Although studies have linked, mucoid Pseudomonas aeruginosa to deteriorating pulmonary function and early mortality, to our knowledge, this is the first observation that irreversible lung structural damage is specifically associated with mucoid P aeruginosa.
Abstract
Purpose:
To correlate the severity of bronchiectasis in children with cystic fibrosis with clinical and microbiologic variables in order to clarify risk factors for the development of irreversible lung disease.
Materials and Methods:
After institutional review board approval and parental informed consents were obtained, a HIPAA-compliant longitudinal epidemiologic evaluation was performed in patients with cystic fibrosis who were enrolled in the Wisconsin trial of newborn screening from 1985 to 2009. Thin-section chest computed tomography (CT) was used in a prospective cross-sectional design to study patients ranging in age from 6.6 to 17.6 years (mean, 11.5 years). Thin-section CT scores were determined objectively on coded images by multiple raters in a standardized fashion. Microbiologic data were obtained by means of culture of respiratory secretions by using methods for differentiation of Pseudomonas aeruginosa (PA) as either nonmucoid or mucoid.
Results:
Eighty-three percent of patients (68 of 82) showed bronchiectasis of varying severity. Of 12 potential risk factors, only respiratory infection with mucoid PA correlated significantly with bronchiectasis (P = .041).
Conclusion:
The severity of bronchiectasis in children with cystic fibrosis is significantly related to respiratory infection with mucoid PA; attempts to prevent bronchiectasis should include reducing exposure to and early eradication of PA.
Patients with cystic fibrosis (CF) are susceptible in early childhood to chronic lung disease associated with recurrent respiratory infections due to unusual respiratory pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa (PA) (1). At a variable age, patients with CF will develop obstructive pulmonary dysfunction (2). In addition, the majority of children with CF show structural bronchopulmonary abnormalities (3–5), including bronchiectasis, mucous plugging, peribronchial thickening, air trapping, and parenchymal opacities. Bronchiectasis is an early and prominent feature of CF lung disease. Bronchiectasis is considered an irreversible abnormality (4,6) and thus indicates the presence of permanent damage to the lungs. Chronologically, bronchiectasis is the first irreversible change seen in children with CF (7). Because the identification of bronchiectasis indicates that treatment can no longer be expected to return the status of the lungs to normal, the prevention of bronchiectasis is of great importance in the care of the child with CF.
Although there has been limited longitudinal research focused on bronchiectasis determinants in patients with CF (4), cross-sectional and short-term studies suggest that bronchiectasis correlates with significantly increased airways obstruction (8,9). In addition, Evans et al (6) reported that patients with bronchiectasis “who become colonized by P aeruginosa have poorer lung function when first colonized than those colonized by other organisms.” Despite its obvious clinical importance, the epidemiology of bronchiectasis has not been well described in CF.
Advances in Knowledge.
Of 12 potential risk factors, only respiratory infection with mucoid Pseudomonas aeruginosa (PA) correlated significantly with the severity of bronchiectasis in children with cystic fibrosis (CF).
CT scores correlated significantly with mucoid PA, while no significant correlations were seen between pulmonary function tests (the most commonly used assessment of lung status) and other potential etiologic factors for bronchiectasis and CF lung disease in children.
Computed tomography (CT) is the imaging modality of choice to identify and quantify bronchiectasis (10) and the other morphologic changes seen with CF lung disease. Pulmonary function tests (PFTs) are the most widely used means of assessing CF lung disease, but CT scanning has been shown to demonstrate bronchiectasis in children with normal PFT findings (7) and to demonstrate progressive disease when PFT results remained stable (11). CT scanning uses ionizing radiation and so involves the risk of radiation exposure but is otherwise noninvasive. The findings cited above support CT scanning as the best available method to identify and quantitate bronchiectasis and other features of CF lung disease in order to assess the determinants of bronchiectasis in children with CF.
Because of Wisconsin's long-term randomized clinical trial (12) of early diagnosis through newborn screening, and especially its unique longitudinal epidemiology component (13), we were presented with an unprecedented opportunity to investigate the determinants of bronchiectasis in children with CF. Our prospective design included thin-section CT scanning in all patients giving consent after they had reached at least 6 years of age. The hypothesis we addressed regarding the epidemiology of bronchiectasis is that extrinsic clinical factors will determine its severity. More specifically, on the basis of our earlier observations (14), we postulated that PA-associated respiratory infections would contribute significantly to the severity of bronchiectasis.
Implication for Patient Care.
Our findings suggest that averting infection with mucoid strains of PA may be critical in preventing bronchiectasis and pulmonary deterioration in children with CF.
In young children, chest imaging is approximately fourfold more sensitive than pulmonary function testing for the evaluation of CF lung disease in children.
Materials and Methods
Patients and Design
The Wisconsin longitudinal cohort study of early diagnosis, a collaborative multidisciplinary project involving the two CF centers of Wisconsin and all newborns, has been described in detail elsewhere (12,13,15). By using randomized newborn screening from April 1985 through June 1994, we enrolled 149 children in whom CF was diagnosed after a sweat chloride level of 60 mmol/L or greater and for whom written informed consent was provided by their parents; thus, patient accrual occurred over approximately a decade. Two groups were then managed and assessed similarly with an evaluation and treatment protocol (13): an early diagnosis, screened group and a concurrent control group with diagnosis obtained by means of the traditional method after signs and/or symptoms of CF appeared or a positive family history was recognized, or through an innovative “unblinding” process in which all the control subjects were identified (12,13). Care was provided by using standard methods of the era (13), with physicians and nurses unaware of the group to which the patient was assigned (3).
