Abstract
Rationale: The rapid diagnosis of pulmonary tuberculosis (TB) is difficult when acid fast bacilli (AFB) cannot be detected in sputum smears.
Objectives: Following a proof of principle study, we examined in routine clinical practice whether individuals with sputum AFB smear-negative TB can be discriminated from those with latent TB infection by local immunodiagnosis with a Mycobacterium tuberculosis–specific enzyme-linked immunospot (ELISpot) assay.
Methods: Subjects suspected of having active TB who were unable to produce sputum or with AFB-negative sputum smears were prospectively enrolled at Tuberculosis Network European Trialsgroup centers in Europe. ELISpot with early-secretory-antigenic-target–6 and culture-filtrate-protein–10 peptides was performed on peripheral blood mononuclear cells (PBMCs) and bronchoalveolar lavage mononuclear cells (BALMCs). M. tuberculosis–specific nucleic acid amplification (NAAT) was performed on bronchoalveolar lavage fluid.
Measurements and Main Results: Seventy-one of 347 (20.4%) patients had active TB. Out of 276 patients who had an alternative diagnosis, 127 (46.0%) were considered to be latently infected with M. tuberculosis by a positive PBMC ELISpot result. The sensitivity and specificity of BALMC ELISpot for the diagnosis of active pulmonary TB were 91 and 80%, respectively. The BALMC ELISpot (diagnostic odds ratio [OR], 40.4) was superior to PBMC ELISpot (OR, 10.0), tuberculin skin test (OR, 7.8), and M. tuberculosis specific NAAT (OR, 12.4) to diagnose sputum AFB smear-negative TB. In contrast to PBMC ELISpot and tuberculin skin test, the BALMC ELISpot was not influenced by previous history of TB.
Conclusions: Bronchoalveolar lavage ELISpot is an important advancement to rapidly distinguish sputum AFB smear-negative TB from latent TB infection in routine clinical practice.
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The rapid diagnosis of active pulmonary tuberculosis is difficult when acid-fast bacilli (AFB) cannot be detected in sputum smears. Local immunodiagnosis by Mycobacterium tuberculosis–specific enzyme-linked immunospot assay (ELISpot) is a promising method for the rapid identification of patients with sputum AFB smear-negative tuberculosis.
What This Study Adds to the Field
In a prospective multicenter TBNET-study, patients with sputum AFB smear-negative pulmonary tuberculosis could rapidly be distinguished from patients with latent tuberculosis infection by the M. tuberculosis–specific ELISpot on cells from the BAL fluid with a high diagnostic sensitivity and specificity. These findings may have significant implications for the rapid decision to initiate antituberculosis treatment where bronchoscopy is routinely performed for individuals suspected to be affected by sputum AFB smear-negative tuberculosis.
Tuberculosis (TB) is among the leading causes of morbidity and mortality worldwide (1). Pulmonary TB is the major manifestation of the disease (2). Despite constant diagnostic improvements, the rapid diagnosis of pulmonary TB is still difficult in a substantial proportion of cases (3). Identification of Mycobacterium tuberculosis by culture is the diagnostic gold standard for active TB, but culture growth of M. tuberculosis may take 2 or more weeks on average (4), and its sensitivity is only approximately 80% (5).
Microscopy for the identification of acid-fast bacilli (AFB) is rapid and inexpensive (6), but AFB are undetectable from the sputum smear in 85 to 90% of children (7) and in approximately 50% of adults (8) with active pulmonary TB. In these cases, the decision to initiate anti-TB treatment can be difficult, especially because sensitivity estimates for the nucleic acid amplification technique (NAAT) to detect nucleic acids of M. tuberculosis from respiratory specimen are too variable and too low to be used to exclude the diagnosis of TB (9).
If combined test results are negative, immunodiagnosis by peripheral blood IFN-γ release assays (IGRAs) and tuberculin skin testing (TST) may be used as rule out tests for active TB in patients with a negative sputum smear result (10, 11). However, positive IGRA results (when performed on peripheral blood) and/or a positive TST result are of limited value because immunodiagnostic tests cannot distinguish individuals with active TB from those with latent TB infection (LTBI) (10, 12).
In active TB, M. tuberculosis–specific lymphocytes are concentrated at the site of the infection (13, 14). Comparison of systemic and local immune responses against antigens of M. tuberculosis may be useful to rapidly distinguish sputum AFB smear-negative cases of active TB from individuals with LTBI. In a pilot study, M. tuberculosis region of difference-1 early secretory antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10 peptide–specific mononuclear cell responses were detectable by enzyme-linked immunospot (ELISpot) assay in bronchoalveolar lavage (BAL) mononuclear cells (BALMCs) only from patients with sputum AFB smear-negative pulmonary TB, whereas these responses were absent in patients with LTBI, in patients with a history of TB without current reactivation, or in patients with pulmonary infiltrates of other origin (15). By enumerating ESAT-6– and CFP-10–specific BALMCs, patients with active TB could be fully distinguished from patients with pulmonary infiltrates of other origin. However, the numbers of enrolled individuals were low in this study, and the setting was monocentric.
To better evaluate the role of BAL ELISpot for the rapid diagnosis of sputum AFB smear-negative TB in countries of low TB incidence, where bronchoscopy and IGRA techniques are available, we performed a large prospective multicenter clinical study within several European centers participating in the TBNET.
