the mechanics using finite element techniques (Zamir and Taber, 2004a, Zamir and Taber, 2004b). Our lab's use of micro-aspiration required us to estimate potential experimental sources of error from imperfect imaging conditions and deviations from the assumptions of the theoretical model used to analyze the data (Boudou et al., 2006, Sato et al., 1990, von Dassow and Davidson, 2009). Experimental measurements may produce “numbers”, but without a clear statement of the assumptions or limitations of the methodology those numbers may not be related to the mechanical properties of the tissue. None of the approaches we discuss are perfect, however, it is important to recognize that these “zeroth-order” measurements are essential first steps in exploring the hidden mechanical properties and forces that shape embryonic tissues.
The basic principles of determining mechanical properties require imaging the initial structure, applying a known perturbing force, and then imaging the deformed structure. The ability to image embryonic tissues during application of a defined force is experimentally very challenging. Laser scanning confocal microscopy has been combined with laser ablation (e.g. Hutson et al., 2003, Peralta et al., 2007) for high resolution description of cell and tissue movement after a stressed tissue is cut by the laser. Lower magnification compound or dissecting stereomicroscopes are more suited to visualizing tissue movements after forces are applied by electromagnets (Selman, 1958, Gustafson and Wolpert, 1963) or magneto-fluids (Desprat et al., 2008). In some cases, the magnitude of these forces can be estimated (Selman, 1958, Farge, 2003), but precise, repeatable control of magnetic-field applied forces is difficult. More exotic technologies such as optical coherence tomography (Filas et al., 2008, Boppart et al., 1996), high resolution magnetic resonance imaging (MRI;Papan et al., 2007b, Papan et al., 2007a), or micro-computed tomography (microCT; Metscher, 2009) hold much promise for visualizing dynamic movements of deep tissues within embryos during embryogenesis and could be useful during mechanical testing of embryonic tissues.
In order to deform embryonic tissues, forces in the nanoNewton to microNewton range must be applied to tissues surfaces from 10 to 10,000 square micrometers. Control of such small forces to study morphogenesis and the method of their application are critical to properly interpret the results (von Dassow and Davidson, 2007). Forces or stresses can be applied to embryonic tissues in tension or compression. Tension-based methods require devices to grip tissues without injury (Benko and Brodland, 2007). Uniaxial compression- (Moore et al., 1995, Zhou et al., 2009), suction- (von Dassow and Davidson, 2009), or indentation- (Nerurkar et al., 2006, Zamir and Taber, 2004a) -based approaches do not require tissue grips and are frequently used due to their relatively simple implementation that make possible studies with larger sample sizes.
Each methodology for applying stresses and visualizing deformations has its own strengths and weaknesses (Table 1). Application of tension or compression to explants allows the most precisely defined application of forces but requires removal of tissues from their normal environment. On the other extreme, studies using recoil following tissue ablation are intended to assess in vivo stresses, therefore the forces are not defined by the experimenter. Because material properties are rarely measured independently (Benko and Brodland, 2007), usually only the relative magnitudes of the forces are determined. Micro-aspiration and -indentation allow application of well-defined forces without requiring cutting the tissue of interest out of the whole structure, however, the interpretation of measurements is more complex than for uniaxial stresses.
2.2 From deformation to material properties
With the ability to apply known forces and image the resulting deformation of the embryonic tissue we can quantify the viscous and elastic properties of these tissues (Box 1, figure 1). To determine Young's modulus requires measurements of the stress and the strain. For most cases modulus, stress, and strain are represented by tensors (Landau and Lifshitz, 1986). For the special case of unconfined uniaxial compression the modulus, stress, and strain can be represented by single numbers. Strain is the deformation of the material along the direction of compression; stress is the axial force applied to the tissue divided by the cross-sectional area perpendicular to the direction of the applied force. In practice each of the values of force, area, and length can be measured during an unconfined uniaxial compression test.
The time-dependent Young's modulus measured from an experimental apparatus can also be represented by a viscoelastic material-model consisting of a network of springs and dashpots (Findley et al., 1989). The Standard Linear Solid Model (SLS; ‘viscoelastic: recoverable creep’ model in figure 1B), consisting of a spring (k1) in parallel to a spring (k2) and dashpot (η) in series, captures both the creep and stress-relaxation behaviors exhibited by the dorsal isolate. The springs are linearly elastic and the dashpot is an idealized Newtonian fluid. The form of the time-dependent Young's modulus (also known as the relaxation modulus) of this network is:
| (1) |
where τ = η/k2. Parameters for k1, k2, and η can be estimated by graphical techniques (Findley et al., 1989), a three-point method (Moore et al., 1995), or by using non-linear regression techniques (Dennis et al., 1981). The SLS model represents a simple way to format data so that mechanical properties of different materials, i.e. biological or synthetic, may be compared (Zhou et al., 2009, Levental et al., 2007). The relaxation time constant τ can also help distinguish tissues that behave more like solids from those that act more like liquids (Fung, 1972). It is important to note that use of the SLS model does not imply spring-like structural or fluid-like mechanical elements within tissues.
The SLS model is just one of many models that one can use to describe materials. An alternative description, commonly used in cell rheology, proposes that the mechanical properties of cells and their component cytoskeleton follow a power-law (Hoffman et al., 2006, Trepat et al., 2007) where the Young's modulus, E(t), takes the following form:
| (2) |
Where A and B are fitted parameters. Few materials are perfectly represented by either and SLS model or a power-law model over the full range of possible time scales (Stamenovic et al., 2007). Although the power-law model is a prediction of soft-glassy rheology (Fabry et al., 2001), models such as the SLS model and the power-law model do not imply particular underlying mechanistic processes (see Section 3.5 below). Instead they are merely useful, simple descriptions of the mechanical response over a particular time scale.
2.3 Extracting structural contribution from bulk properties
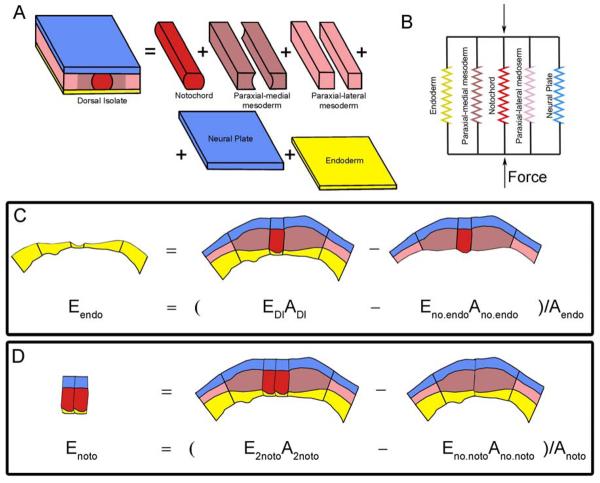
Measurements of tissue stiffness provide data only on the stiffness of intact explants but we have developed methods to infer the relative stiffness of component parts of tissues (Zhou et al., 2009). To quantitatively evaluate the stiffness of tissues that make up the dorsal axis of the early neurula (stage 16) in Xenopus, i.e. the architectural contribution of individual germ layers, we consider the laminar mechanics of composite materials (Christensen, 1991). Considering the contribution of each layer of tissue separately allows us to estimate the contribution of notochord, endoderm, ectoderm, and two domains of prospective somitic mesoderm to the bulk stiffness of an intact block of tissue (figure 2A). Each of these tissues are too small or too thin to measure individually but may be eliminated from a tissue explant by microsurgery. We can remove endoderm from a tissue and compare its stiffness to an intact explant (figure 2C). In another example, we can remove the notochord from a tissue and compare the stiffness with a tissue that has an extra notochord (for a total of 2). We can separate dorsal embryonic tissues into multiple separate layers, each with their own mechanical properties. The separate tissues are bound together as a whole as if they were a set of springs assembled in parallel. Forces (F) applied to the anterior or posterior ends of the dorsal isolate are spread over all the tissue-types that make up the component layers (DI - dorsal isolate, endo - endoderm, MM - medial-most pre-somitic mesoderm, noto- notochord, LL -lateral-most pre-somitic mesoderm, and ecto - ectoderm). Therefore we can write a series of equations where each tissue-type resists an applied strain as if it represented a spring in parallel with springs representing other tissues (figure 2B):
| (3) |
These forces can be re-written in terms of the stiffness of each tissue-type (E) and the cross-sectional area contribution of that tissue-type (A) so the stiffness of the dorsal isolate is a product of all of its constituent tissues and can be written as a single equation:
| (4) |
We can then write an equation for each type of explant, with tissue removed or additional tissues added. Simple algebra allows us to “solve” for the stiffness of the missing or extra pieces (see figure 2C for endoderm example, and 3D for notochord). Measurement of the total stiffness, the stiffness of the explant without the tissue of interest, and their respective areas can be used to estimate the stiffness of the piece of tissue removed by microsurgery. Thus, even though we did not directly measure the stiffness of the piece of tissue that had been excised we can use these equations to estimate its stiffness.
Figure 2. Microsurgery resolves mechanical properties of distinct tissues.
(A) Treating the dorsal isolate as a laminar structure of independent layers allows us to combine microsurgical approaches with the uni-axial compression test to estimate the stiffness of each individual part of the embryo. (B) Each major tissue block can be represented by an individual spring acting in parallel. (C) Simple representation as springs allows us to determine the stiffness of each part by “microsurgical subtraction”; in this case we estimate the stiffness of endoderm in the dorsal isolate (DI). (D) By microsurgical subtraction we can estimate the stiffness of notochord tissue. (Panel A used by permission of the Company of Biologists; Zhou et al., 2009)
It is important to keep in mind, however, that the mechanical properties of complex structures such as the later, tail bud stage notochord (Adams et al., 1990, Koehl et al., 2000; see Section 3.3 below) may not be a simple sum of the properties of the component parts but may require a more careful consideration of how the components are arranged.
