Multiecho iterative decomposition of water and fat with echo asymmetry and least-squares estimation gradient-echo MR is a promising method for rapid evaluation of morphologic characteristics of cartilage.
Abstract
Institutional review board approval and informed consent were obtained for this HIPAA-compliant study. The purpose was to prospectively compare multiecho iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) gradient-echo (GRE) magnetic resonance (MR) imaging with three-dimensional fat-suppressed (FS) spoiled GRE (SPGR) MR imaging to evaluate the articular cartilage of the knee. Six healthy volunteer and 10 cadaver knees were imaged at 1.5 T. Signal-to-noise ratio (SNR), SNR efficiency, and cartilage volume were measured. SNR and SNR efficiency were significantly higher with multiecho IDEAL GRE than with FS SPGR imaging (P < .031). Both methods produced equivalent cartilage volumes (overall concordance correlation coefficient, 0.998) with high precision and accuracy. The use of a cartilage phantom confirmed high accuracy in volume measurements and high reproducibility for both methods. Multiecho IDEAL GRE provides high signal intensity in cartilage and synovial fluid and is a promising technique for imaging articular cartilage of the knee.
Affecting over 20 million people, osteoarthritis is second only to cardiovascular disease as the leading cause of chronic disability in the United States (1,2). The hallmark in diagnosis of osteoarthritis is the detection of articular cartilage degeneration. Magnetic resonance (MR) imaging has become the reference standard in imaging articular cartilage because it noninvasively provides multiplanar images with excellent soft-tissue contrast (3). Accurate diagnosis and tracking of osteoarthritis are needed in the clinical and research settings to direct pharmacologic, surgical, and lifestyle changes, as well as to evaluate the effects of these treatments longitudinally (4,5).
Osteoarthritis research has focused on using three-dimensional MR imaging to study morphologic characteristics of cartilage, with the most popular of these techniques being fat-suppressed (FS) spoiled gradient-echo (SPGR)–recalled acquisition in the steady state (6). Yet, three-dimensional FS SPGR has its limitations: it requires relatively long imaging times of 5–10 minutes and results in dark-appearing synovial fluid owing to the T1 weighting of this pulse sequence. This study compares FS SPGR with a more rapid gradient-echo (GRE) MR imaging pulse sequence recently developed at our institution. Although the T1-weighted contrast of FS SPGR has traditionally been favored for morphologic depiction of cartilage, bright-appearing synovial fluid that can be seen on T2/T1-weighted GRE images produces an arthrographic effect that outlines cartilaginous fissures and surface defects (7).
Advances in Knowledge.
Multiecho iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) gradient-echo (GRE) MR images showed bright-appearing signal from synovial fluid that can be helpful to outline cartilage surface defects.
Multiecho IDEAL GRE compared favorably with fat-suppressed (FS) spoiled GRE (SPGR) imaging, with high reproducibility, accuracy, and equivalent cartilage volume measurements obtained from cadaveric knees.
In both healthy volunteers and cadavers, multiecho IDEAL GRE produced significantly higher cartilage signal-to-noise ratio (SNR) than did FS SPGR (P < .031).
Multiecho IDEAL GRE had an equivalent or faster imaging time than did FS SPGR.
A main challenge of many clinical and research applications of MR imaging is uniform FS in areas of B0 inhomogeneity. Traditional FS techniques include spectrally selective excitation that utilizes radiofrequency pulses to saturate or invert fat signal or excite water signal. These techniques, commonly combined with SPGR, are unfortunately vulnerable to B0 and B1 inhomogeneities. On the other hand, three-point Dixon techniques separate water and fat signals on the basis of their chemical shift differences, thus making Dixon techniques robust to B0 and B1 inhomogeneities.
Similar to a three-point Dixon method, iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) imaging (8) acquires three images at three echo times (TEs) (9) while providing uniform FS by using an iterative least-squares method to estimate B0 field inhomogeneities. IDEAL further improves on traditional Dixon methods by using asymmetrically placed echoes for optimum signal-to-noise ratio (SNR) (10).
IDEAL imaging has been previously combined with pulse sequences such as balanced steady-state free precession and SPGR (11,12); we have developed a sequence that differs from these pulse sequence combinations and from the standard single-echo IDEAL sequences. We propose a multiecho IDEAL method that acquires the three echoes in a single repetition time (TR), instead of the three TRs usually required for conventional IDEAL imaging. This multiecho IDEAL method consequently reduces imaging time and the potential for motion artifact. In our current study, multiecho IDEAL is combined with a GRE sequence to allow fluid to appear bright, instead of dark, as it does at SPGR imaging.
Implication for Patient Care.
In a shorter or an equivalent imaging time compared with FS SPGR, multiecho IDEAL GRE produces accurate cartilage volume measurements, while also having higher SNR and bright-appearing synovial fluid that may highlight cartilage surface pathologic features.
