SUMMARY
Cells in intestinal epithelia turn over rapidly due to damage from digestion and toxins produced by the enteric microbiota. Gut homeostasis is maintained by intestinal stem cells (ISCs) that divide to replenish the intestinal epithelium, but little is known about how ISC division and differentiation are coordinated with epithelial cell loss. We show here that when enterocytes (ECs) in the Drosophila midgut are subjected to apoptosis, enteric infection, or JNK-mediated stress signaling, they produce cytokines (Upd, Upd2, Upd3) that activate Jak/Stat signaling in ISCs, promoting their rapid division. Upd/Jak/Stat activity also promotes progenitor cell differentiation, in part by stimulating Delta/Notch signaling, and is required for differentiation in both normal and regenerating midguts. Hence, cytokine-mediated feedback enables stem cells to replace spent progeny as they are lost, thereby establishing gut homeostasis.
INTRODUCTION
Many animal tissues undergo homeostatic growth in which spent differentiated cells are replaced by the progeny of resident stem or progenitor cells. In the epithelial lining of animal intestines high rates of cell turnover are presumed to vary according to changes in food composition and dietary exposures to toxins, pathogens, and chemical or mechanical injury. To maintain normal gut structure and function (i.e. homeostasis) intestinal stem cells likely respond to variations in cell loss with corresponding changes in rates of self-renewal and differentiation. How this occurs is not well understood. According to a prevalent view of the vertebrate intestine, stem- and transient amplifying cell divisions in the crypts of Lieberkühn, promoted by WNT signaling, drive gut epithelial renewal in a “conveyor-belt” fashion, generating a constant supply of differentiated cells to the villi, where they are autonomously exfoliated (Gregorieff and Clevers, 2005; Sancho et al., 2004). In its simplest form this model does not incorporate feedback from the differentiated epithelium to progenitor cells, and therefore lacks the means to maintain stasis when rates of epithelial cell loss vary. More sophisticated models that do incorporate feedback have been discussed: for instance negative cross-talk between BMP signaling in the villi and WNT signaling in the crypts might allow true homeostasis (Crosnier et al., 2006; Gregorieff and Clevers, 2005). But rigorous tests of the cross-regulatory interactions required have so far not been possible in a vertebrate. In this respect the Drosophila midgut, which is simpler than its vertebrate counterparts but has similar cell types and signaling interactions, is technically advantageous.
The Drosophila adult midgut is maintained by intestinal stem cells (ISCs) that self-renew and also produce the two principal differentiated cell types of the intestinal epithelium, absorptive enterocytes (ECs) and secretory enteroendocrine (EE) cells (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). The midgut also maintains many non-dividing, undifferentiated ISC daughters termed enteroblasts (EBs), which can differentiate directly. Differentiation requires Delta/Notch signaling from the ISC to its EB daughter and, as in mammals, the fate decision taken (absorptive vs. secretory) is thought to depend upon the intensity of Notch signaling received by an EB (Ohlstein and Spradling, 2007). Lineage analysis suggests that differentiated cells in the midgut epithelium turn over roughly weekly in well-fed flies, as in mammals.
Studies of dissociated Lepidopteran midguts found that cell death caused by Bacillus thuringiensis (Bt) endotoxin stimulated the division of a population of cells that were probably ISCs (Hakim et al., 2001; Loeb et al., 2001), and recent reports document mitoses in Drosophila midguts in response to ingested detergent (Amcheslavsky et al., 2009) or bacteria (Buchon et al., 2009). These findings suggest that the loss of damaged ECs stimulates ISC division. Since EB differentiation coincides with a reduction in their contact with a basement membrane, it has also been proposed that this membrane or underlying visceral muscle might provide a niche that promotes stemness and suppresses differentiation (Ohlstein and Spradling, 2007). Consistent with this, the WNT ligand wg is expressed in visceral muscle, and is important for ISC survival (Lin et al., 2008).
We show here that the Drosophila midgut can rapidly regenerate after enterocytes are ablated, or subjected to enteric infection or stress signaling. Damaged or stressed ECs produce the Unpaired cytokines (Upd, Upd2, Upd3). These ligands and their downstream effectors Domeless (dome, an IL-6R-like receptor), Hopscotch (hop, a Janus Kinase; Jak) and Stat92E (a STAT3-like transcription factor) have important roles in germ stem cell maintenance and the immune response in Drosophila. In the midgut, Upds produced by spent ECs trigger Jak/Stat signaling in ISCs and EBs, promoting their division and differentiation respectively, and thereby driving renewal of the gut epithelium.
RESULTS
Progenitor cells are required for midgut maintenance
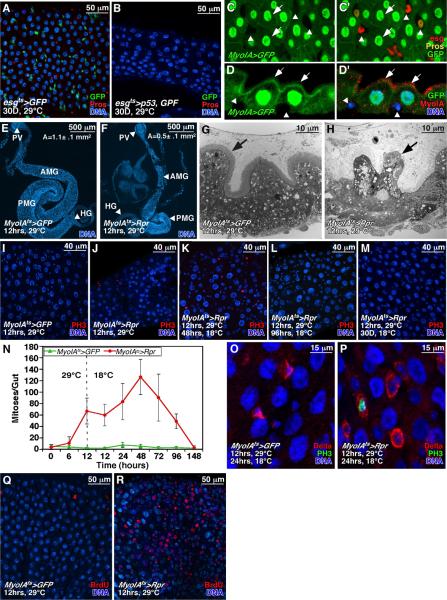
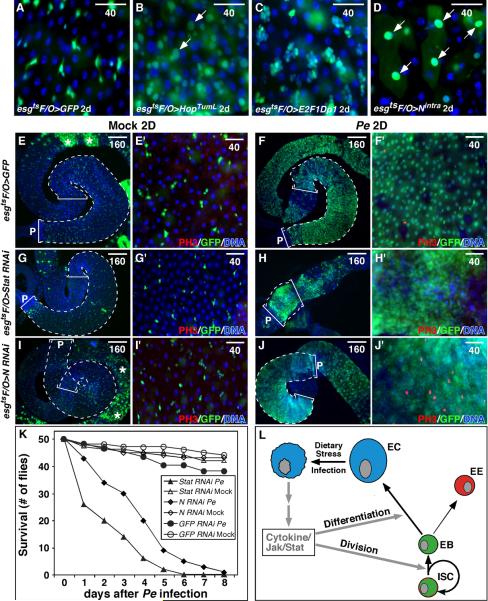
To determine whether ISCs are required for midgut maintenance we sought to ablate them. To express cell death effectors we used esgGal4 and the temperature sensitive Gal4 repressor, tubGal80ts (McGuire et al., 2004) (together referred to as esgts), to allow temporal activation of UAS-linked target genes in ISCs and EBs (Jiang and Edgar, 2008; Micchelli and Perrimon, 2006). Although induction of reaper (rpr, an inhibitor of Drosophila Inhibitor of Apoptosis-1; DIAP-1) had little effect on progenitor cells (i.e. ISCs and EBs), ricin A or Drosophila p53 effectively ablated them (Fig 1A, B). Fifteen days of p53 induction ablated nearly all esg+ progenitor cells and reduced EE numbers, but the midguts were otherwise intact. After 30 days of p53 induction all ISCs, EBs, and EEs and many ECs were lost, and the midguts were shrunken (Fig 1B). Remaining ECs had grown in size, perhaps to compensate for the loss of absorptive surface area. This result concurs with clonal analyses (Ohlstein and Spradling, 2006) showing that the midgut epithelium turns over rapidly and must be constantly replenished by ISC progeny.