METHODS
Patients
After we obtained written informed consent and local ethical committee approval, HIV-seronegative individuals having negative sputum AFB smear results on three consecutive examinations or being unable to produce sputum with pulmonary infiltrates on chest radiography, a medical history, clinical signs, or symptoms compatible with TB were prospectively enrolled between September 2006 and September 2008 at the Medical Clinic of the Research Center Borstel (Germany), the Hospital Großhansdorf (Germany), the Thorax Clinic Heidelberg (Germany), the Diakonessenhuis Utrecht (The Netherlands), the University Hospital of Modena (Italy), the National Institute for Infectious Diseases Rome (Italy), and the Hospital Universitari Germans Trias i Pujol Badalona (Spain).
Standard diagnostic procedures were performed, including PBMC ELISpot, TST (following the national guidelines (16), TST was not performed at the center in The Netherlands; patients with former TB did not receive a TST), bronchoscopy with BAL for microscopy and M. tuberculosis culture, BAL ELISpot, and NAAT (if requested by the treating physician). Transbronchial biopsies were taken for further examinations by the decision of the operating physician.
ELISpot Assays
Venous blood was drawn in preheparinized tubes, and peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll Hypaque density gradient centrifugation.
M. tuberculosis–specific ELISpot (T-SPOT.TB test) was performed based on the recommendations for blood according to the manufacturer's guidelines (OxfordImmunotec Ltd., Abingdon, UK) with 250,000 mononuclear cells per well. BAL was performed with 200 to 300 ml of normal saline from an affected lung segment for mycobacteriological culture, M. tuberculosis–specific ELISpot (T-SPOT.TB test), and M. tuberculosis–specific NAAT. The BAL ELISpot was performed as previously described (14, 15). Mononuclear cells were obtained by passing the BAL fluid through a stainless steel sieve with a mesh aperture of 0.5 mm (Teesieb-Profi-Plus; WMF, Geislingen, Germany). ELISpot assay results were considered positive if more than five spot-forming cells (SFCs) were counted in the ESAT-6 or the CFP-10 well after subtraction of the number of SFCs in the negative control well and if the total number of SFCs in the ESAT-6 or CFP-10 well was at least twice the number of SFCs in the negative control well. ELISpot assay results were considered negative if they did not meet the definition for a positive result and if the number of SFCs in the positive control well was more than 20 SFCs after subtraction of the number of spots in the negative control well and had at least twice the number of spots of the negative control well. Results that did not meet the criteria of positive or negative were considered to be indeterminate. Treating physicians were blinded to the results of the ELISpot assays until the decision for or against anti-TB treatment was made.
Tuberculin Skin Test
Bioequivalent tuberculin skin testing was performed in Germany and Spain with 0.1 ml (2 TU) of tuberculin RT23 (Statens-Serum-Institut, Copenhagen, Denmark) and in Italy with 0.1 ml (5 TU) of tuberculin Biocine (Chiron, Siena, Italy) according to nationally licensed products.
M. tuberculosis–specific Nucleic Acid Amplification Technique
Three different specific NAAT systems were used in the seven centers: (1) a BD Probe Tec ET system (BD Diagnostic Systems, Sparks, MD), (2) an Amplified-MTD (GenProbe, San Diego, CA), and (3) an in-house PCR assay targeting the IS6110 gene (17). All laboratories at the centers participate in regular external quality control surveys.
Statistical Analysis
Data were analyzed using Stata 9.0 (StataCorp, College Station, TX). Comparisons between proportions were performed using χ2 test; the Student's t test was used for continuous variables, and its nonparametric version (Wilcoxon-Mann-Whitney test) was used when appropriate. Differences were considered to be significant when P < 0.05. To avoid a mathematical error, we assigned values of “0” a value of “0.1” when calculating ratios of ESAT-6– and CFP-10–specific cells among lymphocytes from the blood and the BAL.
Reporting of the research findings followed the STARD (standards for the reporting of diagnostic accuracy) guidelines (18).
RESULTS
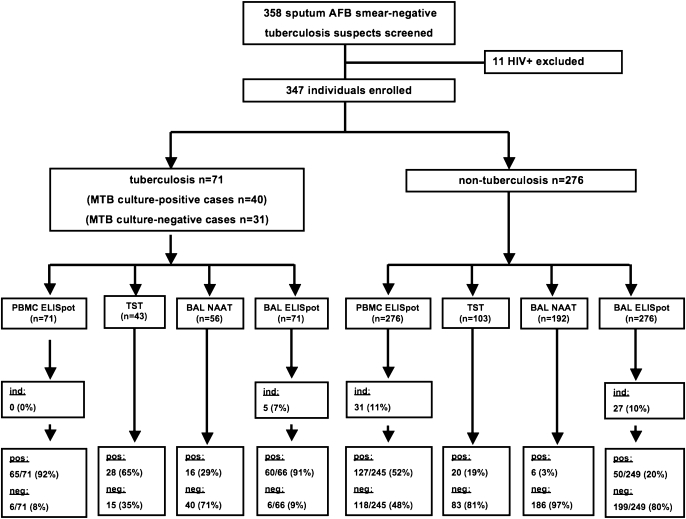
Eleven patients with HIV infection considered for enrolment were excluded. Overall, 347 suspects of active TB unable to produce sputum or with three consecutive negative AFB sputum smears were enrolled in this study. Seventy-one of the subjects with suspected TB were finally diagnosed with active pulmonary TB by one of two case definitions: In 40 (56.3%) subjects, M. tuberculosis was recovered by culture; in 31 (43.7%) patients, TB was diagnosed clinically after alternative diseases were ruled out and patients received anti-TB therapy, which had been prescribed by the treating physician on clinical grounds after the subjects showed no clinical response to antibiotic therapy, in accordance with WHO definitions (19).
