Abstract
Rationale: Increased production of mucus is a prominent feature of asthma. IL-13–driven mucous cell metaplasia is associated with decreased expression of the transcription factor FOXA2 and increased expression of the related transcription factor FOXA3 in animal and cell culture models.
Objectives: Establish how changes in FOXA2 and FOXA3 expression contribute to mucous metaplasia and determine whether FOXA2 and FOXA3 expression is altered in asthma.
Methods: Mice expressing a Foxa2 transgene in airway epithelial cells and mice deficient in Foxa3 were analyzed after allergen sensitization and challenge. Expression of FOXA2, FOXA3, MUC5AC, and the highly IL-13–inducible gene CLCA1 was analyzed in airway biopsies from subjects with asthma and control subjects.
Measurements and Main Results: Expression of a Foxa2 transgene reduced allergen-induced mucous metaplasia by 45% compared with control transgenic mice (P < 0.05) whereas inactivation of Foxa3 had no detectable effects on mucous metaplasia. Expression of FOXA2 was reduced in subjects with asthma and was negatively correlated with MUC5AC and CLCA1 levels in subjects with asthma. In contrast, FOXA3 expression was not significantly correlated with MUC5AC and was positively correlated with CLCA1.
Conclusions: Increasing Foxa2 expression reduced mucous metaplasia in an allergic mouse model. Subjects with asthma had decreased FOXA2 expression, suggesting that therapeutic approaches that increase FOXA2 expression or function could be beneficial for reducing mucus production in asthma. Unlike FOXA2, FOXA3 did not regulate mucous metaplasia.
Keywords: mucus, asthma, transcription factor, lung
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
In mouse asthma models, mucus production is associated with decreased levels of the FOXA2 transcription factor and increased levels of the related protein FOXA3 in airway epithelial cells.
What This Study Adds to the Field
FOXA2 expression is reduced in humans with asthma and increasing FOXA2 expression reduces mucus in a mouse asthma model. FOXA3 did not regulate mucus levels in the same model.
Excessive mucus production is a common feature of asthma and contributes to morbidity and mortality (1–6). Studies using mouse asthma models (7–12) and cultured human bronchial epithelial cells (13, 14) established that the helper T type 2 cytokines IL-4 and IL-13 act directly on epithelial cells to produce mucous metaplasia. In this article, we focus on the functional importance of two related genes, Foxa2 and Foxa3, in this process.
The “Forkhead box a” transcription factors FOXA1, FOXA2, and FOXA3 have overlapping patterns of expression in organs derived from embryonic endoderm such as liver, stomach, and intestine (15). The three FOXA proteins are 95% identical within the DNA-binding domains but less similar in other domains (16, 17). Loss of either FOXA1 or FOXA2 alone does not prevent liver development but hepatic specification was completely abrogated in mice lacking both FOXA1 and FOXA2 in the foregut endoderm (16), indicating that one FOXA family member can sometimes compensate for the loss of another. However, functional relationships between FOXA family members remain incompletely understood.
Foxa2 is expressed at the onset of lung bud formation and continues to be expressed in the pulmonary epithelium in adulthood (15). Disruption of Foxa2 in respiratory epithelial cells caused airspace enlargement, neutrophilic pulmonary infiltrates, and mucous metaplasia (18). Airway epithelial cell FOXA2 expression was decreased by allergen challenge and by IL-4 and IL-13 overexpression in mouse airways (18) and by IL-13 stimulation of human bronchial epithelial cells (14), suggesting that loss of FOXA2 may contribute to mucous metaplasia in these systems. Unlike Foxa2, we found that Foxa3 mRNA was increased in lungs of allergen-challenged mice and FOXA3 mRNA was increased during IL-13–induced mucous metaplasia of cultured human bronchial epithelial cells (14). On the basis of these observations, we hypothesized that increased FOXA3 partially compensates for decreased FOXA2 in allergic airways, thereby limiting mucous metaplasia. An alternative hypothesis was that FOXA3 competes with FOXA2 for DNA binding, and therefore amplifies mucous metaplasia by reducing FOXA2 activity.
In this study, we analyzed how changes in FOXA2 and FOXA3 expression contribute to mucous metaplasia in allergic airway disease and asthma. We used transgenic mice that express a Foxa2 transgene in airway epithelial cells (to counteract allergen-induced loss of FOXA2 expression) and mice deficient in Foxa3 (to prevent allergen-induced FOXA3 expression) (19). We also analyzed airway epithelial FOXA2 and FOXA3 expression in asthma. Our results provide new information about the roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Some of the results of these studies have been previously reported in the form of an abstract (20).