Figure 1. Midgut cell ablation and renewal.
(A, B) Drosophila p53 expressed with esgGal4ts for 30 days ablated esg+ progenitor cells (green), pros+ EEs (red), and decreased ECs (blue; B). Controls overexpressed GFP alone (A).
(C-D’) MyoIAGal4-driven UAS-GFP is expressed in ECs (arrows) but not in small basally located esglacZ+ progenitors (red) or pros+ EEs (yellow; arrowheads). GFP+ ECs were positive for brush border Myosin IA (MyoIA is red in D’).
(E, F) Expression of Reaper (Rpr) with MyoIAts for 12h reduced midgut size (F). Control shown in (E). PV: proventriculus; AMG: anterior midgut; PMG: posterior midgut; HG: hindgut. Mean surface area (A) for 10 such midguts is indicated.
(G, H) TEM of midguts expressing Rpr (H) or GFP control (G). Rpr caused loss of brush border (arrows) and enterocyte.
(I, J) Expression of Rpr with MyoIAts for 12 hours ablated enterocytes and promoted mitoses (PH3+, red). Control in (I).
(K-N) Mitoses and midgut regeneration after enterocyte ablation. During recovery at 18°C, PH3+ mitotic cells (red) increased through 48-72h, but decreased as midguts regained their normal size by 96h. Midguts re-grew to their normal size, due partly to increases in enterocyte size (L). After 1 month cell density and EC size returned to normal (M).
(O, P) Mitotic cells (PH3+, green) in regenerating midguts were positive for the ISC marker, Delta (red, P). ISCs in regenerating midguts (P) were larger than in controls (O), and had more Delta.
(Q, R) EC ablation by Rpr increased BrdU incorporation (red) in small progenitors and large ECs. Blue in all panels is DNA.
Midgut regeneration from stem cells
To determine whether ISC division responds to epithelial cell loss, we sought to ablate ECs. To express genes in ECs we used the MyoIAGal4 driver (NP1-Gal4), an enhancer trap in the gut-specific brush border myosin IA gene (Morgan et al., 1994) in combination with tubGal80ts. UAS-GFP driven by MyoIAGal4 was strongly expressed in all midgut ECs, identified by their large nuclei and expression of brush border Myosin IA. No expression was detected in ISCs, EBs, EEs, or visceral muscle (Fig 1C, D). We used the inducible MyoIAGal4 tubGal80ts system (heretofore referred to as MyoIAts) to express the pro-apoptotic gene reaper (rpr), to trigger EC apoptosis. MyoIAGal4 tubGal80ts UAS-Rpr (MyoIAts>Rpr) animals were raised to adults at 18°C (Gal4 “off”), shifted to 29°C (Gal4 “on”) for 12hrs, and then shifted to 18°C to extinguish rpr expression. 12h induction of Rpr reduced midgut size due to widespread apoptosis (Fig 1F, J). Tissue sections showed the loss of EC brush borders and apical extrusion (Fig 1G, H). Within days, however, the damaged midguts had regenerated substantially (Fig 1K-M). We assayed the mitotic response of ISCs using antibodies to phospho-Ser10-histone 3 (PH3). PH3+ mitotic figures rose to >100/midgut by 48h after a 12h pulse of reaper, whereas controls maintained a mitotic index of 1-3 mitoses/midgut (Fig 1I-K, N). Rpr-induced mitoses could be suppressed by co-expression of the caspase inhibitors p35 or DIAP1 (Fig 2K), indicating that apoptosis was required. Most (88%) PH3+ cells were positive for the ISC marker, Delta, and all PH3+ cells were negative for the EE marker prospero (Fig 1P, Table S1). Delta+ cells in regenerating midguts were enlarged, consistent with increased growth, had higher Delta levels than in controls, and were often paired or clustered (Figs 1P, 5E).
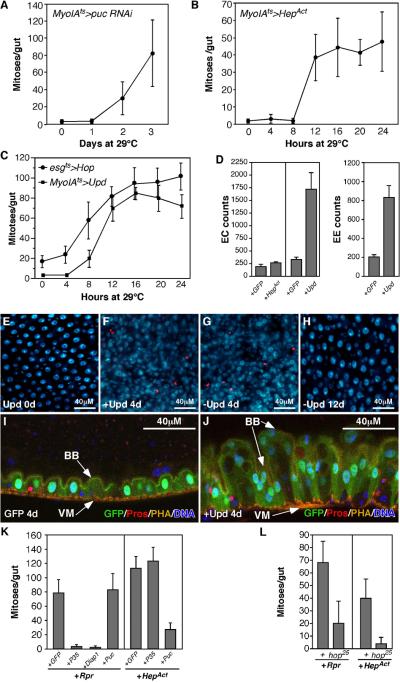
Figure 2. JNK and Cytokine/Jak/Stat signaling induce ISC mitosis.
(A, B) ISC mitoses, quantified by PH3+ mitotic figures/midgut. JNK activation in ECs was achieved by depleting puckered (puc) with RNAi (A), or expressing activated Hemipterous (HepAct, B) using the MyoIAts system.
(C) Expression of Upd using MyoIAts, or Hopscotch (Jak) using esgts induced ISC mitosis.
(D) Quantification of gut hyperplasia (ECs and EEs) induced by JNK (MyoIAts>HepAct 4 days, +HepAct) or Upd (MyoIAts>Upd 4 days, +Upd). pros+ cells were scored as EEs. Large polyploid Pros- Dl- cells were scored as ECs.
(E-H) Upd-induced midgut hyperplasia and recovery. Upd induction in ECs increased PH3+ mitotic figures (red) and midgut cell density (blue: DNA). Midguts recovered normal morphology within 12 days of silencing ectopic Upd by temperature shift (H).
(I, J) Sagittal view of the midgut hyperplasia induced by Upd. Upd induction using MyoIAts increased ECs in the epithelium (J). One side of the midgut epithelium is shown. Actin is stained with phalloidin (PHA, yellow, marks the visceral muscle and brush border), EEs are marked by Pros (red). ECs are marked by GFP (green). DNA is in blue. BB, brush border; VM, visceral muscle.