3. Cell biology and the mechanical control of morphogenesis
What is the role of cell biology in regulating the physical properties of embryos? Embryonic tissues are composed of densely packed cells and extracellular matrix. The cells are inter-connected by cellular adhesions such as cadherin-mediated cell-cell adhesion or integrin-based cell-ECM adhesion. Force exerted on the tissue is transmitted to each individual cell which then can change its shape or rearranges with its neighbors in response to the stress. Subcellular cytoskeletal structures such as the actomyosin network, intermediate filaments and microtubule meshwork maintain the cell shape. Cadherin-based cell-cell adhesions work as “glue” to stick cells together. Without cellular adhesion, tissues would be like sand piles which cannot support any stress. ECM mechanics may also contribute to tissue stiffness by connecting cells together at tissue boundaries.
In this section we will review what is known about the basis for: i) early assembly of biomaterials in the embryo, ii) structural mechanics of embryonic tissues, iii) differences between epithelial and mesenchymal tissues, and iv) the molecular sources of embryonic tissue mechanics. While detailed studies of mechanics are underway on the subcellular and cellular scale we will limit our review to tissue-scale studies.
3.1 Assembly of biomaterials in embryos
Immediately after fertilization the material properties of embryos are determined by maternal contributions to the egg and post-translational events that reorganize the oocyte after sperm-entry (Hiramoto, 1963a, Hiramoto, 1963b). Precise cycles of softening and stiffening correlate to cycles of cell division within early echinoderm eggs (Hiramoto, 1982) and may reflect changes in cytoskeleton (Lucero et al., 2006) or rapid assembly of ECM (Kim et al., 2006, Alliegro and McClay, 1988). Techniques such as micro-aspiration, micro-indentation, or compression between parallel plates allow measurement of single cell stiffness for the first few rounds of cells division as long as deformation of individual cells can be reliably determined. At later stages when cells are small, micro-aspiration and -indentation can be used to look at tissue stiffness.
As cell division continues an increase in cell-cell adhesion and expression of apical-basal polarity factors is thought to drive the process of compaction (Johnson and McConnell, 2004). During compaction the ‘bundle-of-balloons’ appearance of the early embryo is replaced by a single smooth surface of cell-cell junctions and apical membranes. Compaction occurs in many species from invertebrates to vertebrates and mammals yet the mechanical properties of the cells and embryos during this process are unknown.
Cell division continues after compaction and cleaving cells begin to form discrete multicellular structures consisting of contiguous epithelial sheets and mesenchymal masses with fluid-filled and ECM-rich cavities. In mammals these structures include the trophectoderm, the inner cell mass, and the blastocoel; in frogs these structures include the surface ectoderm, deep mesenchymal cells, and the blastocoel. Neither the transition from compact mass to integrated tissue, the sorting of cells into mesenchymal or epithelial structures, nor the formation of fluid-filled cavities in early pre-gastrula embryos are well understood but may involve formation and maturation of tight-junctions by epithelial sheets, and formation of cell-cell adhesions and polarization by mesenchymal cells for fluid transport and directed secretion (Chen and Gumbiner, 2006, Schwartz and DeSimone, 2008).
The timing of ECM assembly parallels the formation of multicellular structures and the earliest morphogenetic movements in vertebrate embryos (Czirok et al., 2006). Extracellular matrices are assembled during early development in mouse (fibronectin, George et al., 1993, and laminin, Miner et al., 2004), chick (fibronectin, Duband and Thiery, 1982, Sanders, 1982; laminin, Bortier et al., 1989), zebrafish (laminin, Scott and Stemple, 2005; fibronectin, Zhao et al., 2001), Xenopus laevis (laminin, Fey and Hausen, 1990; fibronectin, Lee et al., 1984), Ambystoma mexicanum (fibronectin, Boucaut et al., 1985), Ambystoma maculatum (fibronectin, Johnson et al., 1992), and Pleurodeles waltlii (fibronectin and laminin, Boucaut and Darribere, 1983, Darribere et al., 1986). While specific details of matrix assembly differ between vertebrate species, in general, early synthesis or assembly of ECM appears to be required for the success of vertebrate morphogenetic movements.
We focus here on ECM in the frog Xenopus laevis since ECM assembly and function parallel the events of cell differentiation and morphogenesis shared by developing vertebrate embryos. ECM first forms in frog embryos at the boundaries between major tissue types coincident with the start of large-scale movements (Nakatsuji and Johnson, 1983, Davidson et al., 2004). Extracellular matrix first forms a sparse felt-like network becoming denser over a few hours (Davidson et al., 2008). The earliest ECM in the frog is predominately fibronectin but within a few hours fibrillin (Skoglund et al., 2006) and laminin (Krotoski and Bronner-Fraser, 1990, Davidson and Wallingford, 2005) are recruited to regions undergoing active morphogenesis and differentiation.
Extracellular matrix is essential for morphogenesis in the frog (Davidson et al., 2002b, Davidson et al., 2006, Marsden and DeSimone, 2001, Ramos et al., 1996, Rozario et al., 2008, Skoglund and Keller, 2007, Boucaut et al., 1984). Many authors (Davidson et al., 2004, Keller et al., 2008) have hypothesized that a major role of ECM components, such as fibronectin and fibrillin, is to stiffen the embryo so that it can support forces of extension. To test whether ECM contributed to the mechanical properties of early frog embryos (late gastrula to mid-neurula stages; st. 13 to 16) the stiffness of explants was measured by compressing them along the anterior-posterior direction when the ECM was disrupted (Zhou et al., 2009). No difference in stiffness was detected between controls and embryos in which FN, laminin and fibrillin were all disrupted by knocking down FN expression or removed by microsurgery. This indicates that these ECM components are not major contributors to tissue stiffness at early embryonic stages in Xenopus. Collagen, which is a major contributor to notochord stiffness at later tailbud (St. 21-28) stages of development (Adams et al., 1990), was not found at these early stages. Furthermore, the physical formation of an elaborate fibrillar fibronectin matrix is not essential for convergent extension (Rozario et al., 2008). Thus, fibronectin does not appear to transmit forces over long distance and instead appears to play either a signaling role directing cell traction or play a role in transmitting forces at cell-cell interfaces.
In contrast to the early frog embryo, ECM is central to the mechanical properties of several other embryonic tissues. For example, in late blastula stage sea urchins, the external ECM is the major contributor to embryo stiffness and is ≥ 6 times stiffer than the underlying cells (Davidson et al., 1999). In chick embryos, a very soft ECM known as cardiac jelly (primarily hyaluronan) makes up a major fraction of the volume of the developing heart (Zamir and Taber, 2004a). ECM remodeling accompanies morphogenesis in both mouse (Miner et al., 2004) and chick embryos (Czirok et al., 2006, Zamir et al., 2008) but the contribution of ECM to the mechanical properties of these embryos is unknown.
Continued development of the frog embryo into a feeding tadpole consists of straightening, growth, and elaboration of internal organs. Several studies on the mechanics of organogenesis have focused on early steps shaping the chick heart from a single tube (Nerurkar et al., 2006, Voronov and Taber, 2002, Zamir et al., 2003), formation of the gut (Davis et al., 2008, Kurpios et al., 2008) during chick development, and tracheal tube formation in fly (Cheshire et al., 2008, Affolter and Caussinus, 2008). Further development of analytical and experimental tools is sorely needed to test the role of mechanics during these and other events during organogenesis.
3.2 Structural sources of embryonic tissue rheology
How molecular-, cellular-, and tissue-scale structures regulate the mechanical properties of embryonic tissues is poorly understood. For instance, consider the role of ECM. Single fibers of ECM proteins are often very stiff in tension but offer no resistance to compression (Vincent, 1990). Sheets formed from cross-linked or entangled networks of ECM fibers often exhibit non-linear stiffening in tension as more and more fibers align and resist extension (Stella et al., 2008), yet, even dense sheets of ECM fail or collapse under compression. Such failure may be prevented if ECM is integrated with surrounding cells. Such integration can allow cellular tissues reinforced with ECM to convert compressive forces acting on the tissue into distributed tensional-forces acting on the ECM. Key to this conversion is the control of intracellular and interstitial fluid movement.
Control of fluid movement is essential to the control of mechanical properties in plants (Niklas, 1992) but is not well studied in animals. Fluid movement could regulate tissue visco-elasticity by slowing or speeding the flow of fluid in and out of cells or through epithelia that enclose the embryo. Viscous responses to deformation could be due in part to fluid flow within, into, or out of cells. Active pumping of fluid may also stiffen tissues in the same way that turgor pressure increases stiffness in plants. Active pumping of fluid into the frog blastocoel may create hydrostatic pressure within the embryo that stretches the epithelium (Benko and Brodland, 2007) or closely associated ECM basement membranes. A similar process in echinoderms is thought to contribute to the stiffness of the embryo prior to gastrulation (Moore, 1941) and may explain why interfering with osmotic pressure of the external media perturbs formation of the archenteron (Takata and Kominami, 2001b, Takata and Kominami, 2001a). In addition to possibly controlling the mechanical properties of cells or tissues, control of ECM gel swelling by hydration might drive epithelial buckling during the first steps of gastrulation (Lane et al., 1993, Davidson et al., 1995).
3.3 An example integrating materials and structure into mechanical function: stiffening the late notochord
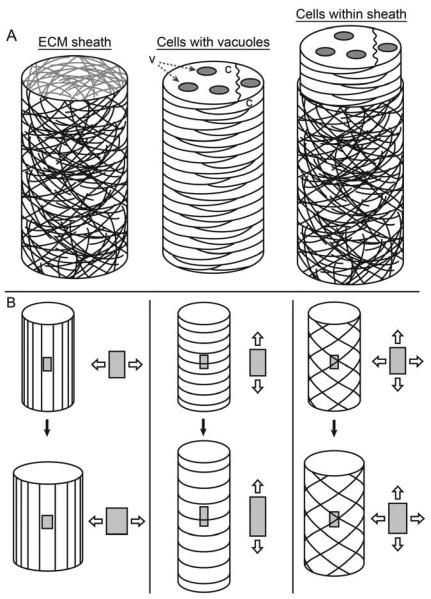
To understand how tissue stiffness may depend on multiple scales we consider the example of the tail bud stage notochord in frog and fish embryos. Early during gastrulation notochord cells separate from surrounding tissues and join together into a column extending from the anterior end to posterior end of the embryo. The notochord is a composite structure consisting of cylinder of tightly-associated stacked notochord cells and a laminar ECM wrapped around the outer surface of the stack (figure 3A). Individual notochord cells contain vacuoles that are filled with glycosaminoglycans that hydrate and swell. Studies by Koehl and co-workers (Adams et al., 1990, Koehl et al., 1990, Koehl et al., 2000) showed that increased osmotic pressure within the notochord could straighten and stiffen the notochord, possibly contributing to straightening the embryo. Stiffening allows the notochord to straighten without buckling (Adams et al., 1990). This stiff notochord ‘backbone’ later provides attachment points for skeletal muscle (Hoff and Wassersug, 2000).