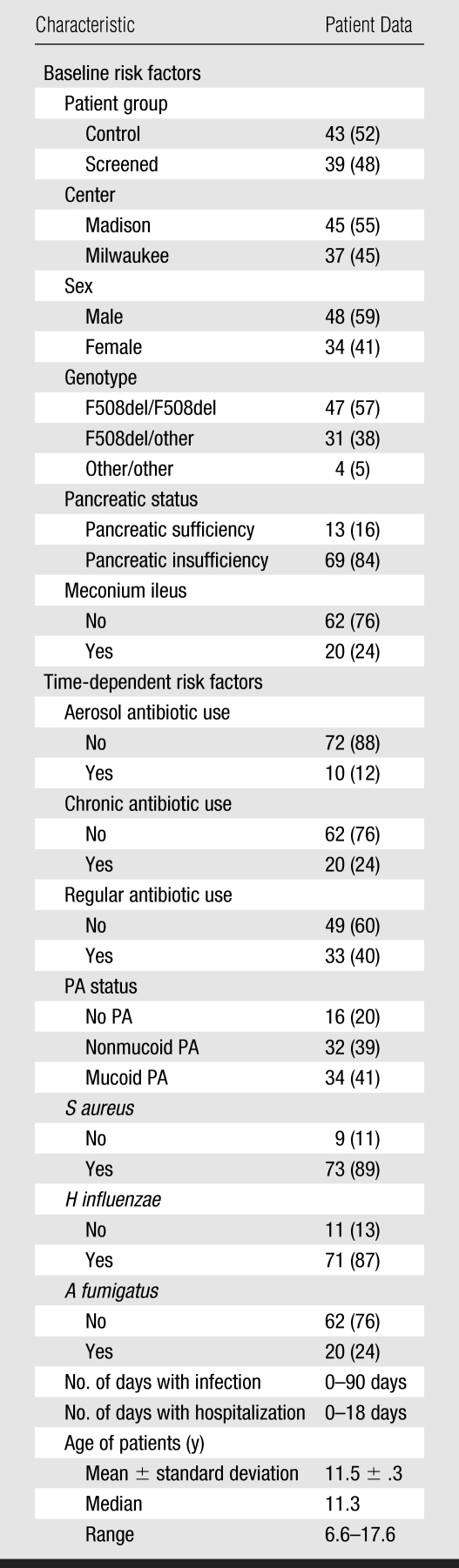
The longitudinal evaluations included quarterly, systematic assessment of respiratory symptoms; cultures of respiratory secretions at least every 6 months; semiannual PFTs beginning at 4 years of age; and annual chest radiographs. An associated cross-sectional thin-section CT study was added in 2000. Of the original 149 patients, 104 who continued to receive care in the protocol at the two CF centers were contacted. Enrollees who were 6 years of age and older and who gave additional informed consent (directly or through their parents) underwent thin-section CT scanning performed as described below. More specifically, 94 patients gave consent to a CF center physician who described the study and chest CT procedure; however, nine patients never came for their scheduled appointment and three scans could not be used. Thus, 82 patients participated in the thin-section CT study. The CT scanning in this research protocol was performed when the patients were at their baseline health status and not for clinical indications. The characteristics of the patients are provided in Table 1. The randomized control trial and all associated studies such as chest imaging were Health Insurance Portability and Accountability Act–compliant and were approved by the institutional review boards of the University of Wisconsin–Madison, the Children's Hospital of Wisconsin, and Cincinnati Children's Hospital, Ohio.
Table 1.
Characteristics of Patients Participating in the Thin-Section CT Study
Note.—Except where otherwise indicated, data are the numbers of patients (n = 82). Data in parentheses are percentages.
Chest CT Scanning and Scoring Procedures
One chest CT scan (Lightspeed; GE Medical Systems, Milwaukee, Wis) was obtained in each of the 82 patients by using a thin-section technique (1.25-mm section thickness, 120 kVp, 40 mAs, and bone reconstruction algorithm). Inspiratory images were obtained at 10-mm intervals from apices through lung bases. Six expiratory images were obtained, evenly spaced from 1 cm above the top of the aortic arch to 2 cm above the higher hemidiaphragm (16). In a 10-year-old child this protocol was calculated to produce an average radiation exposure of approximately 0.55 mSv. All studies were performed without sedation by using verbal commands and voluntary respiratory control. All 82 CT scans scored were of good quality as indicated by the lack of patient motion or any other factor that would interfere with recognition of bronchiectasis, with no patients excluded due to inadequate image quality. Cooperation with instructions by the three patients younger than 8 years, who all produced good-quality inspiratory and expiratory series, was likely facilitated by the PFT training these patients receive beginning at age 4–6 years.
Hard-copy images were scored independently by one adult thoracic radiologist (L.S.B.) and two pediatric radiologists (A.S.B. and one nonauthor), all with more than 5 years postfellowship experience, who trained together by using a previously described scoring system (16). Each lobe, with the lingula scored as a separate lobe, was evaluated for the extent and severity of bronchiectasis, mucous plugging, peribronchial thickening, parenchymal opacity, ground-glass opacity, cysts or bullae, and air trapping. For the parenchymal abnormalities, the score for each lobe was determined by the degree of involvement of the lobe demonstrating the abnormality, using a scale of 1–3. For the remaining findings, the severity of each finding was assessed by using predetermined criteria, and the severity score was multiplied by the degree of involvement as for parenchymal disease. For bronchiectasis, the score reflects the average size of dilated bronchi, the size of the largest bronchus, and the volume of each lobe containing dilated bronchi. Bronchiectasis was defined as an airway lumen diameter greater than the diameter of the accompanying artery or an artery equidistant from the hilum, nontapering bronchi, or a bronchus extending to the pleural surface. Mucous plugging was defined as soft-tissue attenuation filling the bronchial lumen and was identified by multiple factors including relationship to aerated bronchi, location adjacent to pulmonary arteries, and linear or branching configuration not corresponding to pulmonary vessels. The details of the scoring system have been previously reported (17,18).
Reference images chosen (A.S.B.) from prior research subjects were used for quality control purposes and thus were selected from thin-section CT scans obtained in children with CF who were not participants in this study. Reference images were chosen to represent the different findings used in the scoring system and a range of disease severity. The agreement of the three radiologists performing the scoring was excellent, as reported elsewhere (16).