The purpose of this study was to compare multiecho IDEAL GRE and FS SPGR imaging in their evaluation of morphologic characteristics of cartilage with SNR, SNR efficiency, and cartilage volume quantification as means of comparison.
Materials and Methods
GE Healthcare (Milwaukee, Wis) provided research support in the development of our multiecho IDEAL GRE test sequence.
Subjects
Institutional review board approval and informed consent were obtained for this prospective Health Insurance Portability and Accountability Act–compliant study. MR imaging was performed on six knees in four healthy volunteers (two men, two women; age range, 22–39 years). To test the use of the pulse sequences in volunteers without osteoarthritis, we recruited volunteers who were asymptomatic, were less than 40 years of age, and had no history of knee pain or knee surgery. To test the use of the pulse sequences in those with osteoarthritis, MR imaging was performed on 10 knees in seven fresh-frozen cadavers (four men, three women; age range, 60–99 years). Cadaver knees were selected after computed tomographic analysis for another study, where all knees had at least marginal osteophytes and a grade between 1 and 4 on the Kellgren-Lawrence scale (13). Sample sizes for the healthy volunteer and cadaver cohorts were developed on the basis of availability.
MR Imaging
Healthy volunteers
Images were acquired with a 1.5-T unit (Signa TwinSpeed; GE Healthcare, Milwaukee, Wis) with high-performance gradients (maximum gradient strength, 40 mT/m; maximum slew rate, 150 mT/m/sec) by using a commercially available transmit-receive eight-channel knee coil. Acquisition resolution was kept constant for both imaging sequences, with a matrix of 256 × 256, 16-cm field of view, 1.5-mm section thickness, and 64 sagittal sections acquired over the knee joint. FS SPGR images were acquired with the following parameters: TR, 17.3 msec; TE, 3.0 msec; receiver bandwidth, ±31.25 kHz; flip angle, 20°; one acquisition with fat saturation; and imaging time, 4 minutes 44 seconds. Multiecho IDEAL GRE images were acquired with the following parameters: TR, 14.7 msec; TE, 2.7, 5.4, and 8.4 msec (10); receiver bandwidth, ±83 kHz; flip angle, 40°; and imaging time, 4 minutes 4 seconds.
Multiecho IDEAL GRE images were reconstructed online by using the IDEAL algorithm (14). Multiecho IDEAL GRE produced water, fat, and recombined water and fat (in-phase) and water minus fat (out-of-phase) images with no time penalty. The 20° flip angle for FS SPGR was chosen to be close to the Ernst angle for maximum cartilage signal, whereas the 40° flip angle for multiecho IDEAL GRE was chosen to maximize cartilage-to-fluid contrast (15,16).
Human cadavers
Changes in software between imaging the healthy volunteers and cadavers prevented exact replication of imaging parameters. The cadavers were imaged with the same parameters as were the healthy volunteers, with the following minor differences: 68 sagittal sections were acquired with an imaging time of 4 minutes 6 seconds both sequences. Multiecho IDEAL GRE images were acquired with a TR of 15.0 msec. Because there was no significant synovial fluid remaining in the cadavers, a 20° flip angle for multiecho IDEAL GRE was chosen to maximize cartilage signal. FS SPGR images were acquired with a TR of 15.0 msec, a TE of 2.0 msec, and a flip angle of 20°.
Cartilage phantom
To evaluate interimage reproducibility, a novel femoral cartilage phantom (Crescent Phantom, U.S. Patent #6 992 280, March 12, 2003; Synarc, San Francisco, Calif) was imaged with the same parameters used for the cadavers. The phantom was imaged three times with each pulse sequence at different section locations and was repositioned in between the images. The phantom consisted of a solid ball positioned off-center within a larger hollow sphere, forming a crescent-shaped volume in between that was filled with 21.7 mL of contrast material (Magnevist; Schering, Berlin, Germany).
Image Evaluation
Image evaluation was supervised by a radiologist (G.E.G., with 11 years experience with musculoskeletal images). Images for SNR and cartilage volume measurements were presented at random over a 2-month period to prevent memory and learning biases. Cartilage SNR was calculated in both healthy volunteers and human cadavers by measuring the signal intensity in cartilage and noise in five regions of interest in the trochlear cartilage and artifact-free areas of the background, respectively. The region of interest for cartilage measurements was 2 mm in diameter, and the region of interest for noise measurements was 10 mm in diameter. The average signal of the trochlear cartilage was divided by the standard deviation of the noise to produce the SNR. SNR efficiency took into account differences in imaging time and was computed relative to the SNR value of multiecho IDEAL GRE. To calculate the SNR efficiency of FS SPGR, the SNR of FS SPGR was multiplied by the ratio of the square root of the imaging time of multiecho IDEAL GRE to the square root of the imaging time of FS SPGR. By using this calculation, the SNR efficiency of multiecho IDEAL GRE is the same as its SNR value.