METHODS
Mice
Inducible Foxa2 and enhanced green fluorescent protein (EGFP) transgenic mice were produced by coinjection of CCSP-rtTA-hGH (21) and pTRE-Tight-Foxa2 or pTRE-Tight-EGFP (Clontech, Mountain View, CA) into FVB blastocysts. Antisera recognizing FOXA2 (Upstate Biotechnology, Santa Cruz, CA) and MUC5B (kindly provided by C. W. Davis, University of North Carolina, Chapel Hill, NC [22]) were used to detect these proteins in lung sections. Foxa3−/− mice on a C57BL/6 genetic background were generously provided by K. Kaestner (19). To detect FOXA3 protein, lung homogenates were analyzed by immunoblotting, using an antiserum against FOXA3 (Abcam, Cambridge, MA). FVB/N and BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). The University of California San Francisco (San Francisco, CA) Committee on Animal Research approved the use of mice for these experiments. Mice were housed in a specific pathogen–free facility.
Allergen Challenge Model
Six- to 8-week-old transgenic and strain-, age-, and sex-matched control mice were sensitized and challenged with ovalbumin as reported previously (23). In experiments involving Foxa2 transgenic mice, all mice were provided with food containing doxycycline (2 g/kg) to induce transgene expression beginning after the final sensitization and continuing until the mice were killed. Design-based stereology was applied to measure mouse and human epithelial cell mucin stores using point and intercept counting (24), and mucin granule volume using the point-sampled intersect technique (25). Analyses of serum ovalbumin–specific IgE and bronchoalveolar lavage fluid leukocytes (23) and Flexivent measurements of airway reactivity (26) were performed as described previously.
Quantitative Reverse Transcription-Polymerase Chain Reaction
RNA from mouse lungs was reversed transcribed to cDNA and analyzed by SYBR green real-time polymerase chain reaction (PCR). The normalized copy number was determined by comparing the threshold cycle (Ct) of each transcript with the mean Ct for Tubb, Actb, and Gapd. For RNA from human epithelial brushings, cDNA synthesis and two-step quantitative PCR (qPCR) was performed as described previously (27).
MUC5AC Promoter Assay
NCI-H292 cells were transfected with a human MUC5AC promoter-luciferase plasmid (28) together with pcDNA3.1-FOXA2 or pcDNA3.1-FOXA3 expression plasmids, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Luciferase activity was measured 48 hours after transfection. The pSV-βGalactosidase plasmid was included in each transfection and we normalized for transfection efficient by determining the ratio of luciferase activity to β-galactosidase activity. All transfections were performed in triplicate.
FOXA2 Staining in Human Biopsies
Information about the subjects and FOXA2 staining is available in the online supplement.
Statistical Analyses
Data are reported as means ± SEM. For analyses of FOXA2, FOXA3, MUC5AC, and CLCA1 expression in bronchial epithelial cells from subjects with asthma and control subjects, microarray data generated in our previous study (27) were downloaded from the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo; accession number GSE4302). Significance testing was performed by Student t test or by analysis of variance and Tukey-Kramer posttest for multiple groups unless otherwise indicated. Correlations were analyzed by linear regression and analysis of variance.
RESULTS
FOXA2 Expression Is Increased in Airway Epithelial Cells from Foxa2 Transgenic Mice
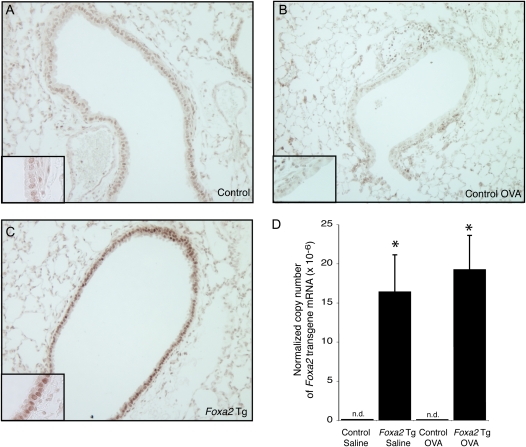
Confirming previous work (18), we found that nuclear FOXA2 protein staining in airway epithelial cells was reduced during allergen-induced mucous metaplasia (Figures 1A and 1B). Quantitative reverse transcription-PCR analysis indicated that allergen challenge resulted in a decrease of Foxa2 mRNA levels in the lungs (44% decrease; P < 0.05). To determine whether persistent Foxa2 expression would inhibit mucous metaplasia, we produced mice with a Foxa2 transgene driven by a doxycycline-regulated protein (reverse tetracycline transregulator, rtTA) expressed under the control of the Clara cell secretory protein (CCSP) promoter. After doxycycline treatment, FOXA2 immunoreactivity was more intense in the Foxa2 transgenic mice than in control mice (Figure 1C). To determine whether transgene expression was affected by allergen challenge, we measured Foxa2 transgene mRNA in the lungs of saline- and ovalbumin-challenged mice. Allergen challenge had no detectable effect on Foxa2 transgene expression (Figure 1D). These results indicate that the Foxa2 transgene was expressed in airway epithelial cells and that transgene expression persisted after allergen challenge.