Other Clinical and Laboratory Assessments
Genotype was determined by means of DNA analysis (19). Pancreatic functional status was assessed with a 3-day fecal fat study. According to protocol, respiratory secretions were obtained and cultured during CF center visits every 6 months or whenever clinically indicated according to the judgment of the patient's physician. The average interval for obtaining protocol-based and clinically indicated cultures was 3.3 months. Most of these respiratory secretions were obtained from vigorous oropharyngeal swabs during forced coughing. Specimens were plated on agar and cultured, with particular attention given to identifying Haemophilus influenzae, S aureus, and PA. The PA identification process included detection of nonmucoid and mucoid species, as described previously (15). In addition, cultures were examined for the presence of Aspergillus fumigatus.
Lung function measurement by means of spirometry was obtained every 6 months, beginning at approximately 4 years of age. The quality of the data was ensured by using the pediatric alternate spirometry system (P.M.F.), as described in detail elsewhere (3). Thus, all pulmonary function data evaluated in this study were of good quality. Predicted values were from reference equations by Wang et al (20).
Statistical Analyses
The effects of baseline risk factors, including patient group, CF center, sex, genotype, pancreatic status, and meconium ileus status were examined. Time-dependent risk factors, including aerosol antibiotic use, chronic antibiotic use (prophylactic antibiotics, defined as antibiotics prescribed for more than 30 days and unrelated to infection), regular antibiotic use (prescribed in response to acute infection), PA status, S aureus status, H influenzae status, A fumigatus status, days with infections before a visit (visits occurred every 3 months), days with hospitalization before a visit, and age of patients were documented 6 months before the CT study was performed. Status of pathogens (eg, nonmucoid PA and mucoid PA) was scored as 0 until the first positive culture and 1 thereafter. The effects of these risk factors on the cross-sectional CT bronchiectasis scores and CT total scores were assessed by means of a general linear model in which all risk factors were examined together. The categoric variables (grouping factors) included patient group, CF center, sex, genotype, pancreatic status, meconium ileus status, aerosol antibiotics use, chronic antibiotic use, regular antibiotic use, PA status, S aureus status, H influenzae status, and A fumigatus status. The continuous variables (covariates) included days with infections, days with hospitalization, and age of patients. In the general linear model, we analyzed all risk factors together in one multiple linear model, so adjustment for multiple comparisons arising from the multiple risk factors is not needed. The collinearity among continuous variables were checked with the COLLIN collinearity option (SAS Institute, Cary, NC) in a regression model with thin-section CT bronchiectasis and total scores as dependent variables, and the proportion of variance greater than 0.5 is considered as collinearity. We did not see significant collinearity.
As we reported in Li et al (15), nonmucoid PA infection occurred after absence of PA infection, and mucoid PA infection occurred after nonmucoid PA infection. They are three distinct stages in the life course of patients with CF (21,22). We were only interested in two comparisons: nonmucoid PA versus no PA infection and mucoid PA versus nonmucoid PA. Our prospective hypothesis was that bronchiectasis at the nonmucoid PA stage is worse than bronchiectasis at the absence of PA infection stage and that bronchiectasis at the mucoid PA stage is worse than bronchiectasis at the nonmucoid PA stage. Thus, we examined only these two comparisons.
Correlation between thin-section CT bronchiectasis scores and the concurrent PFT findings were examined by using Pearson partial correlation coefficients adjusted for all risk factors. Then, the effects of the above various risk factors on the concurrent PFT findings were assessed by means of a general linear model. Since each of the four lung function outcomes is evaluated as a scientifically distinct question, multiple comparison adjustment for multiple outcomes is not needed. The robust locally weighted regression function was used to estimate the mean response in thin-section CT bronchiectasis scores and total scores in different PA groups as a function of age. Results were considered statistically significant if P < .05.
Results
Patient Characteristics
The characteristics of the 82 patients evaluated in the cross-sectional chest CT imaging study are summarized in Table 1. In general, they were a typical population of children with CF, with about half of the CF cases diagnosed through screening (39 of 82 children) and the remainder of the diagnoses being made after the development of signs or symptoms of the disease (43 of 82 children). Gastrointestinal abnormalities were present in the expected proportion (24% with meconium ileus and 84% with pancreatic insufficiency). Aggressive management was used to prevent malnutrition (13). Genotypes of the children in this study were similar to those of U.S. CF patients as a whole: 57% (47 of 82 children) F508del homozygotes, 38% (31 of 82) F508del compound heterozygotes, and 5% (four of 82) lacked this mutation. At the time of CT scanning, the patients ranged in age from 6.6 to 17.6 years (mean, 11.5 years; median, 11.3 years). The mean ages (± standard deviation) were 11.5 years ± 0.48 (range, 7.5–17.6 years) for female patients and 11.5 years ± 0.45 (range, 6.5–17.1 years) for male patients (P = .94). As shown in Table 1, respiratory secretion cultures were often positive, and 89%, 87%, and 80% showed S aureus, H influenzae, and PA, respectively, at the time of the chest CT scan. The PA-positive cultures showed nonmucoid (39%) and mucoid (41%) species.