Cartilage volume measurements were compared between multiecho IDEAL GRE and FS SPGR by segmenting the images from the cadavers. Although FS SPGR is commonly used to measure cartilage volume, cartilage volume measurement for each technique was compared with a cartilage phantom's known volume for an additional measure of accuracy. The cadaveric knees were chosen for cartilage segmentation because they had varying degrees of osteoarthritis and were, thus, more representative of the patient setting than were the healthy volunteers. Femoral, tibial, and patellar cartilage were manually segmented with an image processing software program (3D Slicer, version 2.6; Brigham and Women's Hospital, Boston, Mass) by a single observer (C.A.C., with 2 years experience in cartilage image analysis). Combined fat and water images were used in multiecho IDEAL GRE segmentation. The intraobserver reproducibility was assessed by segmenting the femoral cartilage of one cadaver image set three times. The six image sets of the cartilage phantom were segmented for volume with an image processing software program (OsiriX, version 2.7.5; OsiriX Foundation, Geneva, Switzerland); these segmented volumes were compared with their known physical volume.
Statistical Analysis
Differences between multiecho IDEAL GRE and FS SPGR in SNR and SNR efficiency for healthy volunteers and in SNR for cadavers were tested by using exact two-sided paired Wilcoxon tests. This analysis was performed by using a computer program (R, version 2.7.1 [ http://www.r-project.org ]), with the conditional permutation inference calculated by the “coin” package from the Comprehensive R Archive Network (http://cran.r-project.org ). All other statistical analyses were conducted through the use of another computer program (STATA, release 9.2; Stata, College Station, Tex). In the cadavers, accuracy of the multiecho IDEAL GRE volume measurements relative to the reference standard, FS SPGR, was assessed by using the concordance correlation coefficient (CCC), 95% confidence interval, the Bland-Altman 95% limits of agreement, and the mean and standard deviation of the difference between the two sequences. In the cadavers, intraobserver variability was assessed by calculating the coefficient of variation. For the phantom, interimage reproducibility was assessed by calculating the coefficient of variation and the actual and relative differences between the sequences' volume measurements and the phantom's volume. The actual and relative differences are reported as means and standard errors.
Results
All multiecho IDEAL GRE images had bright-appearing signal from synovial fluid that was helpful in outlining cartilage defects (7). Fluid was not easily visible on FS SPGR images (Fig 1).
Figure 1a:
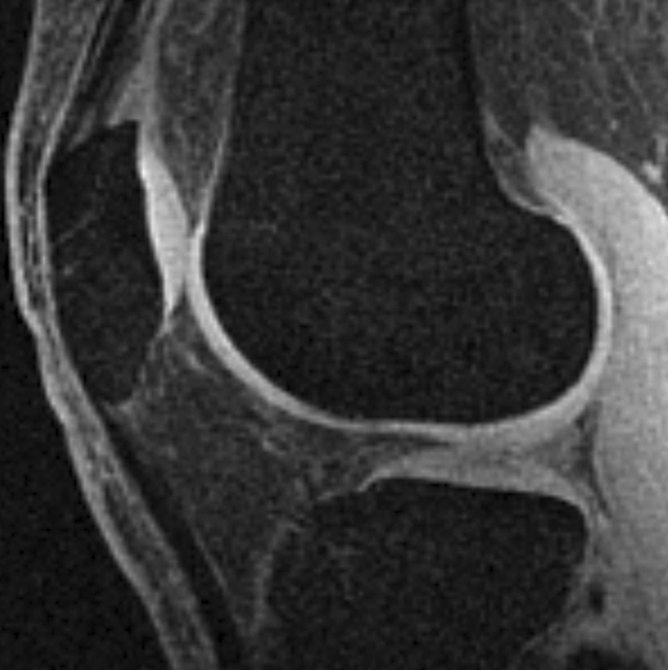
Sagittal MR images in healthy volunteer. (a) FS SPGR (TR/TE, 17.3/3 msec; flip angle, 20°) and (b) multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) water images with bright-appearing synovial fluid (arrow). (c) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) fat image. (d) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) combined image.