(K) Epistasis of Rpr- and JNK-induced mitoses. Midgut mitotic indices 24h after transgene activation. Rpr-induced proliferation was suppressed by co-expressed P35 or DIAP1, but not by co-expressed Puc. Hep-induced proliferation was suppressed by Puc but not P35.
(L) Epistasis showing that Rpr and JNK-induced proliferation require hop (Jak). Midgut mitotic figures were quantified after expressing either Rpr or HepAct for 12h in ECs in hop25 mutants or controls (+).
Figure 5. Rpr, JNK, and Upd signaling up-regulate Delta/Notch signaling.
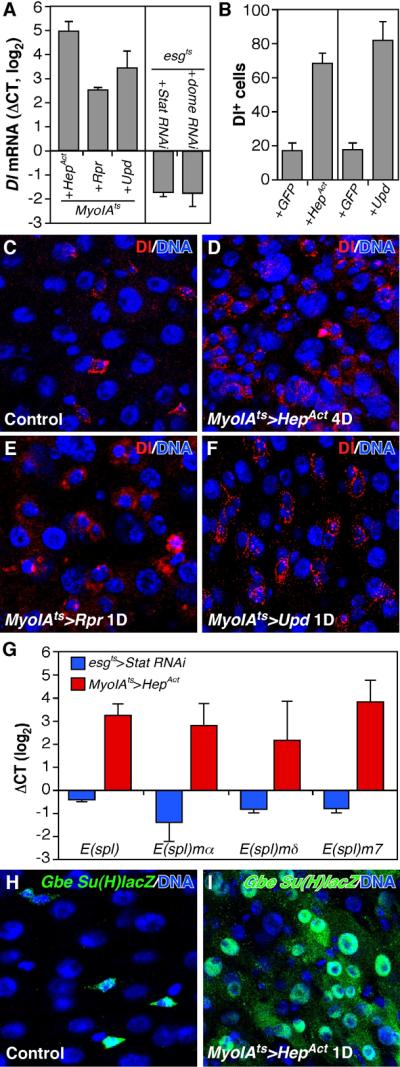
(A) Midgut Dl RNA expression measured by RT-qPCR. UAS-HepAct or UAS-Upd were induced for 24h, whereas UAS-Rpr was induced for 12 hours, in ECs using the MyoIAts system. UAS-Stat RNAi or UAS-Dome RNAi were induced in progenitor cells for 4d using the esgts system. The data were normalized to GFP only controls (MyoIAts>GFP or esgts>GFP respectively).
(B) Quantification of Dl+ progenitor cells in midguts expressing GFP (control), HepAct or Upd using the MyoIAts system.
(C-F) Delta protein (red) after induction of HepAct (D), Rpr (E), or Upd (F) in ECs.
(G) Notch activity as measured by E(spl)-C RNAs using RT-qPCR. UAS-HepAct was induced for 24h with MyoIAts and UAS-Stat RNAi was induced for 4d using esgts. The data were normalized as in (A).
(H, I) The Notch activity reporter, GbeSu(H)lacZ was observed in more gut epithelial cells after induction of HepAct in ECs. Since LacZ is stable its presence in ECs may be due to perdurance.
Midgut mitoses declined after 2 days and reached basal levels within a week (Fig 1L-N). Regenerating midguts re-gained their normal size by 60h of recovery, before the cessation of ISC proliferation or replenishment of the EC population. At this stage the midgut epithelium consisted of fewer ECs than normal, but these ECs were larger and more polyploid than in controls (Fig 1K, L). Following Rpr expression, extensive BrdU incorporation was rapidly induced not only in small cells (ISCs, EBs), but also in large polyploid ECs (Fig 1R). This suggests that existing ECs might respond directly to gut epithelial damage by compensatory EC growth and endoreplication. By one month of recovery Rpr-damaged midguts had regained normal cellularity and EC size (Fig 1M). To summarize, the midgut can compensate for epithelial cell loss by increasing progenitor cell divisions and the consequent generation of new ECs.
JNK signaling in ECs also promotes ISC division
To further investigate midgut regeneration we tested the Jun N-terminal Kinase (JNK) pathway, a MAPK-type kinase cascade that is activated in response to cellular stress, and which is involved in compensatory cell proliferation following injury in both insects and mammals (McEwen and Peifer, 2005; Nakagawa et al., 2008; Prieto, 2008; Ryoo et al., 2004). We activated JNK signaling in ECs by expressing RNAi directed against puckered (puc) using the MyoIAts system. puc encodes Drosophila Jun N-terminal kinase phosphatase. It is a potent suppressor of JNK activity and also a direct downstream target of JNK signaling (Martin-Blanco et al., 1998). Inducing puc RNAi in ECs for 2 days caused a large increase in ISC mitoses (Fig 2A). A similar but more rapid mitotic response was observed when an activated form of hemipterous (HepAct, Drosophila JNK kinase) was used to activate JNK in ECs (Fig 2B). We noted that HepAct induction increased the number and density of small Delta+ cells, suggesting that JNK activation elevated the numbers of ISC-like progenitors (Figs 5B, D, S1B). As observed in other contexts (Kanda and Miura, 2004; McEwen and Peifer, 2005) prolonged JNK activation caused significant cell death (not shown), but the onset of mitoses commenced long before EC apoptosis was observed. Moreover, co-expression of the caspase inhibitor p35 with HepAct did not prevent JNK-mediated mitoses (Fig 2K). Thus apoptosis appeared not to be responsible for JNK-induced ISC divisions. Control experiments showed that co-expressed puc significantly inhibited ISC mitoses induced by HepAct (Fig 2K), but interestingly, puc or another JNK inhibitor, BskDN (Adachi-Yamada et al., 1999), did not suppress ISC divisions induced by Rpr (Fig 2K and not shown). This indicates that stem cell divisions can be triggered by at least two independent pathways: a caspase-independent relay involving JNK signaling, and a caspase-dependent relay.