Figure 3. The notochord as a hydrostat.
(A) The Xenopus notochord consists of a fibrous sheath (left), surrounding a column of flattened cells, “c”, with vacuoles, “v” (middle). The stiffness of the whole (right) requires both components. (B) Fiber orientation affects how the hydrostat changes shape as it inflates. Diagrams illustrate a cylinder wrapped by fibers (dark lines) before (top) and after (bottom) inflation. Insets show changes in shape of a patch of the surface. Left: Longitudinal fibers resist axial extension, but not radial expansion; middle: circumferential fibers allow axial extension, but resist radial expansion; right: fibers oriented at an intermediate angle can resist both extension and expansion. Note that the relationships between fiber orientation and shape change are more complex than illustrated here.
Fibrillar ECM sheaths wound around swelling tissues can create hydrostat-like structures that resist deformation by external forces. Cylindrical hydrostats are common in nature providing stiff structures for plants and animals (Wainwright, 1988). Three mechanical features of the notochord appear to work together allow it to stiffen (figure 3A; Adams et al., 1990, Koehl et al., 2000): 1) Glycosaminoglycans in vacuoles hydrate, swelling the notochord cells. 2) The ECM sheath is put under tension by the swelling cells. Hence the pressure in the notochord increases, thereby stiffening the notochord. 3) The diameter of the notochord contributes to flexural stiffness: it's easier to bend a thin beam than a wide beam (Koehl et al., 2000, Wainwright et al., 1976). Much like a water balloon, the fluid within the notochord and the fibrous ECM sheath are unable to resist compressive forces on their own, but when integrated into a structure they can. The physical properties of the glycosaminoglycans in the vacuoles, the arrangement of cells and ECM assembled around them, and the architecture of the fiber-wound cylinder allow the notochord to stiffen and straighten.
The material properties of both the vacuoles and the ECM sheath regulate the mechanical properties of the notochord. Osmotically reducing pressure within the notochord weakens the flexural rigidity, a measure of stiffness, of the notochord (Adams et al., 1990). Enzymatic digestion of the ECM sheath reduces its ability to maintain the shape and size of the notochord (Adams et al., 1990). The orientation of fibrils within the ECM sheath plays a critical role in determining changes in notochord shape when vacuoles swell within the sheath (figure 3B). If reinforcing fibrils were aligned parallel to the anterior-posterior axis the notochord would widen but resist elongation. If the fibrils were aligned with the circumference of the notochord, the notochord would elongate but resist widening. A mix of fibril orientations could allow the notochord to resist widening and elongation (Adams et al., 1990, Koehl et al., 2000). The late stage stiffening of the notochord provides an example where the mechanical function depends critically on how cells and ECM are assembled into the notochord.
In principle, the strength of cell-cell adhesions during inflation of the notochord may not be important but cell-cell adhesion may be a pre-requisite to positioning notochord cells in the earlier formative stages of the notochord. Mis-positioned notochord cells may well alter the course of ECM assembly and consequently alter the behavior of the notochord/sheath when vacuoles swell. Genetic analysis has revealed multiple mutant lines of zebrafish that develop with kinked or warped notochords (Stemple, 2005). The Chongmague mutation in the ECM protein laminin in ascidians embryos, a group of invertebrate animals closely related to vertebrates that construct a notochord, develops a kinked notochord after vacuoles swell (Veeman et al., 2008) reminiscent of the defects produced by digestion of ECM in Xenopus (Adams et al., 1990). This example demonstrates how physical properties of materials, their organization, and their architectural assembly enable a tissue to serve a mechanical function.
3.4 Epithelial vs. mesenchymal mechanics in embryos
Mesenchymal embryonic tissues differ from epithelial tissues in both the underlying cellular processes and physical mechanics. Epithelial cells form as sheets of cells connected into a tensile array at their apical ends. This tensile array can provide mechanical continuity allowing forces to be transmitted through apical cell-cell junctions. In contrast, mesenchymal cells are loosely connected by sparse, more dynamic cell-cell adhesions. Thus, mesenchymal cells are more likely to exchange neighbors, detaching from one cell to form new attachments with another, than cells within epithelial sheets and can leave the plane to create three-dimensional solid structures and organs. The increased freedom of movement by mesenchymal cells is reflected in the variety of cell behaviors and tissue movements exhibited by mesenchymal tissues. Mesenchymal cells can maintain laminar sheets similar to those maintained by an epithelium. The deep layer of the ectoderm and neural plate epithelium, both mesenchymal tissues in Xenopus, form single laminar sheets of cells (Hausen and Riebesell, 1991). Mesenchymal tissues within the dorsal and ventral leaflets of the pre-somitic paraxial mesoderm also form into single-cell layers (Keller, 2000). Cells within these mono-layered mesenchymal sheets associate with an epithelium or an extracellular matrix and often behave like epithelial cells. For instance, pre-somitic mesoderm as well as deep neural cells can take on wedge shapes as dramatic as those exhibited by apically constricted epithelial cells in the neural groove. In addition to these epithelia-like cell behaviors, mesenchymal cells also engage in directed cell migration and are not restricted to moving within mono-layers (Keller et al., 2000, Davidson et al., 2002b, Carmona-Fontaine et al., 2008). Mesenchymal morphogenesis often appears more complicated than epithelial morphogenesis but is equally important to the generation of embryonic form.
Due to the relative simplicity of epithelia in early embryos of the fruit-fly Drosophila, a growing set of experimental studies combine genetic manipulation, biophysical modeling, and laser micro-dissection to probe the connection between genetics and cellular mechanics during epithelial morphogenesis (Hutson and Ma, 2008) during mesoderm induction (Desprat et al., 2008, Farge, 2003), germ band elongation (Bertet et al., 2004, Blankenship et al., 2006, Rauzi et al., 2008), dorsal closure (Toyama et al., 2008, Peralta et al., 2008), eye formation (Hayashi and Carthew, 2004), and maintenance of embryonic epithelia (Farhadifar et al., 2007, Gibson et al., 2006). Many of these approaches have not yet been applied to the mechanics of mesenchymal morphogenesis where the principles of cell connectivity and transmission of force remain open questions.
Most studies on the mechanical basis of mesenchymal morphogenesis focus on answering two basic questions: What proteins do cells use to “know'” or orient to where they must go? and, What proteins do cells use to get there? Each instance of mesenchymal morphogenesis appears to use a specialized molecular mechanism to orient the cell and establish polarity. For instance, during mediolateral cell intercalation, movements are directed by the Planar Cell Polarity (PCP) pathway (Wallingford et al., 2002). Formation and maintenance of laminar mesenchymal sheets are directed by extracellular matrix (Rozario et al., 2008, Davidson et al., 2006, Marsden and DeSimone, 2001) and nearby epithelial sheets (Hyodo-Miura et al., 2006, Ninomiya and Winklbauer, 2008) and are mediated by integrin- and PAR-signaling pathways respectively. It is clear that polarity-factors influence morphogenesis but how these processes alter the mechanical properties of embryonic tissues remains to be determined.
3.5 Molecular sources of embryonic tissue rheology
Ultimately, the developmental biologist wants to understand how the mechanical properties of tissues are controlled during morphogenesis. Certainly, the physical mechanics of development are controlled through genes. Embryos begin the process of differentiation by expressing unique sets of genes in spatially restricted domains; these genes regulate further differentiation and control the rheological properties of the cells and extracellular matrix that form multicellular structures (Zhou et al., 2009). Our own studies have shown that tissue layers in the developing frog embryo differ in stiffness 9-fold suggesting tissue stiffness is developmentally regulated (Zhou et al., 2009).
While this section aims to review the mechanical properties of tissues we need to briefly consider the properties of biopolymers found inside cells. The rheology of F-actin, microtubules, intermediate filaments are strongly dependent on their 3D conformation, how filaments are bundled or cross-linked, and whether active motors, such as myosin II, remodel the polymer networks (Janmey et al., 2007). These molecular factors are principle targets of gene regulatory networks that control the identity and function of differentiating cells (Sinner et al., 2006). How biopolymer rheology regulates tissue rheology is poorly understood, however, work from our lab has begun to illustrate how actomyosin, fibronectin ECM, and structural stiffness are related (Zhou et al., 2009). Perhaps not surprisingly, inhibition of F-actin polymerization reduces tissue stiffness as does inhibition of myosin II contractility. The regulation and mechanical contribution of many additional “mechanically-relevant” proteins remain to be tested.
The mechanical properties of living animal cells are dependent on the actin cytoskeleton and disruption of the actin network decreases the elastic modulus of the cell (Ananthakrishnan et al., 2006, Kidoaki et al., 2006, Wakatsuki et al., 2000, Wakatsuki et al., 2001). Actin-filament crosslinking or bundling proteins such as filamin-A, α-actinin, and fascin have been shown to increase stiffness of cultured cells (Coughlin et al., 2006, Cunningham et al., 1992, Tseng et al., 2005) but their role in embryonic tissues is unknown. The actin cytoskeleton is also thought to sense substrate stiffness through integrins and cadherins providing feedback to cells (Discher et al., 2005, Engler et al., 2008, Engler et al., 2006, Lo et al., 2000, Pelham and Wang, 1997, Peyton and Putnam, 2005, Schwartz and Ginsberg, 2002, Schwartz and DeSimone, 2008). Few of the principles that regulate F-actin rheology in cytoplasmic extracts (Valentine et al., 2005) or reconstituted gels (Gardel et al., 2006) have been verified within intact embryonic tissues.