Figure 1b:
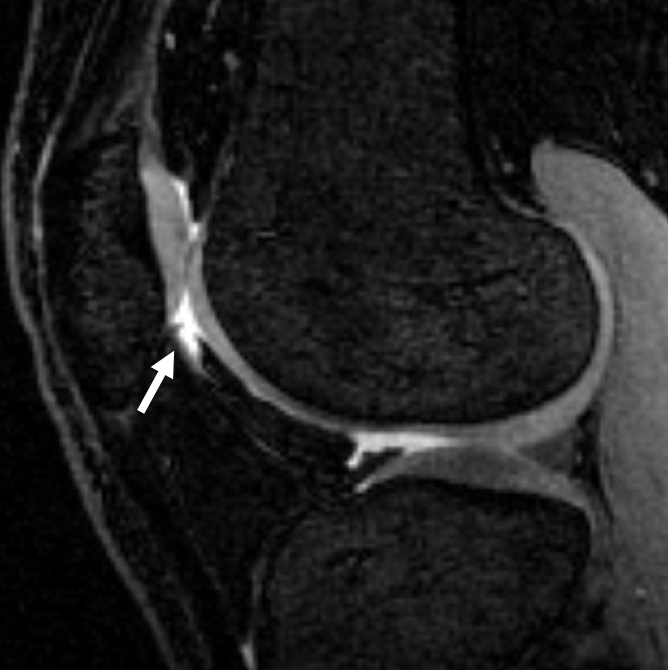
Sagittal MR images in healthy volunteer. (a) FS SPGR (TR/TE, 17.3/3 msec; flip angle, 20°) and (b) multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) water images with bright-appearing synovial fluid (arrow). (c) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) fat image. (d) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) combined image.
Figure 1c:
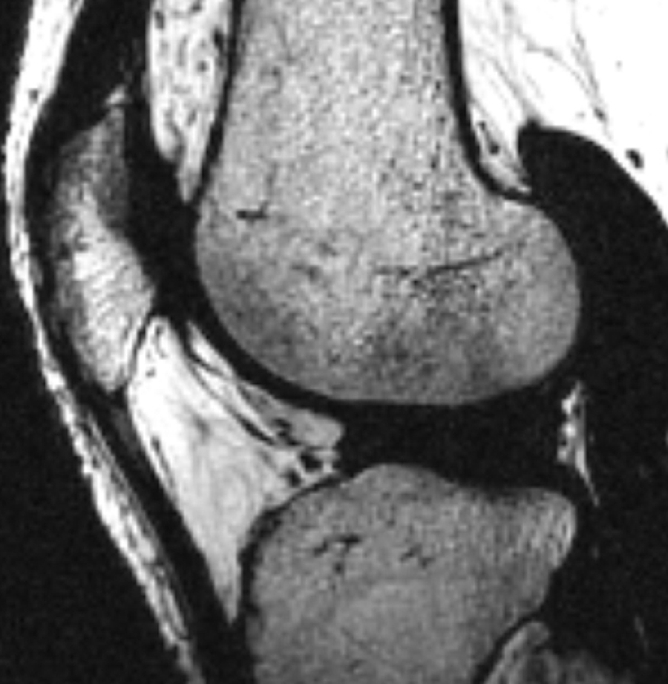
Sagittal MR images in healthy volunteer. (a) FS SPGR (TR/TE, 17.3/3 msec; flip angle, 20°) and (b) multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) water images with bright-appearing synovial fluid (arrow). (c) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) fat image. (d) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) combined image.
Figure 1d:
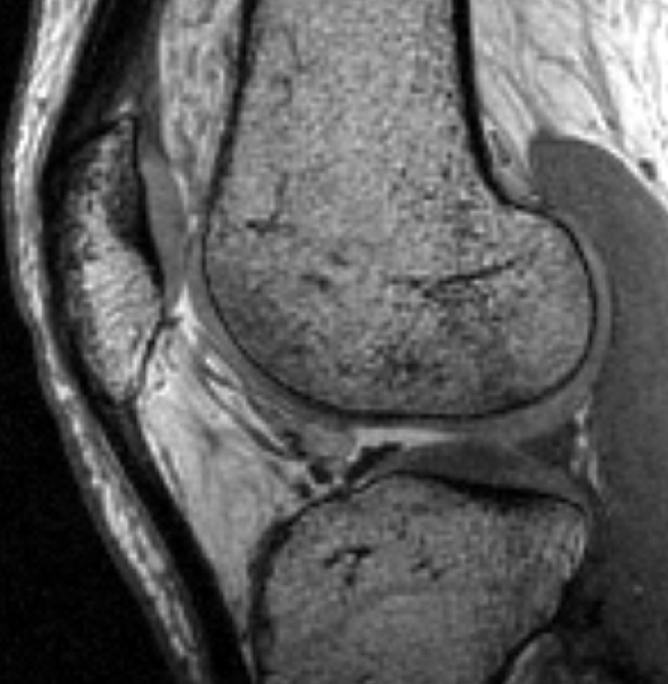
Sagittal MR images in healthy volunteer. (a) FS SPGR (TR/TE, 17.3/3 msec; flip angle, 20°) and (b) multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) water images with bright-appearing synovial fluid (arrow). (c) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) fat image. (d) Multiecho IDEAL GRE (TR, 14.7; flip angle, 40°) combined image.