Upd/Jak/Stat signaling drives midgut renewal
Since cytokine signaling has been implicated in several models of regeneration (Lin and Karin, 2007; Prieto, 2008) we investigated its role in ISC proliferation. Drosophila has three leptin-like (IL-6 family) cytokines called Unpaireds (Upd, Upd2, Upd3). These bind an IL-6R type receptor, Domeless (dome), that activates a Janus kinase (Jak) called Hopscotch (hop), and thereby promotes the translocation of a STAT3-like transcription factor (STAT92E) to the nucleus. Transcriptional targets of STAT92E include the receptor, Dome, and a repressor of receptor/Jak complexes, Socs36E. We first tested this pathway’s effect on ISCs by over-expressing UAS-Upd either in ECs using MyoIAts, or in ISCs+EBs using esgts. Expression of Upd in either cell type induced ISC mitosis (Fig 2C, F, G), and resulted in dramatic gut hyperplasia with large increases in numbers of MyoIA+ ECs (Fig 2D, J), pros+ EEs (Fig 2D, J), and small Delta+ ISCs (Fig 5B, F). Upd2 had similar effects (not shown). We also observed increased midgut mitoses after expressing Hop (JAK) in progenitor cells using esgts (Fig 2C), and in hop gain-of-function mutants (hopTumL; not shown). Thus Upd/Jak/Stat signaling is a potent ISC mitogen, but does not block differentiation. Compared to other signals reported to cause midgut hyperplasia (Wnt signaling (Lin et al., 2008) and loss of Notch signaling (Micchelli and Perrimon, 2006)), Upd or Hop caused a much more rapid, dramatic increase in ISC mitoses and midgut cell numbers. Remarkably, hyperplastic midguts generated by Upd induction returned to normal size, morphology, and cellularity within 2 weeks of silencing the UAS-Upd transgene (Fig 2H). Similarly, JNK-induced hyperplasia was also reversible (Fig S1B-D).
Upd/Jak/Stat mediates apoptosis- and JNK-dependent ISC activation
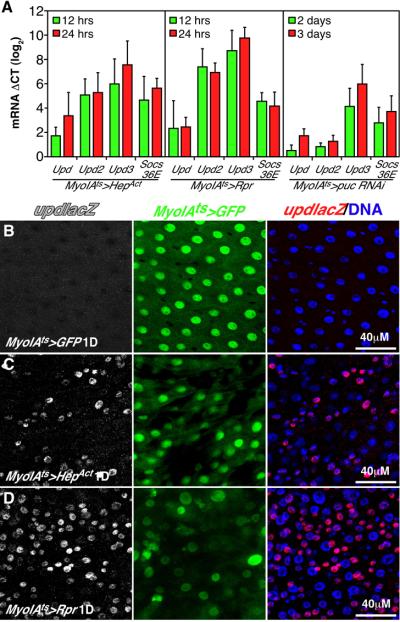
Reverse Transcriptase quantitative PCR (RT-qPCR) assays showed that all three Upd mRNAs were strongly upregulated after EC apoptosis was triggered by Rpr, or after JNK was activated by HepAct or Puc RNAi (Fig 3A). Upd3 was the most induced, to nearly 200 fold. A reporter for Upd transcription (UpdlacZ) (Chao et al., 2004) was also induced after JNK activation or EC ablation (Fig 3B-D), mainly in small progenitor cells and larger MyoIA- cells (GFP-), which we believe are early, partially differentiated ECs. Levels of the STAT target, Socs36E, were also profoundly increased by either JNK signaling or EC apoptosis (Fig 3A).
Figure 3. Apoptosis and JNK activation trigger cytokine production.
(A) RT-qPCR of whole midgut cDNA after activating EC apoptosis (MyoIAts>Rpr) or JNK signaling (MyoIAts>HepAct or puc RNAi). Upd, Upd2, Upd3, and Socs36E were induced within 12h of transgene activation. ΔCT is the difference in calculated normalized Ct (threshold cycle) values between experimental and 0h control samples (∼log2 scale). Error bars indicate std. deviations from 3 independent biological samples.
(B-D) Expression of the upd reporter, UpdlacZ, in control midguts (B), and following expression of Rpr (C) or HepAct (D) 24h after transgene activation by MyoIAts. GFP marks Gal4-expressing ECs.
Epistasis tests showed that ISC mitoses induced by either HepAct or Rpr were strongly reduced in hop25/Y mutant animals (Fig 2L), which have reduced JAK activity. Control hop25/Y mutants had normal numbers of esg+ progenitor cells (not shown), and thus the reduction in induced mitoses was unlikely to be due to decreased ISC numbers. These results indicate that Upd/Jak/Stat signaling is both sufficient and required for triggering ISC mitoses during regeneration.
Dome and Stat are required for EC differentiation
Upd/Dome/Hop signaling drives the nuclear translocation of Stat92E, the sole Drosophila STAT homolog. In normally fed wild type midguts, nuclear Stat92E was observed in esg+ progenitors (ISCs and EBs; Fig 4A), but not in ECs or EEs. STAT activity was also assayed using three transcriptional reporters, 10XStat-DGFP (Bach et al., 2007), (Gas)3lacZ (Gilbert et al., 2005), and an enhancer trap at the domeless locus, domeGal4. In normal midguts each Stat reporter was also expressed only in esg+ progenitor cells (Fig 4B, C, not shown). Thus Stat signaling is normally active in ISCs and EBs, but not in ECs or EEs.
Figure 4. Cytokine/Jak/Stat signaling is required for EB differentiation.
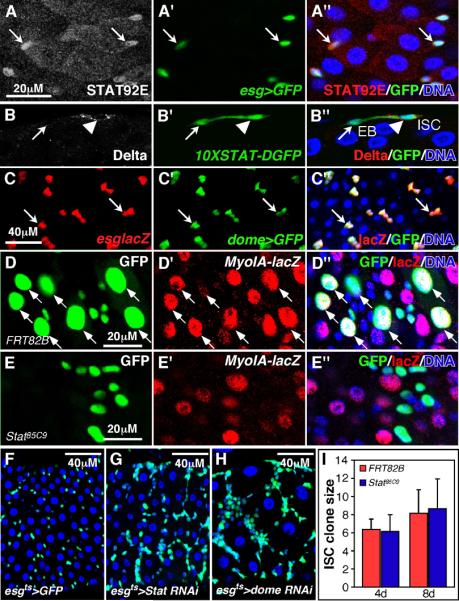
(A) Nuclear STAT92E in esg+ progenitor cells.
(B)10XSTAT-DGFP is active in progenitor cells including Dl+ ISCs (arrowhead) and Dl- EBs (arrow).
(C) domeGal4 drives UAS-GFP in esglacZ+ progenitor cells.
(D, E) MARCM clones showing that Stat85C9 mutant cells fail to enlarge or express the EC differentiation marker MyoIAlacZ (red, E). A control clone (D) has many lacZ+ ECs (red, arrows). GFP-marked clones analyzed 8d post-induction.
(F-H) Expression of Stat92E RNAi (G) or Dome RNAi (H) using the esgts system for 8 days resulted in progenitor cell accumulation and EC depletion.
(I) Cell numbers in control and Stat85C9 mutant MARCM clones.