Figure 1.
Expression of a Foxa2 transgene in airway epithelial cells. (A and B) FOXA2 protein (brown) was identified by immunohistochemistry in the lungs of (A) saline-challenged and (B) ovalbumin (OVA)-challenged nontransgenic mice and (C) Foxa2 transgenic (Tg) mice. Insets show regions of the epithelium at ×2 higher magnification. (D) Expression of Foxa2 transgene mRNA in lungs from saline- and ovalbumin-challenged nontransgenic control and Foxa2 Tg mice was measured by quantitative reverse transcription-polymerase chain reaction (RT-PCR) using PCR primers that amplify Foxa2 transgene mRNAs but not mRNAs derived from the endogenous murine Foxa2 gene. Results represent means ± SEM for two mice per group. *P < 0.05 compared with control mice. n.d. = Not detected.
FOXA3 Protein Is Increased after Allergen Challenge
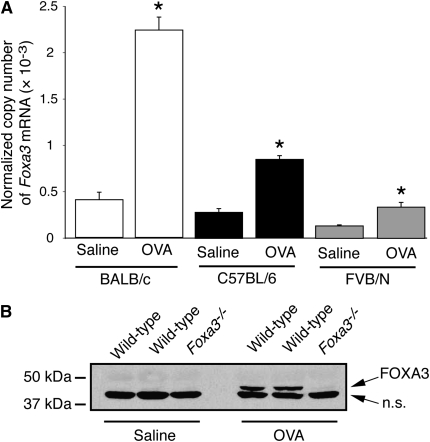
We previously reported that Foxa3 mRNA was increased after allergen challenge of FVB/N mice (14). To determine whether increases were seen in other strains more commonly used for asthma models, we compared the responses of BALB/c and C57BL/6 mice with the response of FVB/N mice (Figure 2A). Foxa3 mRNA expression increased by 5.5-fold in BALB/c mice, by 3.0-fold in C57BL/6 mice, and by 2.5-fold in FVB/N mice after allergen challenge (Figure 2A). For unclear reasons, these fold increases were smaller than the increase we previously reported in FVB/N mice using the same model system. We analyzed lungs from saline- and ovalbumin-treated mice by immunoblotting to determine whether the increase in Foxa3 mRNA was accompanied by an increase in FOXA3 protein. FOXA3 protein expression was detectable in wild-type C57BL/6 mice challenged with ovalbumin but not in saline-challenged mice (Figure 2B). As expected, no FOXA3 protein expression was detectable in Foxa3−/− mice even after allergen challenge. These results show that FOXA3 protein is induced during allergic airway disease and confirm the absence of FOXA3 protein in Foxa3−/− mice. We previously showed that IL-13, a critical helper T cell type 2 cytokine produced during allergic inflammation, induces Foxa3 expression in purified airway epithelial cells. We attempted to use the FOXA3 antiserum to localize FOXA3 protein in the allergic lung but obtained similar staining in wild-type and Foxa3−/− mice, indicating that the staining was not specific for FOXA3.
Figure 2.
Increased lung FOXA3 expression in allergen-challenged mice. (A) Expression of Foxa3 mRNA in lungs from saline- and ovalbumin (OVA)-challenged BALB/c mice (open columns), C57BL/6 mice (solid columns), and FVB/N mice (shaded columns) was measured by quantitative reverse transcription-polymerase chain reaction (RT-PCR). Results represent means ± SEM for five mice per group. *P < 0.05 compared with saline-challenged mice. (B) Immunoblotting for FOXA3 protein in lung extracts from two saline- or OVA-challenged wild-type (Foxa3+/+) and one Foxa3−/− mice. n.s. = Nonspecific.