Potential Risk Factors
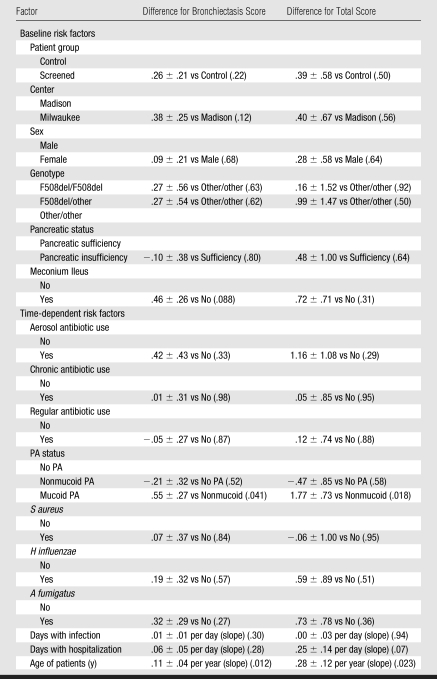
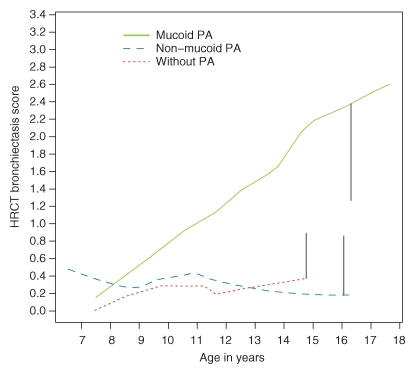
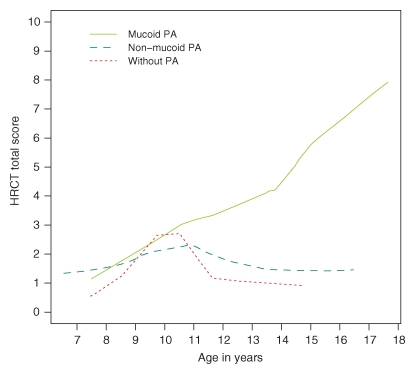
We found that 83% (68 of 82) of the patients had bronchiectasis. The effects of various risk factors on thin-section CT bronchiectasis scores are shown in Table 2. General linear model shows that a significantly higher CT bronchiectasis score (P = .41) was observed in patients with mucoid PA compared to patients with nonmucoid PA and that there is no significant difference in CT bronchiectasis scores between the group with nonmucoid PA and the group with no PA infection. CT bronchiectasis scores increased significantly (P = .012) with age. Analysis of the other potential risk factors revealed no significant effects. Figure 1 demonstrates graphically the prominent role of mucoid PA in the development of bronchiectasis in children with CF. The CT bronchiectasis scores are similar in the patient group with nonmucoid PA and the patient group with no PA infection. In contrast, the group with mucoid PA had apparently higher CT bronchiectasis scores than did the group with nonmucoid PA. Figure 2 demonstrates graphically the effect of mucoid PA and nonmucoid PA on the thin-section CT total scores. The total scores are similar in patients with nonmucoid PA and patients with no PA infection. In contrast, patients with mucoid PA had higher thin-section CT total scores than did patients with nonmucoid PA. Figure 3 illustrates the thin-section CT appearance and scores in representative children without PA infection, with nonmucoid PA infection, and with mucoid PA infection.
Table 2.
General Linear Model Analyses of the Thin-Section CT Bronchiectasis Scores and Total Scores
Note.—Data are differences ± standard error, and numbers in parentheses are P values.
Figure 1:
Graph shows difference in the thin-section CT (HRCT) bronchiectasis scores between patients with nonmucoid PA and those without PA infection and between patients with mucoid PA and those with nonmucoid PA. The vertical bars show average upper or lower 97.5% confidence intervals.
Figure 2:
Graph shows difference in the thin-section CT (HRCT) total scores between patients with nonmucoid PA and those without PA infection and between patients with mucoid PA and those with nonmucoid PA.
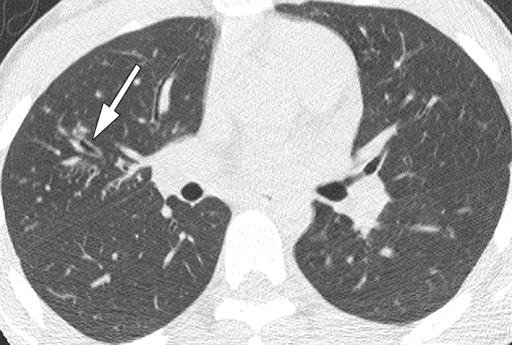
Figure 3a:
Representative 1.25-mm axial thin-section CT images through upper lobes in patients with bronchiectasis scores near the mean for age and PA status. Maximum score for each lobe is 12. Total lung score is mean of the six “lobes.” (a) Scan in 14.7-year-old patient with culture-negative findings for PA. Arrow shows dilated bronchus (lumen larger than accompanying vessel) in right upper lobe (RUL). Scores: RUL bronchiectasis, 0.67; total lung bronchiectasis, 0.44; RUL overall, 1.67; total lung overall, 1.28. (b) Scan in 15.5-year-old patient with culture-positive nonmucoid PA. Dilated bronchi in RUL (arrows) can be compared to normal bronchi in left upper lobe. Scores: RUL bronchiectasis, 1.67; total lung bronchiectasis, 0.51; RUL overall, 4.5; total lung overall, 1.69. (c) Scan in 13.5-year-old patient with culture-positive mucoid PA. Multiple markedly dilated and mucus-filled bronchi are seen in RUL (arrows). Scores: RUL bronchiectasis, 2.25; total lung bronchiectasis, 1.63; RUL overall, 11.25; total lung overall, 5.67.