To further test the function of Jak/Stat signaling we generated ISC clones mutant for strong loss-of-function alleles of Stat92E, Stat85C9 or Stat397 (Bach et al., 2007). While control clones comprised both small diploid progenitors and large polyploid ECs positive for the differentiation marker, MyoIAlacZ, (Fig 4D-D”), all cells in Stat85C9 mutant clones had small nuclei and lacked MyoIAlacZ expression (Fig 4E-E”). Most Stat85C9 mutant cells lacked Pros and Delta (not shown), suggesting that they were EBs that failed to differentiate, rather than ISC-like cells defective in Notch signaling. Stat397 mutant clones showed a similar inability to differentiate into ECs (Fig S2E), and this could be rescued by Gal4-driven Stat92E (Fig S2F). Similar differentiation defects were observed when Stat92E or the Upd receptor, dome, were depleted with RNAi either clonally (Fig S2) or in progenitors using esgGal4ts (Fig 4G, H). Cells homozygous for Stat85C9 or Stat397 or expressing RNAi against Stat92E or dome appeared to divide at rates comparable to WT cells (Figs 4I, S2). Thus Jak/Stat signaling is required for EC differentiation, though it may not be required for basal rates of ISC division.
Next we applied assays of Delta/Notch signaling, which is essential for differentiation of EBs to the EC fate (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007). Delta mRNA was reduced when Stat92E or dome were depleted in progenitor cells (Fig 5A). Conversely, Delta mRNA and protein were increased following induction of Upd, Rpr, or HepAct in ECs (Fig 5A, F). In these cases increased numbers of small Delta+ cells were observed, suggesting that the pool of functional stem cells was expanded (Fig 5B, F). These results suggested that Jak/Stat signaling might promote differentiation by increasing Delta expression and stimulating Notch receptor activity. This notion was supported by RT-qPCR showing that E(spl)-complex genes, which are Notch targets, were upregulated by expressing HepAct in ECs, and downregulated when Stat was depleted in progenitor cells (Fig 5G). Consistently, HepAct expression caused widespread activation of a Notch activity reporter, GbeSu(H)lacZ (Furriols and Bray, 2001) (Fig 5H, I). However, overexpressing Delta in Stat85D9 mutant ISCs, or in progenitor cells expressing Stat RNAi or Domeless RNAi, did not restore the ability of these cells to differentiate (Fig S3F). Thus Stat targets in addition to Delta are required for EC differentiation. The dual function of Upd/Jak/Stat signaling as a mitogen for ISCs and a differentiation factor for EBs may serve to couple these processes.
Enteric infection induces Upd/Jak/Stat signaling and ISC mitoses
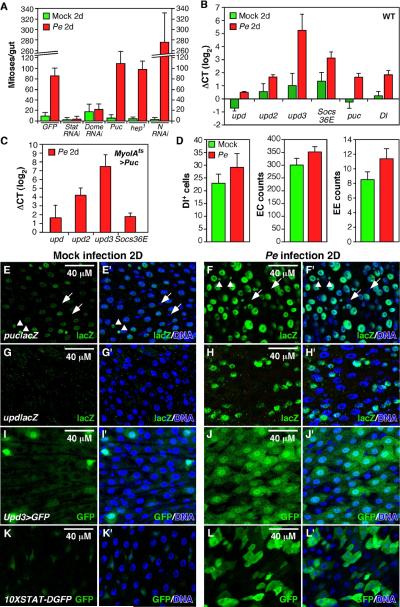
To investigate the physiological relevance of the regenerative responses described above we searched for natural environmental challenges that might stimulate ISC proliferation in Drosophila. Ingestion and enteric infection with Pseudomonas entomophila (Pe), a gram- bacteria, has been reported to kill ECs and activate JNK signaling (Vodovar et al., 2005). Feeding flies Pe for 2 days induced a strong mitotic response in the midgut (Fig 6A), and RT-qPCR showed that this coincided with the induction of the JNK target puc, all three Upd cytokines, the Stat target Socs36E, and delta (Fig 6B). Temporal analysis (not shown) indicated that these genes were appreciably induced by 2h after infection, plateaued by 8h, and that the mitotic response began within 4h. The locations of JNK activation and cytokine induction were assessed using reporter genes. The JNK reporter, puclacZE69, was expressed at low levels in scattered ECs prior to infection and induced to high levels in most ECs after infection (Fig 6E,F). UpdlacZ was not detected prior to infection, and was induced in small esg+ progenitor cells (ISCs/EBs) and slightly larger early ECs (pre-ECs) after infection (Fig 6G, H; see also Fig 3B-D). Upd3Gal4-driven GFP (Agaisse et al., 2003) was found in a few scattered ECs in controls, but was highly induced in almost all ECs after infection (Fig 6I, J). The 10XSTAT-DGFP Stat reporter was heavily induced by Pe, in both small and large cell types (Fig 6K, L). Since these cells turned over rapidly (Fig 7), however, some or all of the DGFP observed in ECs could have been inherited from progenitors.
Figure 6. Pe ingestion induces ISC proliferation.
(A) Stat92E, Dome, or Notch were depleted from progenitor cells by UAS-RNAi using esgts, 2d prior to Pe infection. hep1 is a hypomorphic JNKK allele. JNK signaling was also downregulated in ECs by expressing UAS-Puc using MyoIAts. Midguts were scored for PH3+ mitotic figures 2d after infection (green). “Mock” indicates control animals shifted to food without Pe.
(B) Midgut mRNAs measured in WT by RT-qPCR, 2d after Pe infection (red) or in controls (green). The data were normalized to day 0 (before infection) samples.
(C) Midgut mRNAs measured in MyoIAts>Puc animals by RT-qPCR, 2d after Pe infection. The data were normalized to mock infected controls.
(D) Quantification of Delta+ ISCs, MyoIA>GFP+ ECs and pros+ EEs during gut regeneration induced by Pe.
(E-L) Reporter expression in midguts 2 days after mock (E, G, I and K) or Pe (F, H, J and L) infection.
(E, F) A low level of the JNK reporter, puclacZ, was observed in ECs and EEs (usually paired, arrowheads) in controls (E). Widespread puclacZ after Pe infection (F). puclacZ was not expressed in progenitor cells (arrows).
(G, H) updlacZ was not expressed in controls (G), but was induced in progenitor cells or pre-ECs after Pe infection (H).
(I, J) upd3Gal4-driven GFP in controls (I), or after Pe infection (J).
(K, L) The Stat reporter, 10XSTAT-DGFP, was normally confined to progenitor cells (K), but was present in many cells after Pe infection (L). Blue: DNA in all panels.
Figure 7. Midgut renewal after Pe infection.
Midgut turnover in 3-10d old adult males, measured using the esgtsF/O system.
(A) Gut turnover in a control after 2d. Progenitor cells and their newborn progeny are marked with GFP.
(B) UAS-HopTumL expressed with the esgtsF/O system in progenitor cells for 2 days. These midguts contained many GFP+ cells, including large newborn ECs (arrows).
(C) E2F expressed with the esgtsF/O system caused progenitor cell accumulation, but not EC hyperplasia.