FOXA2, But Not FOXA3, Plays a Role in Allergen-induced Mucous Metaplasia
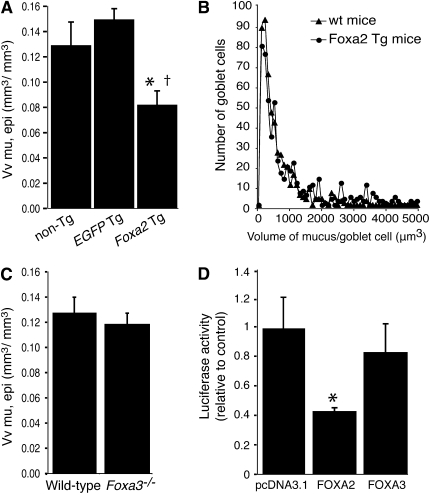
We used Foxa2 transgenic mice and strain-matched wild-type control mice to determine whether transgenic expression of Foxa2 had an effect on mucous metaplasia. Mice were sensitized and challenged with saline or ovalbumin, and received doxycycline throughout the period of allergen challenge to promote transgene expression. Saline-challenged mice had little or no mucin visible in the airway epithelium (data not shown), consistent with previous reports (12, 29). We used computer-assisted stereology to quantify airway epithelial mucin stores in ovalbumin-challenged mice. Airway epithelial mucin stores in ovalbumin-challenged Foxa2 transgenic mice were reduced by 37% compared with ovalbumin-challenged wild-type mice (Figure 3A). Despite the decrease in overall volume of stored mucin within the epithelium, there was no decrease in the volume of mucin within individual cells (Figure 3B). This indicates that the overall decrease in mucin stores reflects a decrease in the proportion of mucin-containing cells within the epithelium and not a decrease in the amount of mucin stored per cell. The Foxa2 transgenic mouse line that we produced includes two transgenes: a Foxa2 transgene and an rtTA transgene that regulates Foxa2 transgene expression. We considered the possibility that expression of the rtTA transgene rather than of Foxa2, in Foxa2 transgenic mice might be responsible for the effect on mucus production. To address this, we measured airway epithelial mucin stores in a control transgenic line. The control transgenic line carried the rtTA transgene and an irrelevant transgene (EGFP) in place of the Foxa2 transgene. After allergen challenge, airway epithelial mucin stores in EGFP transgenic mice were similar to mucin stores in nontransgenic control mice. Mucin stores in Foxa2 transgenic mice were 45% less than in EGFP transgenic mice (Figure 3A). These results indicate that transgenic expression of FOXA2 specifically reduced allergen-induced mucous metaplasia.
Figure 3.
Mucous metaplasia and MUC5AC transcription are decreased by FOXA2 but not by FOXA3. (A) Epithelial mucin stores, represented as means ± SEM of the volume of mucin referenced to the volume of the airway epithelium (Vv mu, epi), in allergen-challenged Foxa2 transgenic (Foxa2 Tg) mice (n = 9), nontransgenic (non-Tg) control mice (n = 9), and enhanced green fluorescent protein (EGFP) transgenic (EGFP-Tg) mice (n = 4). *P < 0.05 compared with non-Tg control mice; †P < 0.05 compared with EGFP-Tg mice. (B) Distribution of the volume of mucin-containing regions. The volume of periodic acid Schiff-stained regions within the epithelium was measured by the point-sampled intercept method. Foxa2 Tg mice (n = 4; circles) were compared with non-Tg wild-type control mice (n = 4; triangles). On average, 125 and 134 PAS-stained regions were measured in each nontransgenic and Foxa2 transgenic mouse, respectively. wt = wild type. (C) Epithelial mucin stores in allergen-challenged Foxa3−/− mice and wild-type control mice (16 per group). (D) NCI-H292 cells were transfected with a MUC5AC promoter-luciferase reporter in combination with either a control (empty) expression vector (pcDNA3.1) or human FOXA2 or FOXA3 expression vector. MUC5AC promoter activity was determined as relative luciferase activity normalized to β-galactosidase activity. Values represent means ± SD from three independent experiments. *P < 0.05 compared with control cells transfected with empty vector.
We analyzed the role of FOXA3 in allergen-induced mucous metaplasia, using Foxa3−/− mice. Foxa3−/− mice and strain-matched wild-type control mice had similar airway epithelial cell mucin stores after ovalbumin challenge (Figure 3C). This result demonstrates that allergen-induced increases in FOXA3 did not affect mucous metaplasia.
FOXA2 has been shown to reduce MUC5AC transcriptional activity in NCI-H292 human lung mucoepidermoid cells (18), which may explain the ability of FOXA2 to inhibit mucous metaplasia in the airways of allergen-challenged mice. We used a similar approach to compare the effects of FOXA2 and FOXA3 on MUC5AC transcription. A luciferase reporter construct containing 3.8 kb of the human MUC5AC promoter was transfected into NCI-H292 cells together with human FOXA2 or FOXA3 expression vectors. FOXA2 decreased reporter expression by 57% (Figure 3D), consistent with the previous report (18). In contrast, expression of FOXA3 had no effect on MUC5AC transcription reporter expression.
In addition to MUC5AC, allergen challenge also induces expression of MUC5B (12). To determine whether FOXA2 overexpression affected MUC5B, we quantified MUC5B staining in lung sections from ovalbumin-challenged nontransgenic, Foxa2 transgenic, and EGFP transgenic mice, using stereology. MUC5B stores in Foxa2 transgenic mice were 36% less than in nontransgenic control mice (P < 0.05), whereas EGFP transgenic mice were similar to nontransgenic control mice (99% relative to the nontransgenic control mice). We did not detect staining for MUC2, a mucin that is found primarily in the intestine, in airways from control or transgenic mice. Taken together, our results indicate that FOXA2 overexpression can reduce production of both major airway mucins, MUC5AC and MUC5B.