Myosin activity is also essential in generating traction forces and cell stiffness (Bhadriraju and Hansen, 2002). Inhibiting myosin activity decreased traction force (Beningo et al., 2006) and cortical stiffness (Pasternak et al., 1989, Engler et al., 2006), while increasing myosin activity increased cortical stiffness (Obara et al., 1995). The state of actomyosin contractility is regulated by Rho family small GTPases such as Rho, Rac and Cdc42, which are in turn controlled by the planar cell polarity pathway (Habas et al., 2003, Tahinci and Symes, 2003). Activation of Rho increases stress fiber assembly and actomyosin contraction force through Rho kinase (ROCK) and diaphanous-related formin, mDia1 (Pellegrin and Mellor, 2007). Our studies show that ROCK inhibitors such as Y-27632 can reduce stiffness of Xenopus embryonic tissues by 50% (Zhou et al., 2009) but little is known concerning the contribution of other Rho GTPases to mechanical properties.
The role of microtubules in determining cell stiffness is controversial: disruption of microtubule network may decrease, increase or have no effects on cell stiffness and may depend on the extent of cell distension (Potard et al., 1997, Sato et al., 1990, Stamenovic, 2005, Stamenovic et al., 2002, Wang, 1998, Trickey et al., 2004, Wu et al., 2000). To complicate matters, microtubules can also regulate actin organization and myosin II contractility (Danowski, 1989). Thus, there are at least two models proposed to explain the effects of microtubules on tissue stiffness. The tensegrity model proposes that eliminating stiff microtubules increases actomyosin contractility (Wang et al., 2001). Alternatively, microtubules may regulate actomyosin contractility through molecular signaling pathways. For example, microtubules physically bind and inactivate GEF-H1, which is a Rho guanine nucleotide exchange factor. When microtubules are depolymerized, released GEF-H1 increases RhoA activation and subsequently phosphorylates a myosin II regulatory light chain (Chang et al., 2008). Further work is needed to resolve the controversial role of microtubules in tissue mechanics.
Intermediate filaments represent another major cytoskeletal component, which provide structural support to the cytoplasm and nucleus of cells and thus affect cellular stiffness (Coulombe et al., 2000, Hutton et al., 1998, Janmey et al., 1991, Wang and Stamenovic, 2000). Intermediate filaments have been shown to interact with actomyosin (Esue et al., 2006, Tint et al., 1991) and microtubule networks (Chang and Goldman, 2004). Keratins, a type of intermediate filament present in Xenopus ectodermal layers, are required for gastrulation in Xenopus laevis (Torpey et al., 1992, Klymkowsky and Parr, 1995). Studies in cultured endothelial cells indicate vimentin, a type of intermediate filament also present in Xenopus mesenchymal layers, can play a major role in mechano-transduction and adaptation to fluid flow (Helmke et al., 2000, Mott and Helmke, 2007, Helmke et al., 2003). However, no studies have investigated the physical mechanical roles of these key cytoskeletal elements in generating force or maintaining stiffness in embryonic tissues.
Cadherin mediated cell-cell adhesion is important to maintain tissue integrity during development, to transmit forces from cell to cell, and to integrate cell-generated forces at the tissue level (Hammerschmidt and Wedlich, 2008). Mutant cadherin proteins which only contain the Catenin-Binding Region (CBR) of cadherin cause a decrease in cell-cell adhesion and even cause cell dissociation of entire embryos at high levels (Espeseth et al., 1998, Kintner, 1992, Riehl et al., 1996). Cadherin expression regulates the apparent surface tension in cell aggregates (Foty and Steinberg, 2005, Forgacs et al., 1998) indicating cadherin mediated cell-cell adhesions play important roles in establishing or maintaining tissue mechanical properties. It is not known whether cadherins affect mechanical properties directly through cell-cell adhesion, or indirectly via the actin cytoskeleton (Kofron et al., 2002, Tao et al., 2007, Tao et al., 2005) in the embryo.
ECM directly contributes to the mechanical properties of tissues such as adult blood vessels, late-embryonic stage notochord, and gastrula-stage sea urchin embryos, and may indirectly contribute to tissue mechanics by regulating cell contractility (Engler et al., 2008, Pelham and Wang, 1997, Peyton and Putnam, 2005, Brooke et al., 2003). Cells may exert forces on their surrounding ECM substrates to generate internal cytoskeletal tension (Wang et al., 2002). Cell stiffness also depends on substrate stiffness (Engler et al., 2006, Solon et al., 2007). Thus raising the stiffness of the underlying ECM may increase the stiffness of closely associated cell layers (Paszek et al., 2005).
Besides acting as mechanical scaffolds, there may be crosstalk between the ECM, cell-cell adhesions and cytoskeletal networks. For example, in Xenopus, fibronectin has been demonstrated to modulate cadherin mediated adhesive activity through integrins (Marsden and DeSimone, 2003, Dzamba et al., in press), while cell-cell and cell-ECM adhesion may be coupled together by the cortical actin network (Skoglund et al., 2008). In addition, over-expression of cadherin may directly down-regulate synthesis of ECM (Finnemann et al., 1995). Furthermore, the actin network is affected by both the microtubule network and C-cadherin expression (Kwan and Kirschner, 2005, Tao et al., 2007). Both cell-cell and cell-matrix adhesion may regulate cytoskeletal effectors through Rho family GTPases (Arthur et al., 2002). With the many opportunities for cross-talk investigation of mechanical roles for these proteins will need to be coordinated with parallel molecular or biochemical studies to evaluate novel interactions that regulate mechanics.
In this section we have outlined what little we know about the molecular regulation of physical mechanical processes in embryonic tissues and highlighted where future research is needed. Clearly, new advances in cell biology obtained through genomics, proteomics, and other high throughput strategies will require complementary studies into the physical roles of key genes required during morphogenesis.
4. Big questions
There is a huge literature on the mechanisms of morphogenesis extending over 130 years. The earliest studies pre-date both molecular descriptions of development as well as modern analyses of biomaterials. The molecular description of morphogenesis is now an advanced experimental field whereas the mechanical analysis of morphogenesis is in its infancy and exists predominantly as a theoretical field. Renewed interest in the mechanics of morphogenesis also comes from the field of cell mechanics and biopolymer dynamics which have both been transformed in the last 10 years into lively experimental fields.
In this section we pose several questions about the mechanics of morphogenesis that can be addressed by combining classical and modern approaches. These questions arise in part from gaps exposed in previous sections and from reconsidering the fundamental roles of mechanics in development raised by recent work on the role of mechanics in guiding progenitor cell differentiation (McBeath et al., 2004, Engler et al., 2006) and in modulating the growth and spread of cancerous tumors (Lopez et al., 2008).
Question #1: Are the mechanical properties of the embryo actually important to morphogenesis?
Based on simple physical arguments we and others have assumed that morphogenesis should be strongly sensitive to the mechanical properties of tissues. Since morphogenesis involves deformations of materials, and deformation depends on the mechanical properties of the deformed material, how could morphogenesis not be sensitive to tissue mechanical properties? Yet several studies hint that mechanical properties are extremely variable among normal embryos (reviewed in von Dassow and Davidson, 2007). Recently we investigated natural biological variability in stiffness in gastrula stage Xenopus laevis embryos (von Dassow and Davidson, 2009). We found that stiffness varied by at least two fold among embryos that successfully completed gastrulation, indicating that morphogenesis can be surprisingly insensitive to variation in tissue stiffness. High levels of variation in apparent tissue surface tension between batches of X. laevis embryos has also been reported (Kalantarian et al., 2009). As we have discussed elsewhere, several physical and biological mechanisms could allow such robust development despite mechanical variability (von Dassow and Davidson, 2007, von Dassow and Davidson, 2009).
To understand how tissue mechanical properties contribute to morphogenesis we need to address the following questions: How mechanically variable are embryos or individual cells of the same type? Does perturbation of tissue mechanical properties also perturb morphogenesis? Finally, what mechanisms allow morphogenesis to be so robust to mechanical variation?
Addressing the first question of how variable are embryos will provide a limit on the sensitivity of morphogenesis to stiffness. It requires careful experimental designs that allow natural variation in mechanical properties to be separated from experimental noise, and to determine the range of mechanical properties within which embryos can undergo normal morphogenesis. Addressing the second question of whether defects in morphogenesis are due specifically to mechanical-defects requires taking account of the fact that any perturbation of stiffness could perturb force production or cell identity. The challenge is to perturb stiffness using multiple different pathways and then test whether the effect is specific to a particular pathway or instead is a result of the change in stiffness. Ideally, one could carry out a “mechanical rescue” of the mutant phenotype by restoring the original stiffness through an independent molecular pathway. To answer the last question on the robustness of morphogenesis is likely to require a combination of theoretical and experimental studies aimed at identifying whether there is a link between force production and tissue stiffness in the embryo and, if so, what is the nature of that link.
Question #2: What scale is important, or at what scale are mechanical properties shaped?
When considering contribution of molecular, cellular, and tissue scale structures to mechanical properties we need to consider the multiple length-scales that cells interact with their local environment. For instance, individual sites of cell adhesion may be as small as the width of a focal adhesion to a single ECM fibril or may be as broad as the cell's entire lateral face of cell-cell adhesions. While tensile forces may act locally through junctional complexes, compressive forces may not, instead being applied over a cell's surface or deforming internal organelles like the nucleus or endoplasmic reticulum. Shear forces require even more complex consideration as these may be transmitted across both surface areas and through junctional complexes. These questions need to be posed and the appropriate scale chosen before any experiments are carried out. For instance mapping the stiffness of regions with a nanometer-scale probe such as an AFM tip may resolve the stiffness of small and sparse attachment sites like stiff ECM fibrils embedded in a low stiffness gel of proteoglycans. However, an AFM tip cannot generate large compressive stresses over broad areas. Similarly, magnetic or fluorescent beads embedded within the cytoskeleton of single cells may report the mechanical conditions of the local nano-environment but may not be useful descriptors of the macroscopic mechanics that propels tissue masses against one another. Similarly, when considering the sources of mechanical stiffness or strength the choice of the scale of the measurement depends critically on the hypothesis. For instance testing the role of adhesion proteins on stiffness may require testing both bulk-stiffness of the whole tissue and the stiffness of apical cell junctions with a higher resolution technique.