(D) Activated Notch (Nintra) promoted the rapid differentiation of progenitor cells into ECs (arrows), depleting the midgut of progenitors.
(E-J) Males of the indicated genotypes were shifted to 29°C for 2d, fed Pe for 2d, and treated with antibiotics for 2d. “Mock” infected controls received no Pe, but were aged identically and received antibiotics. Brackets and dotted lines indicate the posterior midgut. P indicates the posterior end. Asterisks (E, I) indicate induction-independent GFP in the anterior midgut. Midguts were stained for PH3 (red). Pe infection caused almost complete midgut renewal (F), whereas few new ECs were generated in controls (E). Pe-induced cell turnover was inhibited by Stat92E RNAi expressed under esgtsF/O control (G, H). These midguts (H) were shrunken by EC depletion and condensation of the remaining non-mitotic progenitor cells (H’). When Notch RNAi was expressed, Pe infection resulted in midguts comprised mostly of small non-differentiating, proliferative progenitor cells (J, J’).
(K) Survival after Pe infection. 50 flies (25 males, 25 females) were scored for survival after 2d of Pe infection (day 0) followed by another 2d of antibiotic treatment. Flies were then transferred to normal food. Controls (Mock or GFP RNAi with Pe infection) survived after antibiotic treatment, but flies depleted of N or Stat92E died rapidly.
(L) Model for midgut homeostasis.
As in the other cases of midgut regeneration described above, Delta expression and Notch signaling were increased by Pe (Figs 6B, S4), and there were small increases in the numbers of MyoIA+ ECs, pros+ EEs, and Delta+ progenitors (Fig 6D). The relative proportions of these cell types remained essentially normal (Fig 6D, Table S2). To determine the identity of mitotic cells following Pe-infection we scored PH3+ mitotic cells for the ISC marker Delta, the EE marker prospero, and the Notch reporter GbeSu(H)lacZ, an early marker of EC differentiation (Table S2). Most (85%) mitotic cells expressed high levels of Delta, just as in WT (Ohlstein and Spradling, 2007), and all PH3+ cells were negative for GbeSu(H)lacZ and pros (Table S2). This suggests that EE and EB cells do not de-differentiate and re-enter the cell cycle. The expression of GbeSu(H)lacZ and Delta were also mutually exclusive (Table S2), indicating normal Delta/Notch signaling. Clonal analysis showed that after infection there were generally only one or two Delta+ cells/clone, as in controls (Fig S6). Newly generated EEs and ECs occurred at the normal ratio of ∼1:9 (Table S2). These observations all indicate that the ISC lineage and differentiation program are normal in midguts regenerating from Pe infection.
To test whether ISC mitoses induced by Pe required Jak/Stat signaling, we expressed RNAi against either stat92E or Dome in progenitor cells using esgts, and then fed the flies Pe. The mitotic response to infection was entirely suppressed in these animals (Figs 6A, 7H’), indicating that Jak/Stat signaling is required. Pe did, however, induce mitosis in JNK-defective hep1 mutants. Consistently, suppressing JNK in ECs, using MyoIAts to drive Puc or BskDN, also had no detectable affect on ISC mitoses induced by Pe (Fig 6A, not shown), or on the induction of the Upds (Fig 6C). We infer that JNK signaling is not required for ISC activation in response to Pe, but that Jak/Stat signaling is.
Enteric infection drives rapid gut epithelial turnover
We expected the combination of increased EC death and ISC division following Pe infection to result in faster turnover of the gut epithelium. To test this we devised a method to mark all progenitor cells at a specific timepoint with a heritable marker. In this method, which we refer to as esgts Flp-Out (esgtsF/O), UAS-Flp recombinase is induced in progenitor cells by temperature shift using esgGal4ts. Flp excises the CD2 cassette from Act>CD2>Gal4 (or tub>CD2>Gal4), converting it to the ubiquitously expressed heritable driver, ActGal4 (or tubGal4). This marks ISCs and their progeny with Gal4-driven GFP and absence of CD2. The esgtsF/O system proved to be reliable for measuring epithelial turnover in the posterior (but not anterior) midgut. In normally fed adult females, the posterior midgut epithelium renewed itself within about 12 days of temperature shift (Fig S5A-C). In males, significant numbers of newborn GFP+ cells were not observed until ∼3 weeks after inducing Flp (not shown).
Using the esgtsF/O system in males we found that gut renewal was greatly accelerated in the gain-of-function Jak mutant, hopTumL (Fig S5D, E). Similarly, inducing UAS-HopTumL using the esgts F/O system generated many new epithelial cells within 2d, causing hyperplasia (Fig 7B). Consistent with the role of Notch in differentiation, inducing a transcriptionally active intracellular form of Notch (Nintra) with esgtsF/O promoted the rapid differentiation progenitor cells into ECs, depleting the gut of progenitor cells (Fig 7D). We also used esgtsF/O to overexpress the E2F/DP transcription factor, which specifically promotes cell cycle progression. E2F greatly increased the number of small progenitor cells, but did not increase new, GFP-marked ECs (Fig 7C). Thus rates of ISC proliferation and EB differentiation are separable parameters that are likely to be independently regulated.
We further tested the function of Jak/Stat signaling in midgut turnover by combining the esgtsF/O system with Pe infection. First, Stat92E was depleted using RNAi expressed in progenitor cells and their progeny for 2 days, and then the flies (males) were fed Pe for 2 days to generate an enteric infection. These flies were then transferred to food lacking Pe and containing antibiotics (which rescued them from death by infection) for another 2 days. While the midgut epithelium in mock-infected controls did not turn over significantly during this 6-day experiment (Fig 7E), Pe infection induced a virtually complete midgut renewal (Fig 7F). In midguts depleted of Stat92E, however, there was little if any renewal (Fig 7G, H). Instead the midgut lost most of its resident ECs and shrank to a small disorganized structure composed mostly of small non-dividing cells (Figs 7H, H’, 6A). Similarly, Pe infection failed to induce gut renewal in hop25 mutants (Fig S5F, G). Moreover, controls infected with Pe and then cured with antibiotics survived, whereas transient infection was lethal to flies expressing Stat92E RNAi (Fig 7K). Thus Stat signaling is essential for midgut regeneration in response to infection.
We used the same strategy to assess the role of Notch signaling in midgut renewal after Pe infection. When Notch RNAi was expressed in progenitor cells and the flies were infected with Pe, mitotic indices were much higher than in controls (Fig 6A), and the midgut became populated almost entirely with small proliferative progenitor cells (Fig 7J). Thus Notch signaling appears not to be required for ISC mitoses in response to infection, though it is still required for differentiation. As with Stat depletion, animals depleted of Notch in progenitor cells failed to survive after Pe (Fig 7K).