Of the 276 patients in the non-TB group, 250 patients had a definitive alternative diagnosis other than active TB (50 individuals had bacterial pneumonia or lung abscess, 13 had nontuberculous mycobacteria infections, 48 had sarcoidosis, 32 had pulmonary malignancies, 25 had a former history of TB who were re-evaluated for possible reactivation, 15 had cryptogenic organizing pneumonia, 15 had idiopathic pulmonary fibrosis, 15 had collagen vascular diseases, 10 had bronchiectasis, and 27 had miscellaneous identified pulmonary diseases). In 26 individuals, the final alternative diagnosis could not be established. All of these patients did not receive anti-TB therapy and did not develop TB within a 6-month follow–up period. None of the patients enrolled in this study was HIV seropositive. Demographic and microbiological characteristics are shown in Table 1. In 123 patients (41 with TB and 82 from the non-TB group), transbronchial biopsies were taken during bronchoscopy. If available, biopsies were used to make a diagnosis in patients with and without TB.
TABLE 1.
DEMOGRAPHIC, MICROBIOLOGICAL, AND NUCLEIC ACID AMPLIFICATION TECHNIQUE CHARACTERISTICS OF 347 SUBJECTS SUSPECTED TO BE AFFECTED BY ACTIVE TUBERCULOSIS WITH NEGATIVE ACID-FAST BACILLI SMEARS
| Variables | Tuberculosis (n = 71) | Nontuberculosis (n = 276) | P Value |
|---|---|---|---|
| Males, % | 45 (63.4) | 179 (64.9) | 0.87 |
| Age, years (mean ± SD) | 42.4 ± 2 | 56.6 ± 0.95 | <0.001 |
| Positive BAL microscopy result for AFB, n (%) | 3/64 (4.7) | 0/240 (0) | — |
| Positive BAL NAAT result for M. tuberculosis, n (%) | 16/56 (28.6) | 6/192 (3.1) | <0.001 |
| Tuberculin skin testing, mm (mean ± SD) | 16.3 ± 9.8 (n = 43) | 5.5 ± 8.4 (n = 115) | <0.001 |
| Culture confirmation from sputum, BAL or biopsy, n (%) | 40/71 (56.3) | 0/276 (0) | — |
Definition of abbreviations: AFB = acid-fast bacilli; BAL = bronchoalveolar lavage; NAAT = nucleic acid amplification technique.
ELISpot Results
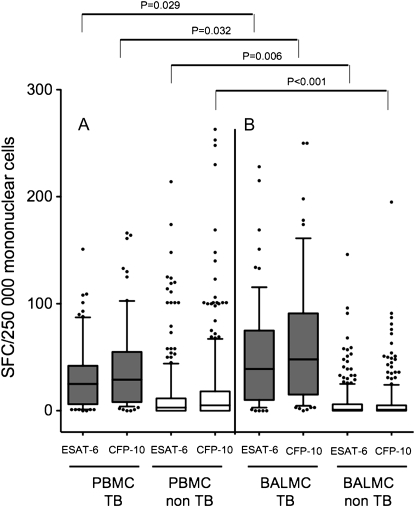
In patients with active TB, ELISpot results on PBMCs were positive in 65 out of 71 (91.5%) and negative in 6 out of 71 (8.5%), respectively. In patients in the non-TB group, ELISpot results on PBMCs were positive in 127 out of 245 (51.8%) and negative in 118 out of 245 (48.2%), respectively. Indeterminate results in the PBMCs ELISpot were observed in 31 out of 347 (8.9%) patients, all belonging to the non-TB group.
ELISpot results on BALMCs were positive in 60 out of 66 (90.9%) and negative in 6 out of 66 (9.1%) of patients with active TB, respectively. In patients in the nonTB group, ELISpot results on BALMCs were positive in 50 out of 249 (20.1%) and negative in 199 out of 249 (79.9%), respectively (Figures 1 and 2; Table 2). In 5 out of 71 (7.0%) of individuals with TB and in 27 out of 276 (9.8%) of individuals without TB, the BALMCs ELISpot results were indeterminate.
Figure 1.
Study design and main outcome.
Figure 2.
Early secretory antigenic target (ESAT)-6– and culture filtrate protein (CFP)-10–specific enzyme-linked immunospot with (A) peripheral blood mononuclear cells (PMBCs) and (B) bronchoalveolar lavage mononuclear cells (BALMCs; B) in suspects with sputum acid-fast bacilli (AFB) smear-negative pulmonary tuberculosis (TB). Gray bars and white bars represent numbers of spot forming cells (SFC) per 250,000 PBMCs and BALMCs in patients with TB and in patients with alternative pulmonary diseases, respectively. Horizontal lines represent median values; whiskers represent 10th to 90th percentiles.
TABLE 2.
COMPARISON OF METHODS FOR THE DETECTION OF ACTIVE TUBERCULOSIS IN SPUTUM ACID-FAST BACILLI SMEAR-NEGATIVE CASES
| Parameter | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Positive Likelihood Ratio | Negative Likelihood Ratio | Area under ROC Curve |
|---|---|---|---|---|---|---|---|
| Blood ELISpot (n = 316) | 0.92 | 0.48 | 0.34 | 0.95 | 1.77 | 0.18 | 0.69 |
| BAL ELISpot (n = 316) | 0.91 | 0.79 | 0.55 | 0.97 | 4.53 | 0.11 | 0.85 |
| TST (n = 146) | 0.65 | 0.81 | 0.58 | 0.85 | 3.35 | 0.43 | 0.76 |
| NAAT (n = 248) | 0.29 | 0.97 | 0.73 | 0.82 | 9.14 | 0.74 | 0.62 |
Definition of abbreviations: BAL = bronchoalveolar lavage; ELISpot = enzyme-linked immunospot; NAAT = nucleic acid amplification technique; TST = tuberculin skin test.