Other Features of Allergic Airway Disease in Foxa2 Transgenic Mice and Foxa3−/− Mice
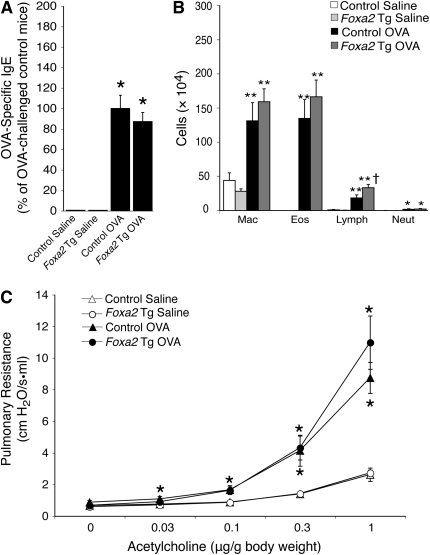
We considered the possibility that the effects of Foxa2 transgene expression might be explained by a general effect on the allergic response rather than a specific effect on epithelial mucus production. To address this possibility, we analyzed other aspects of allergic airway disease in Foxa2 mice. We found that Foxa2 transgenic and strain-matched control mice had similar elevations in serum ovalbumin–specific IgE after allergen challenge (Figure 4A). Bronchoalveolar lavage was performed to assess the effect of allergen challenge on inflammatory cell recruitment (Figure 4B). Allergen challenge induced similar increases in macrophages, eosinophils, and neutrophils in Foxa2 transgenic and control mice, whereas Foxa2 transgenic mice had a significantly greater increase in lymphocytes. Airway reactivity was determined by measuring pulmonary resistance in sedated and mechanically ventilated mice after intravenous administration of increasing doses of acetylcholine (Figure 4C). Foxa2 transgenic mice developed a similar degree of allergen-induced airway hyperreactivity as wild-type control mice. These results indicate that the reduction in mucous metaplasia seen in allergen-challenged Foxa2 transgenic mice was not accompanied by reductions in IgE production, airway inflammation, or airway reactivity.
Figure 4.
Other features of allergic airway disease are not affected in Foxa2 transgenic mice. (A) Serum ovalbumin (OVA)-specific IgE was measured in saline- and OVA-challenged nontransgenic control and Foxa2 transgenic (Foxa2 Tg) mice. (B) Airway inflammation was analyzed by counting macrophages (Mac), eosinophils (Eos), lymphocytes (Lymph), and neutrophils (Neut) in bronchoalveolar lavage fluid. (C) Airway reactivity to intravenously administered acetylcholine was measured with the Flexivent system. Results represent means ± SEM for six to eight mice per group. *P < 0.05, **P < 0.01 compared with saline-challenged mice; †P < 0.05 compared with OVA-challenged mice.
Although FOXA3 deficiency did not affect mucus production, we explored the possibility that FOXA3 might be involved in other aspects of allergic airway disease. We found that allergen-challenged Foxa3−/− mice had increased IgE production and eosinophilic inflammation but reduced airway reactivity compared with nontransgenic control mice (see Figure E1 in the online supplement).
Reduced Airway Epithelial FOXA2 Expression in Subjects with Stable Asthma
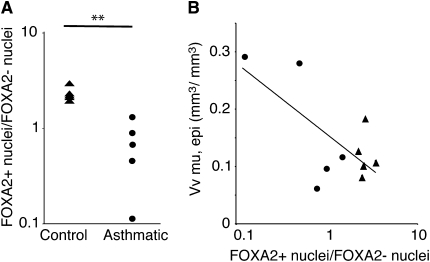
Humans with mild and moderate asthma have increased airway epithelial mucin stores and an increase in the number of goblet cells compared with healthy control subjects (2). To determine whether FOXA2 expression is also altered in asthma, we quantified airway epithelial cell FOXA2 expression in airway biopsies obtained from five subjects with mild or moderate asthma and five healthy control subjects. The ratio of FOXA2-unstained nuclei to FOXA2-stained nuclei was significantly reduced in asthma (Figure 5A). We found a significant negative correlation between MUC5AC and the ratio of FOXA2-stained nuclei to FOXA2-unstained nuclei (Figure 5B). This indicates that asthma and increased mucin stores are associated with reduced FOXA2 expression in airway epithelial cells.
Figure 5.