DISCUSSION
The Drosophila midgut is homeostatic
Rates of cell turnover in the intestine are likely to be in constant flux in response to varying stress from digestive acids and enzymes, chemical and mechanical damage, and toxins produced by both commensal and infectious enteric microbiota. As we show here, feedback from differentiated cells in the gut epithelium to stem- and progenitor cells is a key feature of this system. Genetically directed enterocyte ablation, JNK-mediated stress signaling, or enteric infection with Pseudomonas entomophila all disrupt the Drosophila midgut epithelium and induce compensatory ISC division and differentiation, allowing a compromised intestine to rapidly regenerate. Other recent reports note a similar regenerative response following three additional types of stress: detergent (DSS)-induced damage (Amcheslavsky et al., 2009), oxidative stress by paraquat (Biteau et al., 2008), and enteric infection with another less pathogenic bacterium, Erwinia carotovora (Buchon et al., 2009). Remarkably, the fly midgut can recover not only from damage, but also from severe induced hyperplasia, such as caused by ectopic cytokine (Upd) production (Fig 2F-H). Thus this system is robustly homeostatic.
Each of the three stress conditions we studied induced all three Upd cytokines, and genetic tests showed that Upd/Jak/Stat signaling was both required and sufficient for compensatory ISC division and gut renewal (Figs 2L, 6A, 7H, S5F). Although JNK signaling was also activated in each instance, it was not required for the stem cell response to either EC apoptosis or infection (Figs 2K, 6A, C), implying that other mechanisms can sense EC loss and trigger the cytokine and proliferative responses. JNK signaling may be important in specific contexts that we did not test, such as following oxidative stress, which occurs during some infections (Ha et al., 2005), activates JNK, and stimulates midgut DNA replication (Biteau et al., 2008).
The stem cell lineage during regeneration
Following Pe infection virtually the entire midgut epithelium could be renewed in just 2-3 days (Fig 7), whereas comparable renewal took more than 3 weeks in healthy flies. Despite this radical acceleration of cell turnover the relative proportions of the different gut cell types generated (ISC, EB, EE, EC; Figs 6D, S6; Table S2) remained similar to those in midguts undergoing slow, basal turnover. Our data (Table S2) suggested that de-differentiation did not occur, and we obtained little evidence of symmetric stem divisions (stem cell duplication) induced by enteric infection (Fig S6). Hence we suggest that asymmetric stem cell divisions as described for healthy animals (Ohlstein and Spradling, 2006), together with normal Delta/Notch-mediated differentiation, remain the rule during infection-induced regeneration. The results we obtained using Reaper to ablate ECs are also consistent with this conclusion, as are those from detergent-induced midgut regeneration (Amcheslavsky et al., 2009).
Unlike infection, direct genetic activation of JNK or Jak/Stat signaling promoted large increases not only in midgut mitoses, but also in the pool of cells expressing the stem cell marker Delta (Figs 2, 5B). Cell type marker analysis discounted de-differentiation of EEs or ECs as the source of the new stem cells, but the re-activation of EBs as stem cells seems possible. For technical reasons we did not test whether stem cell duplications occur in response to Jak/Stat or JNK signaling, and this also remains possible. The ability of hyperplastic midguts to recover to normal following the silencing of cytokine expression (Fig 2F-H), suggests that excess stem cells are just as readily eliminated as they are generated. Further studies are required to understand how midgut stem cell pools can be expanded and contracted according to need.
How are the Upd cytokines induced?
How the Upds are induced in the midgut by JNK, apoptosis, or infection remains an open question. Paradoxically, ISC divisions triggered by Reaper required EC apoptosis but not JNK activity, whereas ISC divisions triggered by JNK did not require apoptosis (Fig 2K) , and ISC divisions triggered by infection required neither apoptosis (not shown) nor JNK activity (Fig 6A, C). These incongruent results suggest that different varieties of gut epithelial stress may induce Upd cytokine expression via distinct mechanisms. In the case of EC ablation, physical loss of cells from the epithelium might drive the cytokine response. In the case of infection, we expected the critical inputs to be the Toll and/or IMD innate immunity pathways, which signal via NF-κB transcription factors (Ryu et al., 2008). Functional tests, however, indicated that the Toll and IMD pathways are required for neither Upd/Jak/Stat induction nor compensatory ISC mitoses following enteric infection by gram- bacteria (data not shown and (Buchon et al., 2009). Hence other unknown inputs likely trigger the Upd cytokine response to infection.
Is the cytokine response to infection relevant to normal midgut homeostasis? This seems likely. We observed low levels of Upd3 expression and Stat signaling in healthy animals (Fig 6I,K), and midgut homeostasis required the IL-6R-like receptor Dome and Stat92E even without infection (Fig 4). Wild Drosophila subsist on a diet of rotting fruit, a good source of protein because it is teeming with bacteria and fungi. Given such a diet it seems likely that midgut cytokine signaling is constantly modulated by ever-present factors that impose dietary stress — food composition and commensal micro biota — even in healthy animals.
Jak/Stat in mammalian intestinal homeostasis and cancer
Although studies in mammals have yet to unravel the details of a feedback mechanism underlying gut homeostasis, experimental evidence implies that such a mechanism exists and involves Cytokine/Jak/Stat signaling. As in Drosophila, damage to the mouse intestinal epithelium caused by detergents or infection can stimulate cell proliferation in the crypts, where stem and transient amplifying cells reside (McConnell et al., 2008; Rigby et al., 2007). In a mouse model of detergent(DSS)-induced colitis (Karin, 2008), colon epithelial damage caused by DSS allows exposure to commensal microbes, activating NF-κB signaling in resident macrophage-like Dentritic cells. These cells respond by expressing inflammation-associated cytokines, one of which (IL-6), activates Stat3 and is believed to promote cell proliferation and regeneration. Consistent with a functional role for Jak/Stat, disruption of the Stat inhibitor SOCS3 in the mouse gut increased the proliferative response to DSS, and also increased DSS-associated colon tumorigenesis (Rigby et al., 2007). Also pertinent is the presence of high levels of phospho-Stat3 in a majority of colon cancers, where it correlates with adverse outcome (Kusaba et al., 2005), and the observation that IL-6 can promote the growth of colon cancer cells, which are thought to derive from ISCs or transient amplifying cells (Becker et al., 2005; Schneider et al., 2000). Increased colon cancer incidence is associated with gut inflammatory syndromes, such as inflammatory bowel disease (IBD) and Crohn’s disease (Freeman, 2008), which are likely to involve enhanced cytokine signaling. Whether cytokines mediate gut epithelial turnover in healthy people or only during inflammation is presently unclear, but it nevertheless seems likely that the mitogenic role of IL-6-like cytokines and Jak/Stat signaling in the intestine is conserved from insects to man.