When patients with culture-confirmed sputum AFB smear-negative TB were analyzed separately, sensitivity and specificity of the ELISpot on BALMCs were 87.2 and 79.9%, respectively (area under ROC curve, 0.83; 95% CI, 0.78–0.89).
The diagnostic odds ratio (OR) for the ELISpot on BALMCs was 27.1 (95% CI, 10.1–72.7; P < 0.001). ELISpot results from blood and BAL were not significantly different between culture-positive and culture-negative patients with active TB.
Differentiation of Active TB versus Latent TB Infection
In patients with a positive blood ELISpot result, BAL ELISpot was positive in 55 out of 60 (92%) patients with active TB and negative in 82 out of 116 (71%; P = 0.0012) patients without active TB. A positive result in the BAL ELISpot was highly discriminative to differentiate patients with active TB from individuals with LTBI (OR, 26.5; 95% CI, 9.8–72; P < 0.001).
Concentration of Antigen-specific Lymphocytes at the Site of Infection
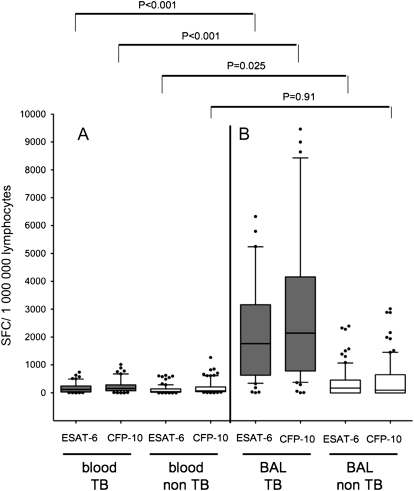
Individuals with positive M. tuberculosis–specific immune responses in PBMCs in the non-TB group were considered to be latently infected with M. tuberculosis. In these individuals, frequencies of ESAT-6– and CFP-10–specific lymphocytes were slightly increased among BAL lymphocytes compared with blood lymphocytes (Figure 3). The mean ratios of ESAT-6– and CFP-10–specific lymphocytes in BAL/blood of patients in the non-TB group were 3.3 and 2.6, respectively (P < 0.0001 for both antigens).
Figure 3.
Numbers of early secretory antigenic target (ESAT)-6- and culture filtrate protein (CFP)-10–specific (A) peripheral blood lymphocytes and (B) bronchoalveolar lavage lymphocytes per 1,000,000 lymphocytes in individuals suspected to be affected by sputum acid-fast bacilli (AFB) smear-negative pulmonary TB. All individuals shown had positive M. tuberculosis specific enzyme-linked immunospot assay results in the blood, compatible with either latent TB infection or active tuberculosis. Gray bars represent patients with sputum AFB smear-negative pulmonary TB; white bars represent patients with alternative pulmonary diseases. Horizontal lines represent median values; whiskers represent 10th to 90th percentiles.
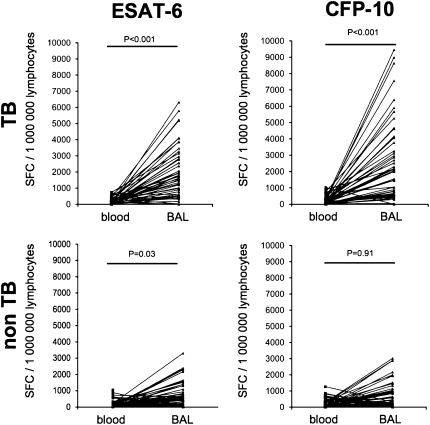
In patients with sputum AFB smear-negative TB, numbers of ESAT-6– and CFP-10–specific lymphocytes were clearly elevated among BAL-lymphocytes (Figure 3). The mean ratios of ESAT-6– and CFP-10–specific lymphocytes in BAL/blood were 16.3 and 16.0, respectively, demonstrating a concentration of antigen-specific T cells at the site of the infection in active TB (Figure 4).
Figure 4.
Comparison of numbers of early secretory antigenic target (ESAT)-6- and culture filtrate protein (CFP)-10–specific peripheral blood lymphocytes and bronchoalveolar lavage lymphocytes in patients with sputum acid-fast bacilli (AFB) smear-negative tuberculosis (TB) (upper row) and alternative pulmonary diseases (bottom row) by enzyme-linked immunospot.
Tuberculin Skin Test
TST was performed in 146 out of 347 (42.1%) patients, 43 with active TB and 103 with alternative diseases. In 28 out of 43 (65.1%) patients with active TB and 20 out of 103 (19.4%; P < 0.0001) with alternative diseases, the TST was positive, corresponding to a sensitivity and specificity of 65.1 and 80.6%, respectively (area under ROC curve, 0.73; 95% CI, 0.65–0.81) (Table 2). The diagnostic OR for TST was 7.8 (95% CI, 3.5–17.2; P < 0.001).