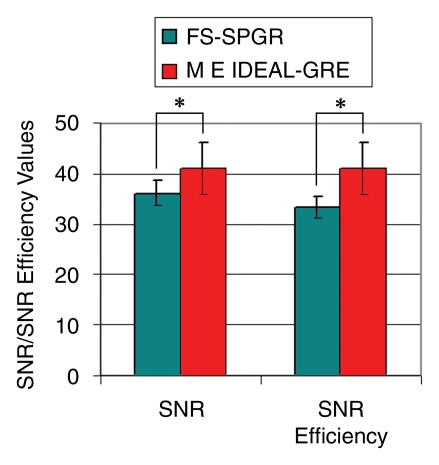
In data obtained from the healthy volunteers, mean cartilage SNR was 41.0 ± 5.2 in multiecho IDEAL GRE images, while mean cartilage SNR was 36.0 ± 2.5 in FS SPGR images (P < .031). Mean cartilage SNR efficiency was also significantly higher (P < .031) with multiecho IDEAL GRE (41.0 ± 5.2) than with FS SPGR (33.3 ± 2.3). Figure 2 demonstrates a higher SNR and SNR efficiency of multiecho IDEAL GRE than for FS SPGR.
Figure 2:
Graph shows cartilage SNR and SNR efficiency values for multiecho (ME) IDEAL GRE and FS SPGR images of healthy volunteers. Cartilage SNR and SNR efficiency measurements were significantly higher (P < .031) for multiecho IDEAL GRE than for FS SPGR.
In data obtained from the cadavers, mean cartilage SNR was the same as the mean cartilage SNR efficiency because imaging times for both sequences were the same. Like the measurements in the healthy volunteers, mean cartilage SNR was again significantly higher (P < .002) with multiecho IDEAL GRE (52.3 ± 9.6) than with FS SPGR (40.0 ± 9.3).
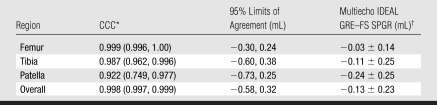
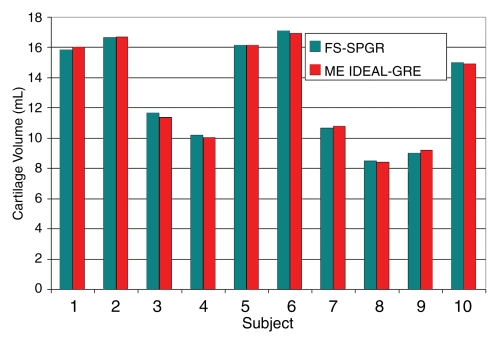
Table 1 displays the measures of accuracy for femoral, tibial, and patellar cartilage volume measurements determined by using multiecho IDEAL GRE and FS SPGR. The CCC compared volume measurements by using the two sequences per knee region per cadaver knee. The overall CCC of 0.998 (95% confidence interval: 0.997, 0.999) demonstrated the high accuracy of multiecho IDEAL GRE in measuring cartilage volumes, compared with the reference standard for morphologic characteristics of cartilage, FS SPGR. The CCC was particularly high for both femoral cartilage (CCC, 0.999) and tibial cartilage (CCC, 0.987) but was lower for patellar cartilage (CCC, 0.922). Figure 3 displays the similar femoral cartilage volume measurements produced by using the two sequences in all 10 cadaver knees.
Table 1.
Comparison between Multiecho IDEAL GRE and FS SPGR of Segmented Volumes of Human Cadaveric Knees
* Data are the CCCs; numbers in parentheses are the 95% confidence intervals.
† Data are the mean ± standard deviation.
Figure 3a:
(a) Graph shows comparison of femoral cartilage volume measurements between multiecho (ME) IDEAL GRE and FS SPGR imaging, which produced equivalent cartilage volume measurements, with overall CCC of 0.999 for femoral, patellar, and tibial cartilage in 10 cadaveric knees. (b) Model created from cartilage segmentation of multiecho IDEAL GRE (TR, 15 msec; flip angle, 20°) water images from human cadaver. Femoral (red), tibial (yellow), and patellar (blue) cartilage were easily visible on multiecho IDEAL GRE images.
Figure 3b:
(a) Graph shows comparison of femoral cartilage volume measurements between multiecho (ME) IDEAL GRE and FS SPGR imaging, which produced equivalent cartilage volume measurements, with overall CCC of 0.999 for femoral, patellar, and tibial cartilage in 10 cadaveric knees. (b) Model created from cartilage segmentation of multiecho IDEAL GRE (TR, 15 msec; flip angle, 20°) water images from human cadaver. Femoral (red), tibial (yellow), and patellar (blue) cartilage were easily visible on multiecho IDEAL GRE images.