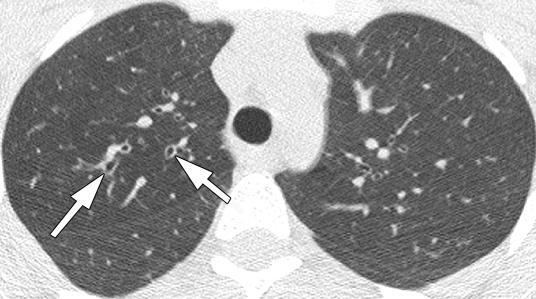
Figure 3b:
Representative 1.25-mm axial thin-section CT images through upper lobes in patients with bronchiectasis scores near the mean for age and PA status. Maximum score for each lobe is 12. Total lung score is mean of the six “lobes.” (a) Scan in 14.7-year-old patient with culture-negative findings for PA. Arrow shows dilated bronchus (lumen larger than accompanying vessel) in right upper lobe (RUL). Scores: RUL bronchiectasis, 0.67; total lung bronchiectasis, 0.44; RUL overall, 1.67; total lung overall, 1.28. (b) Scan in 15.5-year-old patient with culture-positive nonmucoid PA. Dilated bronchi in RUL (arrows) can be compared to normal bronchi in left upper lobe. Scores: RUL bronchiectasis, 1.67; total lung bronchiectasis, 0.51; RUL overall, 4.5; total lung overall, 1.69. (c) Scan in 13.5-year-old patient with culture-positive mucoid PA. Multiple markedly dilated and mucus-filled bronchi are seen in RUL (arrows). Scores: RUL bronchiectasis, 2.25; total lung bronchiectasis, 1.63; RUL overall, 11.25; total lung overall, 5.67.
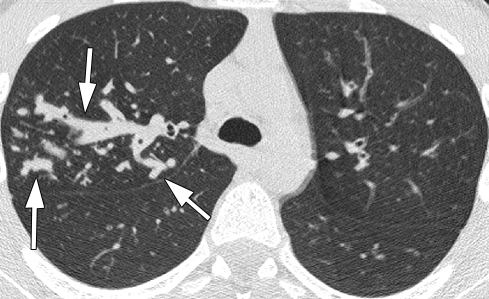
Figure 3c:
Representative 1.25-mm axial thin-section CT images through upper lobes in patients with bronchiectasis scores near the mean for age and PA status. Maximum score for each lobe is 12. Total lung score is mean of the six “lobes.” (a) Scan in 14.7-year-old patient with culture-negative findings for PA. Arrow shows dilated bronchus (lumen larger than accompanying vessel) in right upper lobe (RUL). Scores: RUL bronchiectasis, 0.67; total lung bronchiectasis, 0.44; RUL overall, 1.67; total lung overall, 1.28. (b) Scan in 15.5-year-old patient with culture-positive nonmucoid PA. Dilated bronchi in RUL (arrows) can be compared to normal bronchi in left upper lobe. Scores: RUL bronchiectasis, 1.67; total lung bronchiectasis, 0.51; RUL overall, 4.5; total lung overall, 1.69. (c) Scan in 13.5-year-old patient with culture-positive mucoid PA. Multiple markedly dilated and mucus-filled bronchi are seen in RUL (arrows). Scores: RUL bronchiectasis, 2.25; total lung bronchiectasis, 1.63; RUL overall, 11.25; total lung overall, 5.67.
Correlations of Lung Disease Measures
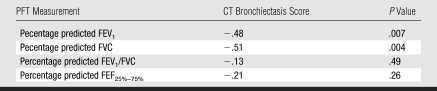
Table 3 shows the Pearson correlation coefficients between CT bronchiectasis scores and the concurrent pulmonary function measurements. The most commonly used measure of lung function, percentage predicted FEV1, was above 0.8 in 82.3% of patients (67 of 82) at the time of the chest thin-section CT procedure, and therefore the group as a whole had mild pulmonary dysfunction. Correlation coefficients for the various pulmonary function results—percentage predicted FEV1, percentage predicted FVC, percentage predicted FEV1/FVC, and percentage predicted FEF25%–75%—ranged from −0.44 to −0.59 (P < .001). The negative correlation indicates that the higher the CT bronchiectasis score, the lower the PFT result. These midrange correlation coefficients support our understanding that while there is significant correlation between thin-section CT and PFT findings, the two modalities reflect different information.
Table 3.
Pearson Partial Correlation Coefficients between CT Bronchiectasis Scores and Concurrent PFT Measurements
Note.—Adjusted for all variables in Table 1. FEF25%–75% = midexpiratory phase of forced expiratory flow, FEV1 = forced expiratory volume in 1 minute, FVC = forced vital capacity.
Pulmonary Function Measurements
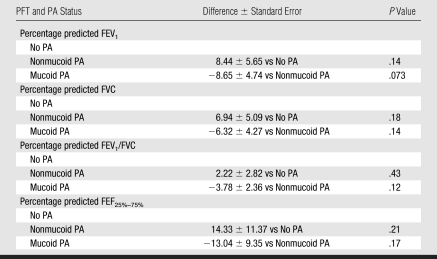
Table 4 shows that there are no significant differences in percentage predicted FEV1, percentage predicted FVC, percentage predicted FEV1/FVC, and percentage predicted FEF25%–75% in patients with nonmucoid PA compared with patients without PA infection. However, percentage predicted FEV1 showed a strong trend of lower values in patients with mucoid PA compared with those with nonmucoid PA (P = .07).
Table 4.
General Linear Model Analyses of the Cross-sectional PFT Measurements
Note.—Adjusted for all risk factors as shown in Table 1.
Discussion
Children with CF are born with morphologically normal lungs. A cycle of inflammation and infection begins within the first few months of life that leads to progressive lung damage (3,14,15). Lung disease is the cause of death of 95% of people with CF, with a mean survival currently at 37 years of age (23). To plan health care to slow or stop this progressive damage, it is essential to know which potential risk factors are associated with more severe lung disease so that these factors can be specifically addressed.
Children with CF are prone to develop irreversible lung disease at a young age (3,5). Long-term epidemiologic studies show that even when the diagnosis is made early through newborn screening and malnutrition is prevented (13), CF patients infected with respiratory pathogens are likely to develop chronic cough and radiographic chest abnormalities (2,3,14). However, to our knowledge, prior to this study the potential determinants of specific morphologic abnormalities such as bronchiectasis had not been analyzed.