Nucleic Acid Amplification Technique
Results for the NAAT were available for 248 patients (56 with active TB and 192 with other diseases). In 16 out of 56 (28.6%) of patients with active TB and in 6 out of 192 (3.1%; P < 0.001) of patients with alternative diseases, the results of the M. tuberculosis–specific NAAT were positive, corresponding to a sensitivity and specificity of NAAT for the diagnosis of paucibacillary active TB of 29 and 97%, respectively (OR, 12.4; 95% CI, 4.6–33.6; P < 0.001; area under ROC curve, 0.62; 95% CI, 0.56–0.68) (Table 2).
Direct Comparison of M. tuberculosis–specific BAL and NAAT for the Diagnosis of Active TB
In 228 out of 347 (65.7%) patients (50 with active TB and 178 with alternative diseases), M. tuberculosis–specific ELISpot and NAAT were performed in parallel on BAL.
In 16 out of 50 (32%) of patients with active TB, NAAT on BAL was positive, and in 6 out of 178 (3.4%) of patients with alternative diseases, NAAT on BAL was positive, corresponding to a sensitivity and specificity of M. tuberculosis–specific NAAT on BAL of 32 and 97%. In 45 out of 50 (90%) of patients with active TB, ELISpot on BAL was positive and in 30 out of 178 (16.9%; P < 0.001) of patients with alternative diseases, ELISpot on BAL was positive, corresponding to a sensitivity and specificity of M. tuberculosis–specific ELISpot on BAL of 90 and 83% (OR, 44.4; 95% CI, 16.3–121.2; P < 0.001; area under ROC curve, 0.87) (Table 3).
TABLE 3.
COMPARISON OF MYCOBACTERIUM TUBERCULOSIS–SPECIFIC NUCLEIC ACID AMPLIFICATION TECHNIQUE AND ENZYME-LINKED IMMUNOSPOT FROM BRONCHOALVEOLAR LAVAGE FOR THE DIAGNOSIS OF SPUTUM ACID-FAST BACILLI SMEAR-NEGATIVE TUBERCULOSIS*
| Parameter | BAL ELISpot | BAL NAAT |
|---|---|---|
| Sensitivity | 0.9 | 0.32 |
| Specificity | 0.83 | 0.97 |
| Positive predictive value | 0.6 | 0.73 |
| Negative predictive value | 0.97 | 0.84 |
| Positive likelihood ratio | 5.34 | 9.49 |
| Negative likelihood ratio | 0.12 | 0.7 |
| Area under ROC curve | 0.87 | 0.64 |
| Odds ratio | 44.4 | 13.49 |
Definition of abbreviations: BAL = bronchoalveolar lavage; ELISpot = enzyme-linked immunospot; NAAT = nucleic acid amplification technique.
Smear-negative tuberculosis, n = 228; tuberculosis, n = 50; alternative diseases, n = 178.
When comparing the assay agreement between BAL ELISpot and NAAT, Cohen's kappa index was 0.012, corresponding to slight agreement according to the interpretation of Landis and Koch. The percentage of patients with TB not diagnosed by NAAT was 64.8, whereas 14.1% patients with TB were not diagnosed by BAL ELISpot. The percentage reduction is 50.7% (95% CI, 36.9–64.5). The inverse of the percentage reduction, comparable to the number needed to treat, is 2. This means that about one in every two patients with AFB smear-negative pulmonary TB will “benefit” from BAL ELISpot (95% CI, 1.6–2.7).
Comparison of Methods for the Rapid Detection of Sputum AFB Smear-negative Active TB
In logistic regression, the OR of a positive BAL ELISpot result to be associated with active TB was 40.4 (95% CI, 16.5–98.9; P < 0.001), compared with 12.4 for the BAL-NAAT (95% CI, 4.6–33.7; P < 0.001), 7.8 for the TST (95% CI, 3.5–17.2; P < 0.001), and 10.1 for the blood ELISpot (95% CI, 4.2–24.1; P < 0.001) (Table 4).
TABLE 4.
LOGISTIC REGRESSION ANALYSIS FOR THE DIAGNOSIS OF SPUTUM ACID-FAST BACILLI SMEAR-NEGATIVE TUBERCULOSIS
| Method | Diagnostic Odds Ratio (95% confidence interval) | P Value |
|---|---|---|
| Blood ELISpot | 10.1 (4.2–24.09) | <0.001 |
| BAL ELISpot | 40.4 (16.54–98.93) | <0.001 |
| TST | 7.8 (3.5–17.15) | <0.001 |
| NAAT | 12.4 (4.56–33.65) | <0.001 |
Definition of abbreviations: BAL = bronchoalveolar lavage; ELISpot = enzyme-linked immunospot; NAAT = nucleic acid amplification technique; TST = tuberculin skin test.
The correlation between TST results and BAL ELISpot and blood ELISpot results was only moderate (Spearman's rho, 0.32 and 0.3, respectively). A poor relationship was found when BAL NAAT results were correlated to the BAL ELISpot (qualitative variable; Spearman's rho, 0.08).
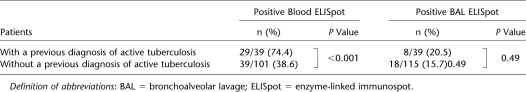
Influence of Previous TB on Blood and BAL ELISpot Results
In patients with a previous medical history of active TB, M. tuberculosis–specific PBMC and BALMC ELISpot results were positive in 29 out of 39 (74.4%) and 8 out of 39 (20.5%), respectively. In patients without a previous medical history of active TB, M. tuberculosis–specific PBMC ELISpot and BALMC ELISpot results were positive in 39 out of 101 (38.6%; P = 0.0001) and in 18 out of 115 (15.7%; P = 0.49), respectively. Previous active TB was significantly related to a positive M. tuberculosis–specific ELISpot result in the blood but not in the BAL fluid (Table 5).