Reduced airway epithelial FOXA2 staining in subjects with asthma is associated with increased mucin stores. Airway epithelial biopsies from five healthy control subjects and five subjects with mild or moderate asthma were stained with FOXA2 antiserum. (A) Ratios of FOXA2-stained (FOXA2+) to FOXA2-unstained (FOXA2–) nuclei were determined by analyzing all the epithelium within multiple biopsies from each subject (mean of 367 nuclei per subject). **P < 0.01 compared with healthy subjects. (B) Correlation between mucin stores and the ratios of FOXA2+ to FOXA2– nuclei for all subjects (five control subjects [triangles] and five subjects with asthma [circles]; R = 0.47, P = 0.027). Vv mu, epi = epithelial mucin stores, represented as means ± SEM of the volume of mucin referenced to the volume of the airway epithelium.
Expression of FOXA2, FOXA3, and MUC5AC mRNA in Bronchial Epithelial Cells from Control Subjects and Subjects with Asthma
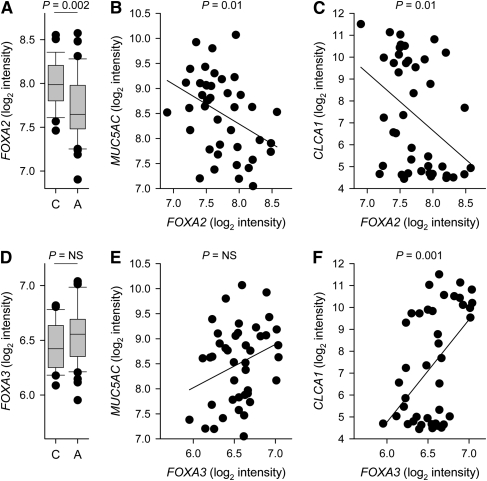
To further analyze FOXA2 and FOXA3 expression in human subjects, we analyzed data from our genome-wide study of mRNA transcript levels in bronchial epithelial cells from 42 subjects with stable mild or moderate asthma and 28 healthy control subjects (27). FOXA2 expression was significantly lower in subjects with asthma compared with control subjects (Figure 6A). Within the asthma group, FOXA2 expression was negatively correlated with MUC5AC expression (Figure 6B). FOXA2 expression was also negatively correlated with the expression of CLCA1, a gene that is highly induced by IL-13 (14, 27) and is expressed at high levels in epithelial cells from many subjects with asthma (Figure 6C). FOXA3 expression tended to be modestly higher in subjects with asthma compared with control subjects, but this did not reach statistical significance (Figure 6D). Within the asthma group, there was no significant correlation between FOXA3 and MUC5AC expression (Figure 6E). In contrast, there was a highly significant positive correlation between FOXA3 and CLCA1 (Figure 6F). These results suggest that IL-13 (and/or other stimuli that increase CLCA1 expression) decrease FOXA2 expression and increase FOXA3 expression.
Figure 6.
Expression of FOXA2, FOXA3, MUC5AC, and CLCA1 mRNAs in bronchial epithelial cells from control subjects and subjects with asthma. Transcript levels in endobronchial brush samples (97 ± 3% epithelial cells) were measured with microarrays as previously reported (27). (A) FOXA2 mRNA expression in 28 control subjects (C) and 42 subjects with asthma (A) were compared by Wilcoxon rank-sum test. (B and C) Correlation between FOXA2 and MUC5AC (B: R = −0.39) or CLCA1 (C: R = −0.40) mRNA expression in samples from subjects with asthma. (D) FOXA3 mRNA expression in control subjects and subjects with asthma. NS = not significant. (E and F) Correlation between FOXA3 and MUC5AC (E: R = 0.30) or CLCA1 (F: R = 0.49) mRNA expression in samples from subjects with asthma.
DISCUSSION
Our goal was to evaluate the functional importance of changes in FOXA2 and FOXA3 expression that occur during allergic airway disease and asthma (14). FOXA2 expression is decreased during allergic airway disease, and we investigated the importance of this decrease by producing and characterizing mice with a transgene that increased FOXA2 expression in airway epithelial cells. These studies demonstrated that persistence of FOXA2 expression during allergic responses reduces airway mucus without detectable effects on other aspects of the allergic response that we analyzed. FOXA3 expression is increased during allergic airway disease, and we investigated the importance of this increase by studying FOXA3-deficient mice. Lack of FOXA3 had no detectable effect on allergen-induced increases in airway mucus. In human subjects, we showed that FOXA2 expression was decreased in asthma and that the decrease in FOXA2 expression is associated with increased mucin levels. In contrast, FOXA3 expression was not associated with mucin expression. Taken together, these studies suggest that loss of FOXA2 expression contributes to mucous metaplasia in asthma, whereas FOXA3 is not involved in this aspect of the disease.