The results of this study indicate an association between bronchiectasis and mucoid PA, but do not address causation. A causal relationship between mucoid PA and CF lung disease, however, has been suggested by other studies (14,15). Longitudinal studies have shown that early colonization with mucoid PA is associated with a more rapid decline in lung function (24), increased morbidity and mortality (25), and a 2.6 times greater 8-year risk of death (26). Li et al (15) found that increased cough scores, worsening abnormalities on chest radiographs, and deterioration of lung function correlated best with the transition of patients with culture-positive nonmucoid PA to culture-positive mucoid PA, suggesting a specific role for mucoid PA. In our study, neither S aureus nor nonmucoid PA, which are known to colonize bronchiectatic airways, were associated with increased bronchiectasis scores. These results suggest that mucoid PA is more than a marker for the presence of bronchiectasis. Mucoid PA was the only one of 12 factors studied that was associated with increased bronchiectasis severity. Factors that relate to overall morbidity, such as antibiotic use, days with infection, and days with hospitalization, did not correlate with the severity of bronchiectasis.
Value of Quantitative Chest Imaging
In this study, no significant correlations were seen between PFTs and the potential etiologic factors for bronchiectasis and CF lung disease, while both CT bronchiectasis score and overall disease severity scores demonstrated significant correlation with colonization with mucoid PA. This is not surprising, since PFTs depend on the behavior of the entirety of both lungs, while the anatomic localization provided by using CT allows reliable identification of disease affecting as little as a single bronchopulmonary segment. A previous study found that bronchiectasis was found in three or more lobes in 17% of children ages 6–10 years with normal PFT results (7). Previous studies have demonstrated that, in addition to being sensitive, CT scanning is reliable and reproducible (16,27). The current study supports the value of CT scanning for studies investigating the severity of CF lung disease in children.
Currently, no other imaging modality, including magnetic resonance imaging (28), provides the sensitivity and specificity for the changes of CF lung disease provided by CT scanning. The technique used in this study resulted in a dose of 0.55 mSv, approximately the amount of radiation exposure from 9 weeks of background radiation. Since this study began, the value of reducing voltage as a means of further reducing radiation exposure has been explored (29). It is likely that the voltage could be reduced from 120 to 100 kVp and still allow for adequate image quality. This would reduce radiation exposure by an additional 30%.
Pathogenesis of Bronchiectasis
The prevalence of bronchiectasis, its early onset in CF, and its causes have not been well understood. Furthermore, the lack of longitudinal bronchiectasis-focused epidemiologic investigations beginning during infancy has left a gap in knowledge about the determinants of this structural lesion in children with CF. Nevertheless, there is a body of literature (30–33) incriminating recurrent lower respiratory tract infections in children who develop bronchiectasis as a result of conditions other than CF. Although there has been limited research on specific respiratory pathogens and their association with bronchiectasis, which is considered an “orphan disease” (32), it seems that many different bacteria and perhaps some viruses are capable of bronchial damage that is sufficient to cause bronchiectasis (30–33). However, to our knowledge, the relationship between PA and bronchiectasis has not been studied in an epidemiologic investigation.
Our CF cohort data accrued over the course of more than 20 years and analyses of potential bronchiectasis determinants revealed a striking observation—only mucoid PA accounted for the irreversible, structural bronchopulmonary lesion of bronchiectasis. In this study we found that the severity of bronchiectasis and of morphologic changes of CF lung disease overall measured at CT scanning is significantly related to colonization with mucoid PA. Colonization with nonmucoid PA and multiple other potential risk factors, including antibiotic use and days of hospitalization, were not significantly associated with increased lung disease.
Although this is an original discovery, perhaps it should not be surprising when one considers the following: (a) Mucoid PA with its protective biofilm is generally resistant to antibiotics (15,34,35), and (c) mucoid PA produces many toxins and virulence factors (36). In addition, our previous observations revealed that mucoid PA respiratory infections accelerate CF lung disease (14) and pulmonary dysfunction (15). On the other hand, our assessment of pulmonary function data compared with thin-section CT evaluation demonstrated that the former surrogate of CF lung disease in children is much less sensitive (quantitatively, about fourfold more revealing).
Clinical Implications
PA, which is ubiquitous in the environment, very effectively targets the CF lung (34,37). The acquisition of mucoid PA occurs only after acquisition of nonmucoid PA (21–23), and the transition from absence of PA infection to mucoid PA occurs over a period of years in most patients (15). This provides a window of opportunity to prevent or delay the acquisition of mucoid PA, which cannot be eradicated by current antibiotics (38,39).
Accordingly, we studied a variety of factors that could conceivably be associated with irreversible lung disease (manifested by bronchiectasis) and tested the hypothesis that PA would contribute significantly. There are study limitations, however, that relate to our enrolled population and design. The number of patients investigated and the cross-sectional evaluation of 82 patients could be limiting, as well as the genotype and clinical variations shown in Table 1. In addition, the cultures of respiratory secretions were based largely on oropharyngeal swabs, which have limited sensitivity (34). One other limitation of our analyses is the possible effect of collinearity and/or confounding among the independent variables. The P values for the individual terms reported in Table 2 are type III P values—that is, they reflect the incremental gain in the model when the term is added to a model already including some of the other terms. As such, a nonsignificant P value does not imply that the term is unrelated to the outcome; rather, it only implies that the term provides nothing more than the other terms.
The association of bronchiectasis with mucoid PA has many implications for diagnosis and treatment in children with CF. Because bronchiectasis may develop early during infancy (40), and newborns with CF are not colonized with PA, these findings add to the arguments for diagnosis through newborn screening combined with aggressive care to avoid chronic and especially mucoid PA infections. Although studies have linked mucoid PA to deteriorating pulmonary function (15,41) and early mortality (25), to our knowledge this is the first observation that irreversible lung structural damage is specifically associated with mucoid PA. Thus, care to prevent PA exposure is essential, especially in infants and young children diagnosed with CF through newborn screening. This follow-up care component was emphasized in the Center for Disease Control's recommendation for nationwide implementation of newborn screening (42). The risk of PA cross-infection is substantial (25,43–46) and the impact of early, nosocomial PA acquisition may be catastrophic (25,46,47). In addition, methods of infection control are well described in the literature (48), but need to be applied rigorously. Although early recognition of nonmucoid PA is important, cultures are somewhat insensitive (49) when obtained in the routine fashion from young children with CF who typically do not expectorate sputum (50,51). Consequently, reliable methods of PA serology are sorely needed (51,52).