The mean differences in volume measurements between the two sequences showed that FS SPGR consistently measured slightly higher cartilage volumes than did multiecho IDEAL GRE by about 0.10 mL. The variability in differences between the two sequences was low, with the highest standard deviation of the differences across the knee regions being ±0.25 mL. The Bland-Altman 95% limits of agreement indicated that 95% of the volume measurement differences between multiecho IDEAL GRE and FS SPGR are (−0.30, 0.24 mL) for femoral cartilage, (−0.60, 0.38 mL) for tibial cartilage, and (−0.73, 0.25 mL) for patellar cartilage.
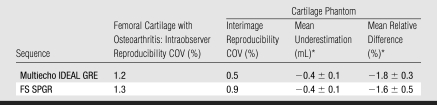
In the phantom experiments, the cartilage volume segmented with multiecho IDEAL GRE and FS SPGR showed high accuracy. The mean absolute underestimation of the cartilage volume was 0.4 mL ± 0.1 for both multiecho IDEAL GRE and FS SPGR. The corresponding relative differences were −1.8% ± 0.3 for multiecho IDEAL GRE and −1.6% ± 0.5 for FS SPGR (Table 2).
Table 2.
Comparison of Multiecho IDEAL GRE and FS SPGR in Reproducibility and Accuracy of Volumetric Quantification
Note.—COV = coefficient of variation.
* Data are the mean ± standard deviation.
Precision was high for our observer who segmented the femoral cartilage volumes of the cadavers; the coefficients of variation for intraobserver reproducibility were 1.2% for multiecho IDEAL GRE and 1.3% for FS SPGR. Interimage reproducibility was also high, with coefficients of variability of 0.5% for multiecho IDEAL GRE and 0.9% for FS SPGR (Table 2).
Discussion
Multiecho IDEAL GRE is a pulse sequence that was recently developed to facilitate rapid MR imaging with fat-water separation. Multiecho IDEAL gives additional flexibility in choice of image parameters and imaging time when compared with single-echo IDEAL (12). Multiecho IDEAL acquires all three echoes with one TR, reducing the potential for motion between echoes that is inherent in single-echo IDEAL imaging. The actual imaging time of a multiecho IDEAL acquisition (depending on receiver bandwidth) is shorter than a comparable single-echo IDEAL image. Because of the need for three acquisitions, single-echo IDEAL images usually require longer imaging times than do conventional FS SPGR images. However, multiecho IDEAL has an imaging time similar to that of FS SPGR.
Multiecho IDEAL GRE provides high signal intensity levels for both hyaline cartilage and synovial fluid. The bright-appearing fluid in multiecho IDEAL GRE imaging is generated by the optimization of the flip angle for maximal cartilage-to-fluid contrast and by the gradient spoiling in GRE imaging that results in a mixed T2/T1 contrast (17). On the other hand, SPGR uses radiofrequency spoiling to reduce signal intensity from fluid, producing T1-weighted contrast with dark-appearing fluid (18). Sequences with bright-appearing fluid, such as dual-echo steady-state imaging (19), and sequences with dark-appearing fluid, such as FS SPGR imaging (20), have been previously used for cartilage assessment.
We present evidence that multiecho IDEAL GRE performs well in evaluating cartilage volume. Multiecho IDEAL GRE compares favorably with FS SPGR, with high reproducibility and accuracy in their equivalent cartilage volume measurements. Measurements of replaceability, such as the CCC, were extremely high for the femoral and tibial cartilage, indicating the similar performance of the two sequences for cartilage volume measurement. The CCC for patellar cartilage was lower, likely owing to the curvature of the patella in the sagittal plane of acquisition leading to partial volume effects.
Comparison of volume differences between our test sequence and FS SPGR showed that multiecho IDEAL GRE was precise, with low variability as measured by using standard deviations and Bland-Altman 95% limits of agreement. Furthermore, multiecho IDEAL GRE yielded an intraobserver reproducibility similar to that reported for FS SPGR (21–23). Reproducibility between images was greater than 99% for both sequences. The interimage reproducibility of multiecho IDEAL GRE was comparable with previously reported SPGR results in a cartilage phantom study (24) and, as expected, was higher than that reported in osteoarthritic femoral cartilage studies at a similar resolution (25,26).
Our results show that multiecho IDEAL GRE has a statistically higher cartilage SNR and SNR efficiency than does FS SPGR (P < .031). Previous work with IDEAL has found that its three echoes can be optimally spaced among the three required acquisitions to maximize SNR performance (10). Our multiecho IDEAL method further maximizes SNR performance by acquiring all optimally chosen echoes in a single acquisition. Thus, multiecho IDEAL GRE uses more time per TR for data acquisition, resulting in a higher acquisition duty cycle than for FS SPGR. In contrast, FS SPGR is relatively inefficient, as it has only one echo per TR and the length of its fat saturation pulse consumes a large fraction of the TR.
The equivalent or faster imaging time of multiecho IDEAL GRE, as compared with FS SPGR, also results from the sequence's multiple echoes per TR. Previous studies have shown that single-echo IDEAL can be combined with parallel imaging or partial k-space acquisition to reduce imaging time (12,27,28). In future studies, multiecho IDEAL GRE could be accelerated in the same manner at 3.0 T, as IDEAL has been successfully applied to 3.0-T imaging (11,12).