The late stage notochord (discussed in section 3.3 above) provides an excellent example of the importance of scale in the measurement of mechanical properties in embryos (Adams, Keller and Koehl, 1990). The capacity of the notochord to resist bending as it extends the embryo comes from the structure of the whole notochord. Measurements at the level of the individual collagen fiber or fluid filled cell that make up the structure would not reveal the mechanical properties of the whole notochord. However, in combination with theoretical and structural analyses, these measurements could help reveal the mechanisms that give rise to the mechanical properties of the notochord and extend our understanding of its role in shaping the embryo.
Question #3: Can we separate processes that generate force from those that make tissues stiff?
Morphogenesis can be surprisingly insensitive to variation in tissue stiffness (discussed in Question #1 above). One possible mechanism for this would be that cells within stiffer tissues exert larger forces. Many studies on cultured cells indicate that cells exert higher forces on stiff substrates than they do on soft substrates (Lo et al., 2000, Wang et al., 2000, Paszek et al., 2005, Saez et al., 2005). Such mechano-transduction could serve to make development robust despite mechanical variability. The central role of actin and myosin in both generating and resisting forces might also contribute to this by linking force production to cell stiffness: increased levels of polymerized actin or higher levels of myosin activity might simultaneously drive increased force production and increased stiffness without any need for feedback. Exploring the links between stiffness and force generation is therefore a fundamental question in morphogenesis.
A different sort of link between stiffness and force production will also be important in designing experiments to measure stiffness. Depending on the type of test done, the stresses on a material can strongly affect its apparent stiffness (Zamir and Taber, 2004a, Zamir and Taber, 2004b). In addition, stress can affect the stiffness of structures in other ways, as we discussed in section 3.3, hydrostats require internal pressure to be stiff, and fibrous materials may be much stiffer when stretched a lot than when stretched a little. Hence, mechanical tests should be done under as close to in vivo stresses and strains as possible.
The question of whether one can distinguish “active” from “passive” mechanical properties comes up repeatedly in cell and developmental biomechanics. Cells are under internal pre-stresses and generate traction forces on their substrates (Wang et al., 2002, Stamenovic et al., 2002). Embryonic tissues actively generate force to deform themselves and their surroundings, and are under stresses produced by their surrounding tissues (Benko and Brodland, 2007, Hutson et al., 2003). Cell stiffness may depend on pre-stress (Wang et al., 2002), as can apparent tissue stiffness (Zamir and Taber, 2004a, Zamir and Taber, 2004b). Both stiffness and force generation require cellular energy supply since both actin polymerization and myosin II contraction depends on ATP (Alberts et al., 2008). Finally, several molecular pathways (e.g. Ca++, Rho-family GTPases, etc.) that control actin polymerization, cross-linking, and myosin activity are shared. Hence “active” and “passive” processes may not be easily defined, much less distinguished.
Disentangling the contributions of underlying tissue architecture, complex material properties, and pre-stresses, requires comparing experimental data to theoretical models that can predict the behavior of complex tissues constructed from various materials. Models have been developed that can distinguish simple surface tension-like and simple solid-like behavior of cells using micro-aspiration (Hochmuth, 2000); and models have been developed that can distinguish the role of pre-stress from elastic material properties in micro-indentation experiments for specific tissue arrangements (Zamir and Taber, 2004b). Given the compound nature of the problem and lack of knowledge about embryonic tissue mechanics, we advocate starting with the simplest mechanical models possible that can still capture the major features under investigation; working with them until we know how they fail; and then adding more realistic features in small steps. The developmental biomechanist is often concerned only with first order questions (e,g, does protein X contribute something to the mechanical properties of the system?), so simple models will often be adequate.
Currently, measurements of forces produced by embryos are even more rare than measurements of tissue mechanical properties (Selman, 1958, Moore, 1994, Benko and Brodland, 2007). Several studies in Drosophila have explored the relative magnitudes of forces using laser ablation (Peralta et al., 2007) but cannot as yet resolve the absolute magnitude of forces, as required for exploring the relationships between force and stiffness. Several studies have measured strains following laser ablation (Hutson et al., 2003, Peralta et al., 2007, Kiehart et al., 2000, Toyama et al., 2008), or cutting (Jacobson and Gordon, 1976, Davidson et al., 2002a, Beloussov and Grabovsky, 2006, Benko and Brodland, 2007, Zhou et al., 2009). From these studies it appears that the cytoskeleton can exert high forces over short time scales, for instance, during recoil of a wounded epithelial sheet after incision by laser-ablation. Such high forces applied over short time-scales may induce non-linear or major changes in the material properties; sudden high forces may induce signaling cascades (Na et al., 2008) or sudden forces or high strains may “liquify” the cytoskeletal network (Gardel et al., 2008, Janmey et al., 1990).
Hence, exploring the link between force production and stiffness requires development of methods for measuring forces produced by embryonic tissues. Future studies need to determine (1) whether substrate stiffness affects force production in embryonic cells in their native environment; (2) whether embryonic tissues exert higher forces when mechanical resistance of their surroundings is greater; (3) whether the stiffness of the tissue itself is related to how much force it can generate.
Question #4: How much mechanical ‘cross-talk’ is there between a cell and its micro-environment?
Cells are a major component of early embryonic tissues so they serve as a major fraction of the substrate for each other. Intriguingly, in culture studies cell stiffness can vary with the stiffness of the substrate (Engler et al., 2006, Solon et al., 2007). Hence cells may adjust their stiffness based on the stiffness of their neighbors. Additionally, traction forces exerted by cells on their substrate and the speed of cell movement depend on substrate stiffness, and the pattern of substrate stiffness can determine the direction of cell movement (Lo et al, 2000; Saez et al, 2007). Thus, tissue stiffness, force generation, and cell movement could be controlled by a complex set of feedback loops between the cell and its environment.
However, there are several important caveats when extrapolating findings on complex feedback loops from cultured cell studies. Most importantly, few principles of mechano-transduction have been demonstrated in developing embryos. In contrast to single cultured cells isolated on homogeneous substrates, all cells in the embryo interact with other neighboring cells and a heterologous complex ECM. Previous studies suggest that cell-cell interactions can alter or eliminate the effects of substrate stiffness (Yeung et al., 2005), although other studies (e.g. Guo et al., 2006, or Paszek et al., 2005) still found strong effects of substrate stiffness on aggregated cells. Cellular responses to substrate stiffness also depend on cell type (Yeung et al., 2005, Wang et al., 2000, Georges et al., 2006). Vertebrate embryos are extremely soft in comparison to most adult tissue types (Moore et al., 1995, von Dassow and Davidson, 2009, Zhou et al., 2009) and many differentiated cells cannot be cultured on such soft substrates. Hence, it remains unknown whether embryonic cells respond to substrate stiffness under the conditions they encounter in the embryo the same as cultured cells do.
Question #5: How does histology relate to mechanical properties?
Immunofluorescence and ultra-structural analyses of tissues can provide tempting hypotheses about the sources of stiffness. However, our own studies have shown us how misleading assumptions based on histology can be. Consider the role of fibrillar fibronectin, the first extracellular matrix in the frog embryo. Histology of frog embryos reveal assembly of fibronectin fibrils coincide with the start of gastrulation and that remodel into a “sheath” surrounding prospective somites prior to neurulation (Davidson et al., 2004). Furthermore, other ECM proteins such as fibrillin (Skoglund et al., 2006) and laminin (Krotoski and Bronner-Fraser, 1990) join fibronectin at the dorsal midline. The onset of ECM assembly coincident with gastrulation and axis elongation suggested a major mechanical role for ECM in these processes. However, direct tests of explant stiffness (Zhou et al., 2009) and inhibition of fibril assembly, leaving the signaling function of fibronectin intact (Rozario et al., 2008) suggest a more complex role for fibronectin during morphogenesis. Our work on the contribution of ECM to mechanical properties of gastrulating frog tissues contrasts with earlier work on the contribution of ECM to the properties of sea urchin blastula and tail bud stage frog notochord. Tests of the stiffness of the ECM in sea urchin embryos demonstrated that reducing ECM produced a commensurate reduction in the stiffness of epithelium in urchins (Davidson et al., 1999), however, the case of the early frog embryo, fibrillar ECM does not appear to contribute to the bulk stiffness of embryonic tissues (Zhou et al., 2009). These two apposed findings highlight an important caveat that the specific mechanics of morphogenesis in one organism might not extend to another. This caveat highlights an important point: even though molecular mechanisms of development may be conserved, the mechanical processes of morphogenesis and organogenesis may be quite different yet critical to the generation of diverse animal forms and the creation of specialized physiological adaptations.
5. Conclusion - integrating mechanics with molecular genetics of morphogenesis
In this paper we have reviewed biomechanical principles of morphogenesis, presented examples of the application of these principles drawn from our own and other's work on frog embryos, and suggested a set of questions that need to addressed by theorists, developmental biologists, and biomechanicians. This review was not intended to summarize cellular mechanisms of morphogenesis, nor was it intended to review the extensive field of cell mechanics and biopolymers. In these fields there is now extensive biophysical theory, molecular biology perturbations, and detailed empirical rheology studies. However, we take from these allied fields the notion that experimental manipulation of embryogenesis, visualization of protein dynamics in embryos and embryonic tissues, and measurement of biophysical properties will need to be coordinated to understand the principles of mechanics in embryos.