Finally, treating nonmucoid PA with antibiotics capable of eradicating this organism (43,53) before mucoid transformation occurs is clearly desirable to prevent early development of progressive lung disease with bronchiectasis in children with CF. Although no long-term placebo-controlled trial exists to examine this, and the issue of bronchiectasis prevention awaits further research, several relatively short-term, randomized controlled trials (43,53–55) have suggested that initial colonization of PA in the respiratory tract can be at least temporarily eradicated by the early use of antibiotics. Our findings suggest that averting infection with mucoid strains of PA is critical in the prevention of chronic bronchiectasis and pulmonary deterioration in patients with CF.
Acknowledgments
We thank the radiology department personnel at the University of Wisconsin–Madison and the Children's Hospital of Wisconsin and all the investigators of the Cystic Fibrosis Neonatal Screening Study Group.
Received October 22, 2008; revision requested December 1; revision received February 9, 2009; accepted February 13; final version accepted February 16.
Supported by Cystic Fibrosis Foundation grant A001-5-01.
Funding: This work supported by the National Institutes of Health (grants DK34108, M01 RR03186).
Authors stated no financial relationship to disclose.
See also the article by McDermott et al and the editorial by Saavedra and Lynch in this issue.
Abbreviations:
- CF
- cystic fibrosis
- FEF25%–75%
- midexpiratory phase of forced expiratory flow
- FEV1
- forced expiratory volume in 1 minute
- FVC
- forced vital capacity
- PA
- Pseudomonas aeruginosa
- PFT
- pulmonary function test
References
- 1.Burns JL, Emerson J, Stapp JR, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 1998; 27( 1): 158– 163 [DOI] [PubMed] [Google Scholar]
- 2.Farrell PM, Li Z, Kosorok MR, et al. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med 2003; 168( 9): 1100– 1108 [DOI] [PubMed] [Google Scholar]
- 3.Farrell PM, Li Z, Kosorok MR, et al. Longitudinal evaluation of bronchopulmonary disease in children with cystic fibrosis. Pediatr Pulmonol 2003; 36( 3): 230– 240 [DOI] [PubMed] [Google Scholar]
- 4.Brody AS, Tiddens HA, Castile RG, et al. Computed tomography in the evaluation of cystic fibrosis lung disease. Am J Respir Crit Care Med 2005; 172( 10): 1246– 1252 [DOI] [PubMed] [Google Scholar]
- 5.Brody AS, Sucharew H, Campbell JD, et al. Computed tomography correlates with pulmonary exacerbations in children with cystic fibrosis. Am J Respir Crit Care Med 2005; 172( 9): 1128– 1132 [DOI] [PubMed] [Google Scholar]
- 6.Evans SA, Turner SM, Bosch BJ, et al. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. Eur Respir J 1996; 9( 8): 1601– 1604 [DOI] [PubMed] [Google Scholar]
- 7.Brody AS, Klein JS, Molina PL, et al. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr 2004; 145( 1): 32– 38 [DOI] [PubMed] [Google Scholar]
- 8.Twiss J, Stewart AW, Byrnes CA. Longitudinal pulmonary function of childhood bronchiectasis and comparison with cystic fibrosis. Thorax 2006; 61( 5): 414– 418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miszkiel KA, Wells AU, Rubens MB, et al. Effects of airway infection by Pseudomonas aeruginosa: a computed tomographic study. Thorax 1997; 52( 3): 260– 264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheehan RE, Wells AU, Copley SJ, et al. A comparison of serial computed tomography and functional change in bronchiectasis. Eur Respir J 2002; 20( 3): 581– 587 [DOI] [PubMed] [Google Scholar]
- 11.de Jong PA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 2004; 23( 1): 93– 97 [DOI] [PubMed] [Google Scholar]
- 12.Fost N, Farrell PM. A prospective randomized trial of early diagnosis and treatment of cystic fibrosis: a unique ethical dilemma. Clin Res 1989; 37( 3): 495– 500 [PubMed] [Google Scholar]
- 13.Farrell PM. Improving the health of patients with cystic fibrosis through newborn screening. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Adv Pediatr 2000; 47: 79– 115 [PubMed] [Google Scholar]
- 14.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol 2001; 32( 4): 277– 287 [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005; 293( 5): 581– 588 [DOI] [PubMed] [Google Scholar]
- 16.Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging 2006; 21( 1): 14– 21 [DOI] [PubMed] [Google Scholar]
- 17.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004; 125( 2): 509– 521 [DOI] [PubMed] [Google Scholar]
- 18.Brody AS, Molina PL, Klein JS, et al. High-resolution computed tomography of the chest in children with cystic fibrosis: support for use as an outcome surrogate. Pediatr Radiol 1999; 29( 10): 731– 735 [DOI] [PubMed] [Google Scholar]
- 19.Braun AT, Farrell PM, Ferec C, et al. Cystic fibrosis mutations and genotype-pulmonary phenotype analysis. J Cyst Fibros 2006; 5( 1): 33– 41 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993; 15( 2): 75– 88 [DOI] [PubMed] [Google Scholar]
- 21.Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 2001; 358( 9286): 983– 984 [DOI] [PubMed] [Google Scholar]
- 22.Sokol PA, Luan MZ, Storey DG, et al. Genetic rearrangement associated with in vivo mucoid conversion of Pseudomonas aeruginosa PAO is due to insertion elements. J Bacteriol 1994; 176( 3): 553– 562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cystic Fibrosis Foundation. Cystic fibrosis patient registry 2007 annual data report. Bethesda, Md: Cystic Fibrosis Foundation, 2008 [Google Scholar]