Our study had several limitations. This study tested the two pulse sequences in ex vivo cartilage with varying degrees of osteoarthritis to model the volume measurement capabilities of these sequences in a clinical setting. However, ex vivo osteoarthritic changes measured by these pulse sequences may not be the same as those in vivo. Future studies can test multiecho IDEAL GRE in evaluating osteoarthritic cartilage in vivo, as well as diseased ligaments, menisci, and tendons.
Another limitation of this study was that the observer who segmented the cartilage volumes could not be blinded from the inherent bias of being able to distinguish multiecho IDEAL GRE images from FS SPGR images. Future studies can validate this study's high precision with the use of more than one observer for interobserver variability. Additionally, a phantom was used as the reference standard of cartilage volume measurement. The cadaveric cartilage volume was not measured through water displacement because the tissues of the knees had by then degraded such that this method would have produced inaccurate results.
Multiecho IDEAL GRE can be further studied by testing its recombined images in quantification of subchondral bone and cartilage thickness. As this study was an initial analysis of multiecho IDEAL GRE, cartilage thickness was not measured because its longitudinal tracking requires a point-by-point comparison. In addition, previous research has found IDEAL imaging to be robust to the presence of iron in the decomposition of water and fat (29). Future studies can examine whether multiecho IDEAL can compensate for the susceptibility of GRE imaging to blooming artifacts. Future research can also investigate multiecho IDEAL GRE imaging with a matrix of 512 × 512, while keeping imaging times low and maintaining robust fat-water separation. This study's resolution was chosen to maintain high SNR for cartilage volume segmentation and was based on previous SPGR cartilage morphologic studies with comparable voxel sizes (25,26,30).
In conclusion, multiecho IDEAL GRE is a promising method for the rapid evaluation of morphologic characteristics of cartilage. Multiecho IDEAL GRE has high SNR efficiency, an imaging time comparable with FS SPGR, cartilage volume measurements equivalent to FS SPGR, and bright-appearing fluid that highlights cartilage pathologic features. Future studies that test the capabilities of multiecho IDEAL GRE in measuring in vivo osteoarthritic tissue and cartilage thickness at higher resolution are important to validate this technique.
Acknowledgment:
We thank Jarrett K. Rosenberg, PhD, Department of Radiology, Stanford University, for his assistance with statistical analysis.
Received August 11, 2008; revision requested September 24; revision received December 24; final version accepted January 30, 2009.
Funding: This research was supported by the National Institutes of Health (grants 1R01-EB002524 and 1R01-EB005790).
See Materials and Methods for pertinent disclosures.
Abbreviations:
- CCC
- concordance correlation coefficient
- FS
- fat suppressed
- GRE
- gradient echo
- IDEAL
- iterative decomposition of water and fat with echo asymmetry and least-squares estimation
- SNR
- signal-to-noise ratio
- SPGR
- spoiled GRE
- TE
- echo time
- TR
- repetition time
References
- 1.Lawrence RC, Hochberg MC, Kelsey JL, et al. Estimates of the prevalence of selected arthritic and musculoskeletal diseases in the United States. J Rheumatol 1989; 16: 427– 441 [PubMed] [Google Scholar]
- 2.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006; 20: 3– 25 [DOI] [PubMed] [Google Scholar]
- 3.Disler DG, Recht MP, McCauley TR. MR imaging of articular cartilage. Skeletal Radiol 2000; 29: 367– 377 [DOI] [PubMed] [Google Scholar]
- 4.McCauley TR, Disler DG. Magnetic resonance imaging of articular cartilage of the knee. J Am Acad Orthop Surg 2001; 9: 2– 8 [DOI] [PubMed] [Google Scholar]
- 5.Sangha O. Epidemiology of rheumatic diseases. Rheumatology (Oxford) 2000; 39( suppl 2): 3– 12 [DOI] [PubMed] [Google Scholar]
- 6.Disler DG, McCauley TR, Kelman CG, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol 1996; 167: 127– 132 [DOI] [PubMed] [Google Scholar]
- 7.Mosher TJ, Pruett SW. Magnetic resonance imaging of superficial cartilage lesions: role of contrast in lesion detection. J Magn Reson Imaging 1999; 10: 178– 182 [DOI] [PubMed] [Google Scholar]
- 8.Reeder SB, Wen Z, Yu H, et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med 2004; 51: 35– 45 [DOI] [PubMed] [Google Scholar]