Multi-scale modeling efforts are being adopted to make sense of the complex observations of morphogenesis in both invertebrates (eg. Farhadifar et al., 2007) and vertebrates (eg. Chen and Brodland, 2008). Modeling serves several distinct functions: 1) to help interpret biomechanical experiments, 2) to demonstrate the plausibility of hypotheses, and 3) to identify key physical parameters that are crucial to the success of morphogenetic movements. Meeting the first goal of modeling involves building simple models to account for single studies. The second goal of modeling is to extend human intuition to the world of viscoelastic materials and complex mechanics. The last function serves to focus experimental efforts toward testable hypotheses. In all cases models must seek more than to mimic morphogenesis. The best and most fruitful of these efforts will rigorously capture the self-organizing principles of cell mechanics, yet be easy-to-use and flexible enough to allow experiments by practicing developmental biologists (Schnell et al., 2008).
abbreviations and acronyms
- AFM
atomic force microscopy
- ECM
extracellular matrix
- MRI
magnetic resonance imaging
- PAR
proline and amino-acid rich
- PCP
planar cell polarity
- ROCK
Rho Kinase
- SLS
Standard Linear Solid
References
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110(1):115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- Affolter M, Caussinus E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development. 2008;135:2055–2064. doi: 10.1242/dev.014498. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; New York: 2008. [Google Scholar]
- Alliegro MC, McClay DR. Storage and mobilization of extracellular matrix proteins during sea urchin development. Developmental Biology. 1988;125:208–216. doi: 10.1016/0012-1606(88)90074-7. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan R, Guck J, Wottawah F, Schinkinger S, Lincoln B, Romeyke M, Moon T, Kas J. Quantifying the contribution of actin networks to the elastic strength of fibroblasts. J Theor Biol. 2006;242:502–516. doi: 10.1016/j.jtbi.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–246. doi: 10.4067/s0716-97602002000200016. [DOI] [PubMed] [Google Scholar]
- Beloussov LV, Grabovsky VI. Morphomechanics: goals, basic experiments and models. Int J Dev Biol. 2006;50:81–92. doi: 10.1387/ijdb.052056lb. [DOI] [PubMed] [Google Scholar]
- Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch Biochem Biophys. 2006;456:224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko R, Brodland GW. Measurement of in vivo stress resultants in neurulation-stage amphibian embryos. Ann Biomed Eng. 2007;35:672–681. doi: 10.1007/s10439-006-9250-1. [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Bhadriraju K, Hansen LK. Extracellular matrix- and cytoskeleton-dependent changes in cell shape and stiffness. Exp Cell Res. 2002;278:92–100. doi: 10.1006/excr.2002.5557. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Boppart SA, Brezinski ME, Bouma BE, Tearney GJ, Fujimoto JG. Investigation of developing embryonic morphology using optical coherence tomography. Dev Biol. 1996;177:54–63. doi: 10.1006/dbio.1996.0144. [DOI] [PubMed] [Google Scholar]
- Bortier H, De Bruyne G, Espeel M, Vakaet L. Immunohistochemistry of laminin in early chicken and quail blastoderms. Anat Embryol (Berl) 1989;180:65–69. doi: 10.1007/BF00321901. [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T. Presence of fibronectin during early embryogenesis in amphibian Pleurodeles waltlii. Cell Differ. 1983;12:77–83. doi: 10.1016/0045-6039(83)90059-3. [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Boulekbache H, Thiery JP. Prevention of gastrulation but not neurulation by antibodies to fibronectin in amphibian embryos. Nature. 1984;307:364–367. doi: 10.1038/307364a0. [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Li SD, Boulekbache H, Yamada KM, Thiery JP. Evidence for the role of fibronectin in amphibian gastrulation. J Embryol Exp Morphol. 1985;89(Suppl):211–227. [PubMed] [Google Scholar]
- Boudou T, Ohayon J, Arntz Y, Finet G, Picart C, Tracqui P. An extended modeling of the micropipette aspiration experiment for the characterization of the Young's modulus and Poisson's ratio of adherent thin biological samples: numerical and experimental studies. J Biomech. 2006;39:1677–1685. doi: 10.1016/j.jbiomech.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–613. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 Couples Nocodazole-induced Microtubule Disassembly to Cell Contractility via RhoA. Mol Biol Cell. 2008;19:2147–2153. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Brodland GW. Multi-scale finite element modeling allows the mechanics of amphibian neurulation to be elucidated. Phys Biol. 2008;5:15003. doi: 10.1088/1478-3975/5/1/015003. [DOI] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006;18:572–578. doi: 10.1016/j.ceb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Cheshire AM, Kerman BE, Zipfel WR, Spector AA, Andrew DJ. Kinetic and mechanical analysis of live tube morphogenesis. Dev Dyn. 2008;237:2874–2888. doi: 10.1002/dvdy.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen RM. Mechanics of Composite Materials. Krieger Publishing Company; Malabar, Florida: 1991. [Google Scholar]
- Coughlin MF, Puig-de-Morales M, Bursac P, Mellema M, Millet E, Fredberg JJ. Filamin-a and rheological properties of cultured melanoma cells. Biophysical Journal. 2006;90:2199–2205. doi: 10.1529/biophysj.105.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA, Bousquet O, Ma L, Yamada S, Wirtz D. The ‘ins’ and ‘outs’ of intermediate filament organization. Trends Cell Biol. 2000;10:420–428. doi: 10.1016/s0962-8924(00)01828-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255(5042):325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Czirok A, Zamir EA, Filla MB, Little CD, Rongish BJ. Extracellular matrix macroassembly dynamics in early vertebrate embryos. Curr Top Dev Biol. 2006;73:237–258. doi: 10.1016/S0070-2153(05)73008-8. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. Journal of Cell Science. 1989;93(Pt 2):255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Darribere T, Riou JF, Shi DL, Delarue M, Boucaut JC. Synthesis and distribution of laminin-related polypeptides in early amphibian embryos. Cell Tissue Res. 1986;246:45–51. doi: 10.1007/BF00218997. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Dzamba BD, Keller R, Desimone DW. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn. 2008;237:2684–2692. doi: 10.1002/dvdy.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Ezin AM, Keller R. Embryonic wound healing by apical contraction and ingression in Xenopus laevis. Cell Motil Cytoskeleton. 2002a;53:163–176. doi: 10.1002/cm.10070. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha5beta1, fibronectin, and tissue geometry. Dev Biol. 2002b;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Keller R, Desimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn. 2004;231:888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Koehl MA, Keller R, Oster GF. How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development. 1995;121:2005–2018. doi: 10.1242/dev.121.7.2005. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Marsden M, Keller R, Desimone DW. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Oster GF, Keller RE, Koehl MA. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1999;209:221–238. doi: 10.1006/dbio.1999.9249. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Wallingford JB. Visualizing morphogenesis in the frog embryo. In: Yuste R, Konnerth A, editors. Imaging in Neuroscience and Development: A Laboratory Manual. Cold Spring Harbor Laboratory Press; NY: 2005. [Google Scholar]
- Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell. 2008;15:134–145. doi: 10.1016/j.devcel.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JE, Gay DM, Welsch RE. An adaptive nonlinear least-squares algorithm. ACM Transactions on Mathematical Software. 1981;7:348–368. [Google Scholar]
- Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Duband JL, Thiery JP. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Dev Biol. 1982;93:308–323. doi: 10.1016/0012-1606(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Dzamba B, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and non-canonical wnt signaling regulate gastrulation movements through matrix organization. Developmental Cell. doi: 10.1016/j.devcel.2009.01.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Espeseth A, Marnellos G, Kintner C. The role of F-cadherin in localizing cells during neural tube formation in Xenopus embryos. Development. 1998;125:301–312. doi: 10.1242/dev.125.2.301. [DOI] [PubMed] [Google Scholar]
- Esue O, Carson AA, Tseng Y, Wirtz D. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. J Biol Chem. 2006;281:30393–30399. doi: 10.1074/jbc.M605452200. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87:148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Farhadifar R, Roper JC, Aigouy B, Eaton S, Julicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17:2095–2104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Fey J, Hausen P. Appearance and distribution of laminin during development of Xenopus laevis. Differentiation. 1990;42:144–152. doi: 10.1111/j.1432-0436.1990.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Filas BA, Knutsen AK, Bayly PV, Taber LA. A new method for measuring deformation of folding surfaces during morphogenesis. J Biomech Eng. 2008;130:061010. doi: 10.1115/1.2979866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley WN, Lai JS, Onaran K. Creep and relaxation of nonlinear viscoelastic materials. Dover Publications, Inc; New York: 1989. [Google Scholar]
- Finnemann S, Kuhl M, Otto G, Wedlich D. Cadherin transfection of Xenopus XTC cells downregulates expression of substrate adhesion molecules. Mol Cell Biol. 1995;15:5082–5091. doi: 10.1128/mcb.15.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophysical Journal. 1998;74:2227–2234. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Fung YC. Stress strain history relations of soft tissues in simple elongation. In: Fung YC, Perrone N, Anliker M, editors. Biomechanics, Its Foundations and Objectives. Prentice Hall; Englewood Cliffs, NJ: 1972. [Google Scholar]
- Gardel ML, Kasza KE, Brangwynne CP, Liu J, Weitz DA. Chapter 19: Mechanical response of cytoskeletal networks. Methods Cell Biol. 2008;89:487–519. doi: 10.1016/S0091-679X(08)00619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci U S A. 2006;103:1762–1767. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophysical Journal. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MC, Patel AB, Nagpal R, Perrimon N. The emergence of geometric order in proliferating metazoan epithelia. Nature. 2006;442:1038–1041. doi: 10.1038/nature05014. [DOI] [PubMed] [Google Scholar]
- Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophysical Journal. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T, Wolpert L. The cellular basis of morphogenesis and sea urchin development. Int. Rev. Cytol. 1963;15:139–214. doi: 10.1016/s0074-7696(08)61117-1. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, Wedlich D. Regulated adhesion as a driving force of gastrulation movements. Development. 2008;135:3625–3641. doi: 10.1242/dev.015701. [DOI] [PubMed] [Google Scholar]
- Hausen P, Riebesell M. The early development of Xenopus laevis : an atlas of the histology. Springer Verlag; New York: 1991. [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Helmke BP, Goldman RD, Davies PF. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- Helmke BP, Rosen AB, Davies PF. Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophysical Journal. 2003;84:2691–2699. doi: 10.1016/S0006-3495(03)75074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of sea urchin eggs. I. Surface force and elastic modulus of the cell membrane. Experimental Cell Reseach. 1963a;32:59. doi: 10.1016/0014-4827(63)90069-7. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of sea urchin eggs. II. Changes in the mechanical properties from fertilization to cleavage. Experimental Cell Reseach. 1963b;32:76. doi: 10.1016/0014-4827(63)90070-3. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Rheological properties of echinoderm eggs during cell division. Biorheology. 1982;19(12):71–78. doi: 10.3233/bir-1982-191-208. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Hoff K. v., Wassersug R. Tadpole Locomotion: Axial Movement and Tail Functions in a Largely Vertebraeless Vertebrate. Amer. Zool. 2000;40:62–76. [Google Scholar]
- Hoffman BD, Massiera G, Van Citters KM, Crocker JC. The consensus mechanics of cultured mammalian cells. Proc Natl Acad Sci U S A. 2006;103:10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MS, Ma X. Mechanical aspects of developmental biology: perspectives on growth and form in the (post)-genomic age. Preface. Phys Biol. 2008;5:15001. doi: 10.1088/1478-3975/5/1/015001. [DOI] [PubMed] [Google Scholar]
- Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- Hutton E, Paladini RD, Yu QC, Yen M, Coulombe PA, Fuchs E. Functional differences between keratins of stratified and simple epithelia. J Cell Biol. 1998;143:487–499. doi: 10.1083/jcb.143.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, Nishida E, Natsume T, Ueno N. XGAP, an ArfGAP, is required for polarized localization of PAR proteins and cell polarity in Xenopus gastrulation. Dev Cell. 2006;11:69–79. doi: 10.1016/j.devcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Jacobson AG, Gordon R. Changes in the shape of the developing vertebrate nervous system analyzed experimentally, mathematically, and by computer simulation. Journal of Experimental Zoology. 1976;197:191–246. doi: 10.1002/jez.1401970205. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. Journal of Cell Biology. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Georges PC, Hvidt S. Basic rheology for biologists. Methods Cell Biol. 2007;83:3–27. doi: 10.1016/S0091-679X(07)83001-9. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Hvidt S, Lamb J, Stossel TP. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature. 1990;345(6270):89–92. doi: 10.1038/345089a0. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Darribere T, Boucaut JC. Ambystoma maculatum gastrulae have an oriented, fibronectin-containing extracellular matrix. J Exp Zool. 1992;261:458–471. doi: 10.1002/jez.1402610413. [DOI] [PubMed] [Google Scholar]
- Johnson MH, McConnell JM. Lineage allocation and cell polarity during mouse embryogenesis. Semin Cell Dev Biol. 2004;15:583–597. doi: 10.1016/j.semcdb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kalantarian A, Ninomiya H, Saad SM, David R, Winklbauer R, Neumann AW. Axisymmetric drop shape analysis for estimating the surface tension of cell aggregates by centrifugation. Biophysical Journal. 2009;96:1606–1616. doi: 10.1016/j.bpj.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. The origin and morphogenesis of amphibian somites. Curr Top Dev Biol. 2000;47:183–246. doi: 10.1016/s0070-2153(08)60726-7. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Phil. Trans. R. Soc. Lond. B. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Shook D, Skoglund P. The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:15007. doi: 10.1088/1478-3975/5/1/015007. [DOI] [PubMed] [Google Scholar]
- Kidoaki S, Matsuda T, Yoshikawa K. Relationship between apical membrane elasticity and stress fiber organization in fibroblasts analyzed by fluorescence and atomic force microscopy. Biomech Model Mechanobiol. 2006;5:263–272. doi: 10.1007/s10237-006-0048-8. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Wang CW, Thomas FIM, Sastry AM. Fluid-structure interaction analysis of flow-induced deformation in a two-phase, Neo-Hookean marine egg. Asme-Amer Soc Mechanical Eng. 2006:519–526. [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69(2):225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW, Parr B. The body language of cells: the intimate connection between cell adhesion and behavior. Cell. 1995;83:5–8. doi: 10.1016/0092-8674(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Koehl MAR. Biomechanical approaches to morphogenesis. Seminars in Developmental Biology. 1990;1:367–378. [Google Scholar]
- Koehl MAR, Adams DS, Keller RE. Mechanical development of the notochord in Xenopus early tail-bud embryos. In: Akkas N, editor. Biomechanics of active movement and deformation. Springer-Verlag; Berlin Heidelberg: 1990. pp. 471–485. [Google Scholar]
- Koehl MAR, Quillin KJ, Pell CA. Mechanical design of fiber-wound hydraulic skeletons: The stiffening and straightening of embryonic notochords. American Zoologist. 2000;40:28–41. [Google Scholar]
- Kofron M, Heasman J, Lang SA, Wylie CC. Plakoglobin is required for maintenance of the cortical actin skeleton in early Xenopus embryos and for cdc42-mediated wound healing. J Cell Biol. 2002;158:695–708. doi: 10.1083/jcb.200202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotoski D, Bronner-Fraser M. Distribution of integrins and their ligands in the trunk of Xenopus laevis during neural crest cell migration.PG - 139-50. J Exp Zool. 1990;253:139–150. doi: 10.1002/jez.1402530204. [DOI] [PubMed] [Google Scholar]
- Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Belmonte JC, Tabin CJ. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci U S A. 2008;105:8499–8506. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis. Development. 2005;132:4599–4610. doi: 10.1242/dev.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau LD, Lifshitz EM. Theory of Elasticity. Pergamon Press; Oxford: 1986. [Google Scholar]
- Lane MC, Koehl MAR, Wilt F, Keller R. A role for regulated secretion of apical extracellular matrix during epithelial invagination in the sea urchin. Development. 1993;117:1049–1060. doi: 10.1242/dev.117.3.1049. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Lee G, Hynes R, Kirschner M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell. 1984;36:729–740. doi: 10.1016/0092-8674(84)90353-2. [DOI] [PubMed] [Google Scholar]
- Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- Lin DC, Dimitriadis EK, Horkay F. Robust strategies for automated AFM force curve analysis--I. Non-adhesive indentation of soft, inhomogeneous materials. J Biomech Eng. 2007;129:430–440. doi: 10.1115/1.2720924. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JI, Mouw JK, Weaver VM. Biomechanical regulation of cell orientation and fate. Oncogene. 2008;27:6981–6993. doi: 10.1038/onc.2008.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero A, Stack C, Bresnick AR, Shuster CB. A global, myosin light chain kinase-dependent increase in myosin II contractility accompanies the metaphase-anaphase transition in sea urchin eggs. Mol Biol Cell. 2006;17:4093–4104. doi: 10.1091/mbc.E06-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M, DeSimone D. Regulation of morphogenesis through convergence of Wnt-11 and integrin signaling pathways; 43rd Annual Meeting of American Society of Cell Biology; 2003. abstract. [Google Scholar]
- Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Metscher BD. MicroCT for developmental biology: A versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn. 2009;238:632–640. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Moore AR. On the mechanics of gastrulation in Dendraster excentricus. J. Exp. Zool. 1941;87:101–111. [Google Scholar]
- Moore SW. A fiber optic system for measuring dynamic mechanical properties of embryonic tissues. IEEE Transaction on Biomedical Engineering. 1994;41:45–50. doi: 10.1109/10.277270. [DOI] [PubMed] [Google Scholar]
- Moore SW, Keller RE, Koehl MAR. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus leavis. Development. 1995;121:3130–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1616–1626. doi: 10.1152/ajpcell.00457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji N, Johnson KE. Comparative study of extracellular fibrils on the ectodermal layer in gastrulae of five amphibian species. J Cell Sci. 1983;59:61–70. doi: 10.1242/jcs.59.1.61. [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Ramasubramanian A, Taber LA. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Dev Dyn. 2006;235:1822–1829. doi: 10.1002/dvdy.20813. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. Plant Biomechanics: an engineering approach to plant form and function. University of Chicago Press; Chicago: 1992. [Google Scholar]
- Ninomiya H, Winklbauer R. Epithelial coating controls mesenchymal shape change through tissue-positioning effects and reduction of surface-minimizing tension. Nat Cell Biol. 2008;10:61–69. doi: 10.1038/ncb1669. [DOI] [PubMed] [Google Scholar]
- Obara K, Nikcevic G, Pestic L, Nowak G, Lorimer DD, Guerriero V, Jr., Elson EL, Paul RJ, de Lanerolle P. Fibroblast contractility without an increase in basal myosin light chain phosphorylation in wild type cells and cells expressing the catalytic domain of myosin light chain kinase. J Biol Chem. 1995;270:18734–18737. doi: 10.1074/jbc.270.32.18734. [DOI] [PubMed] [Google Scholar]
- Papan C, Boulat B, Velan SS, Fraser SE, Jacobs RE. Formation of the dorsal marginal zone in Xenopus laevis analyzed by time-lapse microscopic magnetic resonance imaging. Dev Biol. 2007a doi: 10.1016/j.ydbio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Papan C, Boulat B, Velan SS, Fraser SE, Jacobs RE. Two-dimensional and three-dimensional time-lapse microscopic magnetic resonance imaging of Xenopus gastrulation movements using intrinsic tissue-specific contrast. Dev Dyn. 2007b;236:494–501. doi: 10.1002/dvdy.21045. [DOI] [PubMed] [Google Scholar]
- Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Peralta XG, Toyama Y, Hutson MS, Montague R, Venakides S, Kiehart DP, Edwards GS. Upregulation of forces and morphogenic asymmetries in dorsal closure during Drosophila development. Biophysical Journal. 2007;92:2583–2596. doi: 10.1529/biophysj.106.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta XG, Toyama Y, Kiehart DP, Edwards GS. Emergent properties during dorsal closure in Drosophila morphogenesis. Phys Biol. 2008;5:15004. doi: 10.1088/1478-3975/5/1/015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- Potard US, Butler JP, Wang N. Cytoskeletal mechanics in confluent epithelial cells probed through integrins and E-cadherins. Am J Physiol. 1997;272:C1654–1663. doi: 10.1152/ajpcell.1997.272.5.C1654. [DOI] [PubMed] [Google Scholar]
- Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 2008;24:221–230. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Ramos JW, Whittaker CA, DeSimone DW. Integrin-dependent adhesive activity is spatially controlled by inductive signals at gastrulation. Development. 1996;122:2873–2883. doi: 10.1242/dev.122.9.2873. [DOI] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Rozario T, Dzamba B, Weber GF, Davidson LA, DeSimone DW. The physical state of fibronectin matrix differentially regulates morphogenetic movements in vivo. Developmental Biology. 2008 doi: 10.1016/j.ydbio.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Buguin A, Silberzan P, Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophysical Journal. 2005;89:L52–54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EJ. Ultrastructural immunocytochemical localization of fibronectin in the early chick embryo. J Embryol Exp Morphol. 1982;71:155–170. [PubMed] [Google Scholar]
- Sato M, Theret DP, Wheeler LT, Ohshima N, Nerem RM. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. Journal of Biomechanical Engineering. 1990;112:263–268. doi: 10.1115/1.2891183. [DOI] [PubMed] [Google Scholar]
- Schnell S, Maini PK, Newman SA, Newman TJ. Multiscale modeling of developmental systems. Introduction. Curr Top Dev Biol. 2008;81:xvii–xxv. doi: 10.1016/S0070-2153(07)81018-0. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Scott A, Stemple DL. Zebrafish notochordal basement membrane: signaling and structure. Curr Top Dev Biol. 2005;65:229–253. doi: 10.1016/S0070-2153(04)65009-5. [DOI] [PubMed] [Google Scholar]
- Selman GG. The forces producing neural closure in amphibia. Journal of Embryology and Experimental Morphology. 1958;6:448–465. [PubMed] [Google Scholar]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland HR, Zorn AM. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Dzamba B, Coffman CR, Harris WA, Keller R. Xenopus fibrillin is expressed in the organizer and is the earliest component of matrix at the developing notochord-somite boundary. Dev Dyn. 2006;235:1974–1983. doi: 10.1002/dvdy.20818. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Keller R. Xenopus fibrillin regulates directed convergence and extension. Dev Biol. 2007;301:404–416. doi: 10.1016/j.ydbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophysical Journal. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenovic D. Microtubules may harden or soften cells, depending of the extent of cell distension. J Biomech. 2005;38:1728–1732. doi: 10.1016/j.jbiomech.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Mijailovich SM, Tolic-Norrelykke IM, Chen J, Wang N. Cell prestress. II. Contribution of microtubules. Am J Physiol Cell Physiol. 2002;282:C617–624. doi: 10.1152/ajpcell.00271.2001. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Rosenblatt N, Montoya-Zavala M, Matthews BD, Hu S, Suki B, Wang N, Ingber DE. Rheological behavior of living cells is timescale-dependent. Biophysical Journal. 2007;93:L39–41. doi: 10.1529/biophysj.107.116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella JA, Liao J, Hong Y, David Merryman W, Wagner WR, Sacks MS. Tissue-to-cellular level deformation coupling in cell micro-integrated elastomeric scaffolds. Biomaterials. 2008;29:3228–3236. doi: 10.1016/j.biomaterials.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Taber LA. Nonlinear theory of elasticity. Applications in biomechanics. World Scientific Publishing Co; Singapore: 2004. [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Takata H, Kominami T. Ectoderm exerts the driving force for gastrulation in the sand dollar Scaphechinus mirabilis. Dev Growth Differ. 2001a;43:265–274. doi: 10.1046/j.1440-169x.2001.00576.x. [DOI] [PubMed] [Google Scholar]
- Takata H, Kominami T. Shrinkage and expansion of blastocoel affect the degree of invagination in sea urchin embryos. Zoologcal Science. 2001b;18:1097–1105. [Google Scholar]
- Tao Q, Lloyd B, Lang S, Houston D, Zorn A, Wylie C. A novel G protein-coupled receptor, related to GPR4, is required for assembly of the cortical actin skeleton in early Xenopus embryos. Development. 2005;132:2825–2836. doi: 10.1242/dev.01866. [DOI] [PubMed] [Google Scholar]
- Tao Q, Nandadasa S, McCrea PD, Heasman J, Wylie C. G-protein-coupled signals control cortical actin assembly by controlling cadherin expression in the early Xenopus embryo. Development. 2007;134:2651–2661. doi: 10.1242/dev.002824. [DOI] [PubMed] [Google Scholar]
- Tint IS, Hollenbeck PJ, Verkhovsky AB, Surgucheva IG, Bershadsky AD. Evidence that intermediate filament reorganization is induced by ATP-dependent contraction of the actomyosin cortex in permeabilized fibroblasts. Journal of Cell Science. 1991;98(Pt 3):375–384. doi: 10.1242/jcs.98.3.375. [DOI] [PubMed] [Google Scholar]
- Torpey N, Wylie CC, Heasman J. Function of maternal cytokeratin in Xenopus development. Nature. 1992;357:413–415. doi: 10.1038/357413a0. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey WR, Vail TP, Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J Orthop Res. 2004;22:131–139. doi: 10.1016/S0736-0266(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Tseng Y, Kole TP, Lee JS, Fedorov E, Almo SC, Schafer BW, Wirtz D. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochem Biophys Res Commun. 2005;334:183–192. doi: 10.1016/j.bbrc.2005.05.205. [DOI] [PubMed] [Google Scholar]
- Valentine MT, Perlman ZE, Mitchison TJ, Weitz DA. Mechanical properties of Xenopus egg cytoplasmic extracts. Biophysical Journal. 2005;88:680–689. doi: 10.1529/biophysj.104.048025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Citters KM, Hoffman BD, Massiera G, Crocker JC. The role of Factin and myosin in epithelial cell rheology. Biophysical Journal. 2006;91:3946–3956. doi: 10.1529/biophysj.106.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Nakatani Y, Hendrickson C, Ericson V, Lin C, Smith WC. Chongmague reveals an essential role for laminin-mediated boundary formation in chordate convergence and extension movements. Development. 2008;135:33–41. doi: 10.1242/dev.010892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JV. Structural Biomaterials. Princeton University Press; Princeton: 1990. [Google Scholar]
- Vogel S. Comparative biomechanics: life's physical world. Princeton University Press; Princeton: 2003. [Google Scholar]
- von Dassow M, Davidson LA. Variation and robustness of the mechanics of gastrulation: the role of tissue mechanical properties during morphogenesis. Birth Defects Res C Embryo Today. 2007;81:253–269. doi: 10.1002/bdrc.20108. [DOI] [PubMed] [Google Scholar]
- von Dassow M, Davidson LA. Natural variation in embryo mechanics: gastrulation in Xenopus laevis is highly robust to variation in tissue stiffness. Dev Dyn. 2009;238:2–18. doi: 10.1002/dvdy.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov DA, Taber LA. Cardiac looping in experimental conditions: effects of extraembryonic forces. Dev Dyn. 2002;224:413–421. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- Wainwright SA. The role of materials in organisms. In: Bolis L, Maddrell HP, Schmidt-Nielsen K, editors. Comparative Physiology - Functional Aspects of Structural Materials. North-Holland Publishing Co; Amsterdam: 1975. pp. 3–8. [Google Scholar]
- Wainwright SA. Axis and circumference: the cylindrical shape of plants and animals. Harvard University Press; Cambridge MA: 1988. [Google Scholar]
- Wainwright SA, Biggs WD, Currey JD, Gosline JM. Mechanical Design in Organisms. John Wiley and Sons; New York: 1976. [Google Scholar]
- Wakatsuki T, Kolodney MS, Zahalak GI, Elson EL. Cell mechanics studied by a reconstituted model tissue. Biophysical Journal. 2000;79:2353–2368. doi: 10.1016/S0006-3495(00)76481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J Cell Sci. 2001;114:1025–1036. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wang N. Mechanical interactions among cytoskeletal filaments. Hypertension. 1998;32:162–165. doi: 10.1161/01.hyp.32.1.162. [DOI] [PubMed] [Google Scholar]
- Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279:C188–194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282:C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nature Reviews in Molecular Cell Biology. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZZ, Zhang G, Long M, Wang HB, Song GB, Cai SX. Comparison of the viscoelastic properties of normal hepatocytes and hepatocellular carcinoma cells under cytoskeletal perturbation. Biorheology. 2000;37:279–290. [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Zamir EA, Rongish BJ, Little CD. The ECM moves during primitive streak formation--computation of ECM versus cellular motion. PLoS Biol. 2008;6:e247. doi: 10.1371/journal.pbio.0060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir EA, Srinivasan V, Perucchio R, Taber LA. Mechanical asymmetry in the embryonic chick heart during looping. Ann Biomed Eng. 2003;31:1327–1336. doi: 10.1114/1.1623487. [DOI] [PubMed] [Google Scholar]
- Zamir EA, Taber LA. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J Biomech Eng. 2004a;126:823–830. doi: 10.1115/1.1824129. [DOI] [PubMed] [Google Scholar]
- Zamir EA, Taber LA. On the effects of residual stress in microindentation tests of soft tissue structures. Journal of Biomechanical Engineering-Transactions of the Asme. 2004b;126:276–283. doi: 10.1115/1.1695573. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Liu X, Collodi P. Identification and characterization of a novel fibronectin in zebrafish. Exp Cell Res. 2001;268:211–219. doi: 10.1006/excr.2001.5291. [DOI] [PubMed] [Google Scholar]
- Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during critical stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]