- 24.Schaedel C, de Monestrol I, Hjelte L, et al. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 2002; 33( 6): 483– 491 [DOI] [PubMed] [Google Scholar]
- 25.Nixon GM, Armstrong DS, Carzino R, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 2001; 138( 5): 699– 704 [DOI] [PubMed] [Google Scholar]
- 26.Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34( 2): 91– 100 [DOI] [PubMed] [Google Scholar]
- 27.de Jong PA, Ottink MD, Robben SG, et al. Pulmonary disease assessment in cystic fibrosis: comparison of CT scoring systems and value of bronchial and arterial dimension measurements. Radiology 2004; 231( 2): 434– 439 [DOI] [PubMed] [Google Scholar]
- 28.Puderbach M, Eichinger M, Haeselbarth J, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thin-section CT and chest x-ray. Invest Radiol 2007; 42( 10): 715– 725 [DOI] [PubMed] [Google Scholar]
- 29.Paterson A, Frush DP. Dose reduction in paediatric MDCT: general principles. Clin Radiol 2007; 62( 6): 507– 517 [DOI] [PubMed] [Google Scholar]
- 30.Singleton R, Morris A, Redding G, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol 2000; 29( 3): 182– 187 [DOI] [PubMed] [Google Scholar]
- 31.Valery PC, Torzillo PJ, Mulholland K, et al. Hospital-based case-control study of bronchiectasis in indigenous children in Central Australia. Pediatr Infect Dis J 2004; 23( 10): 902– 908 [DOI] [PubMed] [Google Scholar]
- 32.Barker AF, Bardana EJ., Jr Bronchiectasis: update of an orphan disease. Am Rev Respir Dis 1988; 137( 4): 969– 978 [DOI] [PubMed] [Google Scholar]
- 33.Nicotra MB. Bronchiectasis. Semin Respir Infect 1994; 9( 1): 31– 40 [PubMed] [Google Scholar]
- 34.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168( 8): 918– 951 [DOI] [PubMed] [Google Scholar]
- 35.Baltimore RS, Christie CD, Smith GJ. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis: implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis 1989; 140( 6): 1650– 1661 [DOI] [PubMed] [Google Scholar]
- 36.Corech R, Rao A, Laxova A, et al. Early immune response to the components of the type III system of Pseudomonas aeruginosa in children with cystic fibrosis. J Clin Microbiol 2005; 43( 8): 3956– 3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadikot RT, Blackwell TS, Christman JW, et al. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005; 171( 11): 1209– 1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch C. Early infection and progression of cystic fibrosis lung disease. Pediatr Pulmonol 2002; 34( 3): 232– 236 [DOI] [PubMed] [Google Scholar]
- 39.Hoiby N. Prospects for the prevention and control of pseudomonal infection in children with cystic fibrosis. Paediatr Drugs 2000; 2( 6): 451– 463 [DOI] [PubMed] [Google Scholar]
- 40.Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 2004; 144( 2): 154– 161 [DOI] [PubMed] [Google Scholar]
- 41.Parad RB, Gerard CJ, Zurakowski D, et al. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect Immun 1999; 67( 9): 4744– 4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep 2004; 53( RR-13): 1– 36 [PubMed] [Google Scholar]
- 43.Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003; 167( 6): 841– 849 [DOI] [PubMed] [Google Scholar]
- 44.Ojeniyi B, Frederiksen B, Hoiby N. Pseudomonas aeruginosa cross-infection among patients with cystic fibrosis during a winter camp. Pediatr Pulmonol 2000; 29( 3): 177– 181 [DOI] [PubMed] [Google Scholar]
- 45.Farrell PM, Shen G, Splaingard M, et al. Acquisition of Pseudomonas aeruginosa in children with cystic fibrosis. Pediatrics 1997; 100( 5): E2 doi: 10.1542/peds.100.5.e2 Accessed September 2008 [DOI] [PubMed] [Google Scholar]
- 46.Griffiths AL, Jamsen K, Carlin JB, et al. Effects of segregation on an epidemic Pseudomonas aeruginosa strain in a cystic fibrosis clinic. Am J Respir Crit Care Med 2005; 171( 9): 1020– 1025 [DOI] [PubMed] [Google Scholar]
- 47.Armstrong DS, Nixon GM, Carzino R, et al. Detection of a widespread clone of Pseudomonas aeruginosa in a pediatric cystic fibrosis clinic. Am J Respir Crit Care Med 2002; 166( 7): 983– 987 [DOI] [PubMed] [Google Scholar]
- 48.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control 2003; 31( 3 suppl): S1– S62 [PubMed] [Google Scholar]
- 49.Rosenfeld M, Emerson J, Accurso F, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol 1999; 28( 5): 321– 328 [DOI] [PubMed] [Google Scholar]
- 50.West SE, Zeng L, Lee BL, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA 2002; 287( 22): 2958– 2967 [DOI] [PubMed] [Google Scholar]
- 51.Kappler M, Kraxner A, Reinhardt D, et al. Diagnostic and prognostic value of serum antibodies against Pseudomonas aeruginosa in cystic fibrosis. Thorax 2006; 61( 8): 684– 688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farrell PM, Govan JR. Pseudomonas serology: confusion, controversy, and challenges. Thorax 2006; 61( 8): 645– 647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiesemann HG, Steinkamp G, Ratjen F, et al. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr Pulmonol 1998; 25( 2): 88– 92 [DOI] [PubMed] [Google Scholar]
- 54.Munck A, Bonacorsi S, Mariani-Kurkdjian P, et al. Genotypic characterization of Pseudomonas aeruginosa strains recovered from patients with cystic fibrosis after initial and subsequent colonization. Pediatr Pulmonol 2001; 32( 4): 288– 292 [DOI] [PubMed] [Google Scholar]
- 55.Gibson RL, Emerson J, Mayer-Hamblett N, et al. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr Pulmonol 2007; 42( 7): 610– 623 [DOI] [PubMed] [Google Scholar]