The connection to inflammation suggests that our findings may also be relevant to the activity of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, and celecoxib as suppressors of colorectal carcinogenesis. These drugs target the cyclooxygenase activity of prostaglandin H synthases (PGHS, COX), which are rate-limiting for production of prostaglandin E2, a short range lipid signal that promotes inflammation, wound healing, cell invasion, angiogenesis and proliferation (Cha and DuBois, 2007; Gupta and Dubois, 2001). Notably, COX-2 has been characterized as an immediate early gene that can be induced by signals associated with infection and inflammation, including the pro-inflammatory cytokines IL-1β and IL-6, which activate NF-κB and STAT3 respectively (Chun and Surh, 2004; Rummel et al., 2006; Xuan et al., 2003). Whether prostaglandins mediate the effects of Jak/Stat signaling in the fly midgut remains to be tested, but insects do produce prostaglandins and Drosophila has a functional COX homolog, pxt, whose activity can be suppressed by NSAIDs (Tootle and Spradling, 2008).
EXPERIMENTAL PROCEDURES
Genetics
See Supplemental Methods.
Histology
After dissection and fixation midguts were stained with mouse monoclonal anti-Delta (1:100) or anti-Prospero (1:100) (Developmental Studies Hybridoma Bank); rabbit polyclonal anti-phosphoSer10-histone 3 (1:4000) (Upstate Biotechnology), or rabbit polyclonal anti-β-galactosidase (1:2000) (Cappel), anti-STAT92E (1:250, S. Hou). For BrdU incorporation midguts were dissected in Ringer’s soln and incubated with 100μg/ml BrdU for 30 minutes in Schneider’s medium. Midguts were then fixed, treated with 3M HCl and stained with anti-BrdU (1:100, Becton Dickinson). Samples were analyzed on a Nikon Eclipse Ti or a Zeiss LSM510 confocal microscope.
Cell counts
Mitotic indices were quantified by counting PH3+ cells in 10 midguts of the proper genotype (5 females and 5 males unless noted otherwise). For quantification of gut hyperplasia induced by JNK, Jak/Stat or Pe, cells were counted in a defined posterior midgut region between the hindgut and the copper cells, and the values were corrected for changes in the area of this region.
Gut turnover analysis
UAS-transgenes were crossed to an esgtsF/O tester: w; esgGal4 tubGal80ts UAS-GFP; UAS-flp Act>CD2>Gal4. 3-10d old male progeny (raised at 18°C) were shifted to 29°C for 2d and then midguts were dissected and analyzed. For RNAi experiments 3-10d old adult males (raised at 18°C) were shifted to 29°C for 2d before being transferred to fly food laced with either ½ ml of 10X concentrated overnight Pe culture or ½ ml 5% sucrose (mock infection). After 2d the flies were transferred to food containing antibiotics (100μg/ml Ampicilin, 50μg/ml Vancomycin, 100μg/ml Neomycin and 100μg/ml Metronidazole) for an additional 2d before being dissected and analyzed. See Supplemental Methods for additional detail.
RT-qPCR
RNA was extracted from 10 midguts (5 males/5 females) using TRIzol (Invitrogen). RNA was cleaned using RNAeasy (Qiagen) and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). qPCR was performed using the iScript one step RT-PCR SYBR green kit (Bio-Rad). Data were acquired using an iQ5™ System (Bio-Rad). Primer sequences are listed in Supplemental Materials. RT-qPCR was performed in duplicate, and all results are presented with means and STDEV from 3 independent biological samples. We used RpL11 as a normalization control.
Supplementary Material
ACKNOWLEDGEMENTS
We thank G. Schubiger, C. O’Cane, N. Reich, P. Hagan, D. Montell, G. Baeg, P. O’Farrell, M. Van Doren, E. Bach, K. Choi, S. Hou, H. Sun, K. Smith, VDRC, NIG, and the Bloomington DSC for reagents. We thank C. M. Ulrich for comments on the manuscript. Supported by the UW/FHCRC Cancer Consortium and NIH R01 GM51186 to B.A.E. P.H.P. was an American Cancer Society Fellow (PF-08-040-01-DDC). A.K. was a Human Frontiers in Science Program Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–450. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- Chao JL, Tsai YC, Chiu SJ, Sun YH. Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development. 2004;131:3839–3847. doi: 10.1242/dev.01258. [DOI] [PubMed] [Google Scholar]
- Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Freeman HJ. Colorectal cancer risk in Crohn’s disease. World J Gastroenterol. 2008;14:1810–1811. doi: 10.3748/wjg.14.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M, Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Gilbert MM, Weaver BK, Gergen JP, Reich NC. A novel functional activator of the Drosophila JAK/STAT pathway, unpaired2, is revealed by an in vivo reporter of pathway activation. Mech Dev. 2005;122:939–948. doi: 10.1016/j.mod.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, Lee WJ. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Hakim RS, Baldwin KM, Loeb M. The role of stem cells in midgut growth and regeneration. In Vitro Cell Dev Biol Anim. 2001;37:338–342. doi: 10.1007/BF02577567. [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar B. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2008 doi: 10.1242/dev.026955. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Miura M. Regulatory roles of JNK in programmed cell death. J Biochem. 2004;136:1–6. doi: 10.1093/jb/mvh098. [DOI] [PubMed] [Google Scholar]
- Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Nagayasu T, Sekine I. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol. 2005;58:833–838. doi: 10.1136/jcp.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb MJ, Martin PA, Narang N, Hakim RS, Goto S, Takeda M. Control of life, death, and differentiation in cultured midgut cells of the lepidopteran, Heliothis virescens. In Vitro Cell Dev Biol Anim. 2001;37:348–352. doi: 10.1007/BF02577569. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Klapproth JM, Sasaki M, Nandan MO, Yang VW. Kruppel-like factor 5 mediates transmissible murine colonic hyperplasia caused by Citrobacter rodentium infection. Gastroenterology. 2008;134:1007–1016. doi: 10.1053/j.gastro.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morgan NS, Skovronsky DM, Artavanis-Tsakonas S, Mooseker MS. The molecular cloning and characterization of Drosophila melanogaster myosin-IA and myosin-IB. J Mol Biol. 1994;239:347–356. doi: 10.1006/jmbi.1994.1376. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, et al. Deletion of Apoptosis Signal-Regulating Kinase 1 Attenuates Acetaminophen-Induced Liver Injury by Inhibiting c-Jun N-Terminal Kinase Activation. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48:380–381. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1316–1326. doi: 10.1152/ajpregu.00301.2006. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett. 2000;151:31–38. doi: 10.1016/s0304-3835(99)00401-2. [DOI] [PubMed] [Google Scholar]
- Tootle TL, Spradling AC. Drosophila Pxt: a cyclooxygenase-like facilitator of follicle maturation. Development. 2008;135:839–847. doi: 10.1242/dev.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, Boccard F, Lemaitre B. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proceedings of the National Academy of Sciences. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–537. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.