TABLE 5.
INFLUENCE OF PREVIOUS ACTIVE TUBERCULOSIS ON TEST RESULTS OF MYCOBACTERIUM TUBERCULOSIS–SPECIFIC ELISPOT IN BLOOD AND BRONCHOALVEOLAR LAVAGE
DISCUSSION
The results from this study suggest that local immunodiagnosis by M. tuberculosis–specific ELISpot is an important advancement to rapidly distinguish sputum AFB smear-negative active TB from LTBI in routine clinical practice in countries with low TB incidence.
IGRAs, as ELISA (QuantiFERON-TB-Gold In Tube; QFT-GIT test; Cellestis, Carnegie, Australia) and as ELISpot (T-SPOT.TB test; Oxford Immunotec, Abingdon, UK), have been approved in many countries as advanced tools for the immunodiagnosis of LTBI. However, for the diagnosis of active TB, IGRAs are of little clinical value because immunodiagnostic tests are not likely to distinguish active TB from LTBI when performed on cells from the peripheral blood alone (10, 20). A plausible explanation for the lack of discrimination of active TB from LTBI by IGRAs is the limitation relating to the use of peripheral blood for the assays. Immune responses assayed on blood mononuclear cells may only provide background information about effector memory T-cell activity in active TB (21). In contrast, M. tuberculosis–specific T cells are recruited to and expanded among lymphocytes from pleural effusion (13, 22–24), ascites (13), pericardial effusion (25), and cerebrospinal fluid (26) in patients with AFB smear-negative pleural, peritoneal, pericardial, and meningeal TB. Because IFN-γ–secreting T lymphocytes are also expanded in human lungs in active pulmonary TB (27, 28), IGRA responses assayed in mononuclear cells from BAL should provide better discrimination of active TB from LTBI than responses with PBMC alone.
In a pilot study (15), it was reported that sputum AFB smear-negative active TB was highly likely when M. tuberculosis–specific lymphocytes were detectable by ELISpot among cells from the BAL fluid. However, the study was too small to draw definitive clinical conclusions (29). Results from this much larger prospective multicenter study by the TBNET confirm that in the majority of patients suspected to be affected by active TB without detectable AFB in sputum smears, M. tuberculosis–specific ELISpot with peptides of ESAT-6, and CFP-10 performed on mononuclear cells from the BAL fluid can rapidly distinguish patients with active TB from those with LTBI. Although the results of this study are not as clear cut as in the pilot trial, the sensitivity and specificity of the BAL ELISpot for the detection of sputum AFB smear-negative pulmonary TB were 91 and 80%, respectively. The diagnostic accuracy of this BAL ELISpot assay for the diagnosis of sputum AFB smear-negative pulmonary TB is comparable with results from a recent trial using a technically more demanding flow cytometry assay (30).
In patients with a positive blood ELISpot result, the diagnostic OR for active TB versus LTBI was 26.5 (95% CI, 9.8–72) if the BAL ELISpot assay result was also positive. Although a previous history of active TB was a confounder for a positive blood ELISpot assay result, BAL ELISpot results were independent of previous TB. When compared with the blood ELISpot, the TST and the M. tuberculosis–specific NAAT on BAL, BAL ELISpot was superior for rapidly identifying patients with sputum AFB smear-negative TB. When compared with M. tuberculosis–specific NAAT, local immunodiagnosis for mycobacteria-specific T cells was markedly more sensitive for the rapid diagnosis of sputum AFB smear-negative TB, confirming previous findings (15, 30). However, because of the very high specificity of NAAT of 97%, the positive likelihood ratio of NAAT was superior to the BAL ELISpot for the diagnosis of sputum AFB smear-negative TB.
Monocytes and dendritic cells are included together with lymphocytes in the ELISpot assays as antigen presenting cells, but their contribution to IFN-γ production is probably negligible. By adjusting the numbers of spot-forming cells to the numbers of lymphocytes in the blood and BAL ELISpot assays using differential blood and BAL cell counts, we observed a concentration of ESAT-6– and CFP-10–specific lymphocytes in the lungs versus the blood by a factor of 16 in patients with active TB who did have a positive blood ELISpot result. These findings are consistent with previous observations (13, 14). M. tuberculosis antigen-specific lymphocytes were also slightly expanded among BAL lymphocytes versus blood lymphocytes in individuals with LTBI who did not have active TB, presumably because of antigen stimulation at the site of M. tuberculosis persistence in LTBI. It is interesting to speculate whether individuals with positive blood ELISpot responses and absent BAL ELISpot responses may be more likely to have cleared latent M. tuberculosis infection in the lungs completely.
Bronchoscopy is indicated in all individuals suspected to be affected by active TB with negative AFB smears in countries with low incidence of TB (31) because alternative diagnoses, including sarcoidosis, bronchoalveolar carcinoma, and cryptogenic organizing pneumonia, must be considered (32). Only one out of five individuals suspected to be affected by sputum AFB smear-negative TB in this multicenter study was eventually diagnosed with active TB. Simple immunodiagnostic assays that enable to establish a rapid diagnosis of active TB in sputum AFB smear-negative cases would be welcome, but the frequencies of ESAT-6– or CFP-10–specific T cells in induced sputum are too low to be reliably detected by currently available techniques (33).