- 9.Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging 1991; 1: 521– 530 [DOI] [PubMed] [Google Scholar]
- 10.Pineda AR, Reeder SB, Wen Z, Pelc NJ. Cramer-Rao bounds for three-point decomposition of water and fat. Magn Reson Med 2005; 54: 625– 635 [DOI] [PubMed] [Google Scholar]
- 11.Gold GE, Reeder SB, Yu H, et al. Articular cartilage of the knee: rapid three-dimensional MR imaging at 3.0 T with IDEAL balanced steady-state free precession—initial experience. Radiology 2006; 240: 546– 551 [DOI] [PubMed] [Google Scholar]
- 12.Siepmann DB, McGovern J, Brittain JH, Reeder SB. High-resolution 3D cartilage imaging with IDEAL SPGR at 3 T. AJR Am J Roentgenol 2007; 189: 1510– 1515 [DOI] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957; 16: 494– 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Reeder SB, Shimakawa A, Brittain JH, Pelc NJ. Field map estimation with a region growing scheme for iterative 3-point water-fat decomposition. Magn Reson Med 2005; 54: 1032– 1039 [DOI] [PubMed] [Google Scholar]
- 15.Kijowski R, Passov L, Shimakawa A, Yu H, Reeder S. Unspoiled Gradient Recalled-Echo Acquired in the Steady-State (GRASS) with IDEAL fat-suppression for high resolution cartilage imaging at 3T [abstr]. In: Proceedings of the Fifteenth Meeting of the International Society for Magnetic Resonance in Medicine Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2007; 3819 [Google Scholar]
- 16.Chen CA, John CT, Hargreaves BA, et al. Multi-echo IDEAL-GRE water-fat separation for rapid assessment of cartilage morphology [abstr]. In: Proceedings of the Fifteenth Meeting of the International Society for Magnetic Resonance in Medicine Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2007; 2618 [Google Scholar]
- 17.Sekihara K. Steady-state magnetizations in rapid NMR imaging using small flip angles and short repetition intervals. IEEE Trans Med Imaging 1987; 6: 157– 164 [DOI] [PubMed] [Google Scholar]
- 18.Frahm J, Haase A, Matthaei D. Rapid NMR imaging of dynamic processes using the FLASH technique. Magn Reson Med 1986; 3: 321– 327 [DOI] [PubMed] [Google Scholar]
- 19.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008; 16: 1433– 1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein F, Schnier M, Haubner M, et al. Accuracy of cartilage volume and thickness measurements with magnetic resonance imaging. Clin Orthop Relat Res 1998; 352: 137– 148 [PubMed] [Google Scholar]
- 21.Cicuttini F, Forbes A, Asbeutah A, Morris K, Stuckey S. Comparison and reproducibility of fast and conventional spoiled gradient-echo magnetic resonance sequences in the determination of knee cartilage volume. J Orthop Res 2000; 18: 580– 584 [DOI] [PubMed] [Google Scholar]
- 22.Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis Cartilage 1999; 7: 265– 271 [DOI] [PubMed] [Google Scholar]
- 23.Eckstein F, Westhoff J, Sittek H, et al. In vivo reproducibility of three-dimensional cartilage volume and thickness measurements with MR imaging. AJR Am J Roentgenol 1998; 170: 593– 597 [DOI] [PubMed] [Google Scholar]
- 24.Marshall KW, Mikulis DJ, Guthrie BM. Quantitation of articular cartilage using magnetic resonance imaging and three-dimensional reconstruction. J Orthop Res 1995; 13: 814– 823 [DOI] [PubMed] [Google Scholar]
- 25.Peterfy CG, van Dijke CF, Janzen DL, et al. Quantification of articular cartilage in the knee with pulsed saturation transfer subtraction and fat-suppressed MR imaging: optimization and validation. Radiology 1994; 192: 485– 491 [DOI] [PubMed] [Google Scholar]
- 26.Hardya PA, Newmark R, Liu YM, et al. The influence of the resolution and contrast on measuring the articular cartilage volume in magnetic resonance images. Magn Reson Imaging 2000; 18: 965– 972 [DOI] [PubMed] [Google Scholar]
- 27.McKenzie C, Reeder S, Shimakawa A, Pelc N, Brittain J. Abdominal three point Dixon imaging with self calibrating parallel MRI [abstr]. In: Proceedings of the Twelfth Meeting of the International Society for Magnetic Resonance in Medicine Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2004; 917 [Google Scholar]
- 28.Reeder SB, Hargreaves BA, Yu H, Brittain JH. Homodyne reconstruction and IDEAL water-fat decomposition. Magn Reson Med 2005; 54: 586– 593 [DOI] [PubMed] [Google Scholar]
- 29.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 2007; 26: 1153– 1161 [DOI] [PubMed] [Google Scholar]
- 30.Piplani MA, Disler DG, McCauley TR, Holmes TJ, Cousins JP. Articular cartilage volume in the knee: semiautomated determination from three-dimensional reformations of MR images. Radiology 1996; 198: 855– 859 [DOI] [PubMed] [Google Scholar]