Our studies of FOXA2 complement a previous groundbreaking study (18) that demonstrated a major role for this transcription factor in regulating mucus production. In the previous work, conditional deletion of FOXA2 in respiratory epithelial cells induced mucus production in the absence of allergen challenge or other exogenous stimuli. In addition, allergen challenge of wild-type mice was shown to decrease FOXA2 expression, suggesting that allergen-induced increases in mucus might depend on loss of FOXA2. We directly tested this possibility with Foxa2 transgenic mice, and found a significant reduction of allergen-induced airway mucus in these mice. This finding demonstrates that allergen-induced mucus production is at least partially dependent on loss of FOXA2 in airway epithelial cells. The effect of the Foxa2 transgene is likely explained by a cell autonomous effect of FOXA2 on mucus production, because FOXA2 overexpression reduced MUC5AC transcription in cultured lung mucoepidermoid cells ([18] and Figure 3D) and because other aspects of allergic airway disease, including IgE production, airway inflammation, and airway hyperreactivity, were not reduced in Foxa2 transgenic mice. Although we have previously shown that hyperreactivity persists even when mucus production is dramatically reduced in this allergic model, other studies in mouse models (30) and humans (31) indicate that mucus hypersecretion is often a major contributor to airway obstruction in allergic models and in humans with asthma. Foxa2 transgenic mice were not completely protected against allergen-induced mucus production. This might reflect the existence of FOXA2-independent pathways for mucus production. However, it is also possible that the failure to completely repress mucus production is due to incomplete restoration of FOXA2 expression in airway epithelial cells of allergen-challenged transgenic mice. The promoter we used to drive transgene expression is active in Clara cells, which are believed to be the major precursors of mucus-producing cells in allergic airway disease (32), but other cell types may also be capable of transdifferentiation to mucus-producing cells (33).
We found that Foxa3 mRNA was increased during allergic airway disease (14), but the contributions of FOXA3 in this disease have not been explored previously. Here we extended our previous work by showing that the allergen-induced increase in Foxa3 mRNA is accompanied by a substantial increase in FOXA3 protein in the lung. We attempted to identify FOXA3-expressing cells in lung sections, but the antibodies we used were not suitable for immunohistochemistry because they produced similar staining patterns in wild-type and Foxa3−/− mice (data not shown). However, in previous work we showed that the cytokine IL-13, which plays a key role in the airway epithelial response to allergy, substantially increased FOXA3 expression in cultured normal human bronchial epithelial cells (14). This suggests that the FOXA3 protein detected in allergic mouse lungs is at least partially derived from airway epithelial cells. We hypothesized that the increase in FOXA3 expression might partially compensate for loss of FOXA2 in allergic airways, and thereby limit the extent of mucous metaplasia. Alternatively, we considered the possibility that FOXA3 might competitively inhibit FOXA2 and thereby promote mucous metaplasia. To investigate this, we examined airway mucins in allergen-challenged Foxa3−/− mice. We found similar amounts of stored mucins in airways from Foxa3−/− and matched Foxa3+/+ control mice. We also compared the effects of FOXA2 and FOXA3 on MUC5AC promoter activity in cultured cells. FOXA2 inhibited transcription but FOXA3 had no effect. Analysis of mRNA expression data from our study of freshly isolated bronchial epithelial cells (Figure 6) showed a highly significant positive correlation between FOXA3 expression and expression of CLCA1, a highly IL-13–inducible gene that is greatly increased in asthma (14, 27). This suggests that the IL-13–induced increases in FOXA3 expression that have been reported in mice and in cultured human cells (14) also occur in vivo in people with asthma. However, expression of FOXA3 was not significantly correlated with expression of MUC5AC. Taken together, these findings indicate that changes in FOXA3 expression, unlike changes in FOXA2 expression, do not affect airway mucus accumulation in response to allergen. FOXA2 and FOXA3 have substantially different sequences (16, 34) and it seems likely that structural differences between these two family members lead to differences in interaction with DNA or with other proteins involved in the regulation of gene expression. Allergen-challenged Foxa3−/− mice, unlike Foxa2 transgenic mice, had differences in serum OVA-specific IgE, bronchoalveolar lavage eosinophil counts, and airway reactivity compared with allergen-challenged control mice (Figure E1). Further work will be required to determine whether these differences indicate a direct role for airway epithelial cell FOXA3 in these aspects of the allergic response.
FOXA2 is a promising therapeutic target in asthma and other diseases characterized by excessive mucus production. We found that maintaining FOXA2 expression in airway epithelium is sufficient to cause a substantial decrease in airway mucus in vivo in a mouse model of asthma. We also showed that FOXA2 expression is reduced in human subjects with mild to moderate stable asthma and is negatively correlated with the volume of stored mucins and the level of MUC5AC mRNA. Loss of FOXA2 staining has also been reported in areas of mucous metaplasia within airways from humans with cystic fibrosis, chronic obstructive pulmonary disease, and bronchopulmonary dysplasia (18). This suggests that studies of the function of FOXA2 in mice are relevant for common human diseases. Therapies that increase FOXA2 expression or function in airway epithelial cells might inhibit mucus overproduction, which is an important cause of morbidity and mortality in asthma and other airway diseases (35).