The limitations of our study need to be addressed. Active TB was only proven in 56.3% of cases that fulfilled the case definition for active sputum AFB smear-negative TB. Although the results of the ELISpot and NAAT investigations were comparable in the culture-positive and culture-negative cases of sputum AFB smear-negative TB, some patients with negative M. tuberculosis cultures may have been misclassified. In 9.2% of BAL-ELISpot assays, indeterminate results were found. Unstimulated IFN-γ production is frequently observed in BALMCs. To avoid an impairment of the test, we expanded the manufacturer's definition for test results in PBMCs to the BALMCs.
Following national guidelines, TST was not performed at the center in the Netherlands. The tuberculin licensed for the TST in Italy differs from the tuberculin used in Germany and Spain, although the products are thought to be bioequivalent.
The sensitivity of M. tuberculosis–specific NAAT for the detection of sputum AFB smear-negative TB was low in this study. Although the ELISpot method was standardized at all centers participating, different NAAT systems were used according to local practice, causing a limitation in comparability. A recent metaanalysis reported a high variability of NAAT sensitivity among different clinical trials in individuals with sputum AFB smear-negative TB (9).
None of the individuals enrolled in this study had a positive HIV serostatus. Although sputum AFB smear-negative pulmonary TB can be identified by BAL ELISpot in persons with immunosuppression (34), the results from this study cannot be extended to persons with HIV infection without further investigations.
Finally, in individuals living in areas of high incidence of TB, where frequent exposure to M. tuberculosis is likely, pulmonary immune responses to antigens of M. tuberculosis could be different from those observed in individuals from areas of low incidence of TB who were enrolled in this study.
In conclusion, M. tuberculosis–specific ELISpot on cells from the BAL fluid is an important advancement to distinguish sputum AFB smear-negative pulmonary TB from LTBI in routine clinical practice, although the specificity of the test is suboptimal and inferior to NAAT. One in every two patients evaluated in this study would have benefited from BAL ELISpot for the rapid diagnosis of AFB smear-negative pulmonary TB. This approach may be most applicable for a rapid decision to initiate anti-TB treatment where bronchoscopy is routinely performed for individuals suspected to be affected by sputum AFB smear-negative TB and where the technology for ELISpot can be established. However, the gold standard for the diagnosis of pulmonary TB continues to be the direct detection and isolation of M. tuberculosis from respiratory tract specimen because species identification and drug susceptibility testing is currently not possible by immune-based tests.
Acknowledgments
ELISpot test kits (T-SPOT.TB) were supplied by the manufacturer, Oxford Immunotec (Abingdon, UK) free of charge. The manufacturer of the ELISpot test had no influence on the study design, participating clinicians and/or scientists, selection of the study centers, data collection, data analysis, or the decision to publish the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.200904-0557OC on July 9, 2009
Conflict of Interest Statement: C.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.T. has a patent for the Cross-spot technique for recognizing antigens using immune-effector cells. G.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G. has patent that is submitted on the use of RD1 selected peptides for the discrimination of active TB from LTBI. J.A.D.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B. received up to $1,000 from bioMerieux in lecture fees and has a patent for the Cross-spot technique for recognizing antigens using immune-effector cells. I.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. U.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.R. received $1,001 to $5,000 from Cellestis Ltd and $1,001 to $5,000 from Oxford Immunotec in consultancy fees. M.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.L. received $1,001 to $5,000 from AstraZeneca, $1,001 to$5,000 from GlaxoSmithKline, $1,001 to $5,000 from Pfizer, $1,001 to $5,000 from Oxford Immnotec, and $1,001 to $5,000 from Chiesi in lecture fees.
References
- 1.Dye C. Global epidemiology of tuberculosis. Lancet 2006;367:938–940. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA 1999;282:677–686. [DOI] [PubMed] [Google Scholar]
- 3.Pai M, O'Brien R. New diagnostics for latent and active tuberculosis: state of the art and future prospects. Semin Respir Crit Care Med 2008;29:560–568. [DOI] [PubMed] [Google Scholar]
- 4.Hanna BA, Ebrahimzadeh A, Elliott LB, Morgan MA, Novak SM, Rusch-Gerdes S, Acio M, Dunbar DF, Holmes TM, Rexer CH, et al. Multicenter evaluation of the bactec mgit 960 system for recovery of mycobacteria. J Clin Microbiol 1999;37:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bericht zur Epidemiologie der Tuberkulose in Deutschland für 2007. Berlin, Germany; Robert-Koch-Institut: 2009.