Supplementary Material
Acknowledgments
The authors thank A. Barczak and N. Killeen for advice and K. Huang, Y. Wang, X. Bernstein, X. Ren, and S. Kim for technical assistance. C.V. was supported by a fellowship from the Belgian American Educational Foundation and by the Leon Fredericq Fund.
Supported by funding from the NIH and the UCSF Strategic Asthma Basic Research (SABRE) Program.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200811-1768OC on July 23, 2009
Conflict of Interest Statement: S.-W.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; L.T.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; Y.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; X.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; P.G.W. receives $200,000 per year as a research grant from Genentech Inc and is a coinventor on a patent related to asthma biomarkers; J.V.F. received $1,001–$5,000 from Amira, up to $1,000 from Gilead, $1,001–$5,000 from Merck, $1,001–$5,000 from Roche, up to $1,000 from Aerovance for consulting, $5,001–$10,000 from Cytokinetics as a member of their scientific advisory board, more than $100,001 in grants for research in asthma and cystic fibrosis from Genentech, a $50,001–$100,000 grant for research in asthma from Roche, and a grant of more than $100,001 for a clinical trial in COPD from Boehringer Ingelheim, and is the coinventor on a patent for the development of biomarkers of asthma; D.J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921. [DOI] [PubMed] [Google Scholar]
- 2.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523. [DOI] [PubMed] [Google Scholar]
- 3.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol 2004;4:241–250. [DOI] [PubMed] [Google Scholar]
- 4.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 5.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665. [DOI] [PubMed] [Google Scholar]
- 6.Tesfaigzi Y. Regulation of mucous cell metaplasia in bronchial asthma. Curr Mol Med 2008;8:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temann UA, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of muc5ac gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol 1997;16:471–478. [DOI] [PubMed] [Google Scholar]
- 8.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889. [DOI] [PubMed] [Google Scholar]
- 12.Kuperman DA, Huang X, Nguyenvu L, Holscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in clara cells is required for allergen-induced mucus production. J Immunol 2005;175:3746–3752. [DOI] [PubMed] [Google Scholar]
- 13.Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L226–L236. [DOI] [PubMed] [Google Scholar]
- 14.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol 2007;36:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of foxa1 and foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 2004;5:193–208. [DOI] [PubMed] [Google Scholar]
- 16.Friedman JR, Kaestner KH. The foxa family of transcription factors in development and metabolism. Cell Mol Life Sci 2006;63:2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai E, Clark KL, Burley SK, Darnell JE Jr. Hepatocyte nuclear factor 3/fork head or “Winged helix” proteins: a family of transcription factors of diverse biologic function. Proc Natl Acad Sci USA 1993;90:10421–10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 2004;131:953–964. [DOI] [PubMed] [Google Scholar]
- 19.Kaestner KH, Hiemisch H, Schutz G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3γ results in reduced transcription of hepatocyte-specific genes. Mol Cell Biol 1998;18:4245–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaeghe C, Park SW, Nguyenvu LT, Eisley C, Erle DJ. Distinct roles of foxa2 and foxa3 in allergic airway disease [abstract]. Am J Respir Crit Care Med 2009;179:A6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase– and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 2008;586:1977–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol 2005;116:305–311. [DOI] [PubMed] [Google Scholar]
- 24.Bolender RP, Hyde DM, Dehoff RT. Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. Am J Physiol 1993;265:L521–L548. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988;96:857–881. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Huang X, Sheppard D. Adam33 is not essential for growth and development and does not modulate allergic asthma in mice. Mol Cell Biol 2006;26:6950–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007;104:15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longphre M, Li D, Gallup M, Drori E, Ordonez CL, Redman T, Wenzel S, Bice DE, Fahy JV, Basbaum C. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest 1999;104:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagami Y, Favoreto S Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA, Avila PC, et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol 2008;181:2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol 2007;102:399–405. [DOI] [PubMed] [Google Scholar]
- 31.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 2009;15:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006;116:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaestner KH, Hiemisch H, Luckow B, Schutz G. The hnf-3 gene family of transcription factors in mice: gene structure, cDNA sequence, and mRNA distribution. Genomics 1994;20:377–385. [DOI] [PubMed] [Google Scholar]
- 35.Henke MO, Shah SA, Rubin BK. The role of airway secretions in COPD: clinical applications. COPD 2005;2:377–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.