- 6.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006;6:664–674. [DOI] [PubMed] [Google Scholar]
- 7.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis 2008;8:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel TM. The rapid diagnosis of tuberculosis: a selective review. J Lab Clin Med 1990;116:277–282. [PubMed] [Google Scholar]
- 9.Sarmiento OL, Weigle KA, Alexander J, Weber DJ, Miller WC. Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis. J Clin Microbiol 2003;41:3233–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goletti D, Stefania C, Butera O, Amicosante M, Ernst M, Sauzullo I, Vullo V, Cirillo D, Borroni E, Markova R, et al. Accuracy of immunodiagnostic tests for active tuberculosis using single and combined results: a multicenter TBNET-study. PLoS ONE 2008;3:e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dosanjh DP, Hinks TS, Innes JA, Deeks JJ, Pasvol G, Hackforth S, Varia H, Millington KA, Gunatheesan R, Guyot-Revol V, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med 2008;148:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez J, Ruiz-Manzano J, De Souza-Galvao M, Latorre I, Mila C, Blanco S, Jimenez MA, Prat C, Lacoma A, Altet N, et al. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin Vaccine Immunol 2008;15:168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, Maskell N, Davies R, Pasvol G, Lalvani A. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis 2005;40:184–187. [DOI] [PubMed] [Google Scholar]
- 14.Jafari C, Ernst M, Strassburg A, Greinert U, Kalsdorf B, Kirsten D, Lange C. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur Respir J 2008;31:261–265. [DOI] [PubMed] [Google Scholar]
- 15.Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, Marienfeld K, Lalvani A, Lange C. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med 2006;174:1048–1054. [DOI] [PubMed] [Google Scholar]
- 16.Verbon A, Cobelens FG. [Indications for, and the significance of, the tuberculin test in the Netherlands]. Ned Tijdschr Geneeskd 2003;147:539–543. [PubMed] [Google Scholar]
- 17.Savelkoul PH, Catsburg A, Mulder S, Oostendorp L, Schirm J, Wilke H, van der Zanden AG, Noordhoek GT. Detection of mycobacterium tuberculosis complex with real time PCR: comparison of different primer-probe sets based on the is6110 element. J Microbiol Methods 2006;66:177–180. [DOI] [PubMed] [Google Scholar]
- 18.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG. The stard statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003;138:W1–W12. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Treatment of tuberculosis: Guidelines for national programmes. Geneva: WHO; 2003.
- 20.Dominguez J, De Souza-Galvao M, Ruiz-Manzano J, Latorre I, Prat C, Lacoma A, Mila C, Jimenez MA, Blanco S, Maldonado J, et al. T-cell responses to the mycobacterium tuberculosis-specific antigens in active tuberculosis patients at the beginning, during, and after antituberculosis treatment. Diagn Microbiol Infect Dis 2009;63:43–51. [DOI] [PubMed] [Google Scholar]
- 21.Jafari C, Lange C. Suttons's law: local immunodiagnosis of tuberculosis. Infection 2008;36:510–514. [DOI] [PubMed] [Google Scholar]
- 22.Losi M, Bossink A, Codecasa L, Jafari C, Ernst M, Thijsen S, Cirillo D, Ferrarese M, Greinert U, Fabbri LM, et al. Use of a T-cell interferon-{gamma} release assay for the diagnosis of tuberculous pleurisy. Eur Respir J (In press) [DOI] [PubMed]
- 23.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun 1993;61:3482–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, Modlin RL. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol 1989;142:1114–1119. [PubMed] [Google Scholar]
- 25.Biglino A, Crivelli P, Concialdi E, Bolla C, Montrucchio G. Clinical usefulness of elispot assay on pericardial fluid in a case of suspected tuberculous pericarditis. Infection 2008;36:601–604. [DOI] [PubMed] [Google Scholar]
- 26.Thomas MM, Hinks TS, Raghuraman S, Ramalingam N, Ernst M, Nau R, Lange C, Kosters K, Gnanamuthu C, John GT, et al. Rapid diagnosis of mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int J Tuberc Lung Dis 2008;12:651–657. [PMC free article] [PubMed] [Google Scholar]
- 27.Schwander SK, Torres M, Sada E, Carranza C, Ramos E, Tary-Lehmann M, Wallis RS, Sierra J, Rich EA. Enhanced responses to mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J Infect Dis 1998;178:1434–1445. [DOI] [PubMed] [Google Scholar]
- 28.Nemeth J, Winkler HM, Zwick RH, Rumetshofer R, Schenk P, Burghuber OC, Graninger W, Ramharter M, Winkler S. Recruitment of mycobacterium tuberculosis specific CD4+ T cells to the site of infection for diagnosis of active tuberculosis. J Intern Med 2009;265:163–168. [DOI] [PubMed] [Google Scholar]
- 29.Sood A, Schuyler M. Finally, a perfect diagnostic test for pulmonary tuberculosis–or is it? Am J Respir Crit Care Med 2006;174:963–964. [DOI] [PubMed] [Google Scholar]
- 30.Breen RA, Barry SM, Smith CJ, Shorten RJ, Dilworth JP, Cropley I, McHugh TD, Gillespie SH, Janossy G, Lipman MC. Clinical application of a rapid lung-orientated immunoassay in individuals with possible tuberculosis. Thorax 2008;63:67–71. [DOI] [PubMed] [Google Scholar]
- 31.Schoch OD, Rieder P, Tueller C, Altpeter E, Zellweger JP, Rieder HL, Krause M, Thurnheer R. Diagnostic yield of sputum, induced sputum, and bronchoscopy after radiologic tuberculosis screening. Am J Respir Crit Care Med 2007;175:80–86. [DOI] [PubMed] [Google Scholar]
- 32.Lange C, Ernst M, Strassburg A, Sarrazin H, Jafari C. Diagnosis of smear negative tuberculosis in low prevalence countries: keeping the scope on the bronchoscope [letter, 2008 Feb 20]. PLoS ONE 2007;2:e1335.18092001 [Google Scholar]
- 33.Breen RA, Hardy GA, Perrin FM, Lear S, Kinloch S, Smith CJ, Cropley I, Janossy G, Lipman MC. Rapid diagnosis of smear-negative tuberculosis using immunology and microbiology with induced sputum in HIV-infected and uninfected individuals. PLoS ONE 2007;2:e1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strassburg A, Jafari C, Ernst M, Lotz W, Lange C. Rapid diagnosis of pulmonary TB by BAL enzyme-linked immunospot assay in an immunocompromised host. Eur Respir J 2008;31:1132–1135. [DOI] [PubMed] [Google Scholar]