Abstract
Objective
To develop an evidence base for recommendations on the use of atypical antipsychotics for patients with schizophrenia.
Design
Systematic overview and meta-regression analyses of randomised controlled trials, as a basis for formal development of guidelines.
Subjects
12 649 patients in 52 randomised trials comparing atypical antipsychotics (amisulpride, clozapine, olanzapine, quetiapine, risperidone, and sertindole) with conventional antipsychotics (usually haloperidol or chlorpromazine) or alternative atypical antipsychotics.
Main outcome measures
Overall symptom scores. Rate of drop out (as a proxy for tolerability) and of side effects, notably extrapyramidal side effects.
Results
For both symptom reduction and drop out, there was substantial heterogeneity between the results of trials, including those evaluating the same atypical antipsychotic and comparator drugs. Meta-regression suggested that dose of conventional antipsychotic explained the heterogeneity. When the dose was ⩽12 mg/day of haloperidol (or equivalent), atypical antipsychotics had no benefits in terms of efficacy or overall tolerability, but they still caused fewer extrapyramidal side effects.
Conclusions
There is no clear evidence that atypical antipsychotics are more effective or are better tolerated than conventional antipsychotics. Conventional antipsychotics should usually be used in the initial treatment of an episode of schizophrenia unless the patient has previously not responded to these drugs or has unacceptable extrapyramidal side effects.
Introduction
The most pressing clinical uncertainty arising from recent advances in the management of schizophrenia1 is the role of atypical antipsychotics. The term “atypical” was originally used to describe drugs that in animal models predict antipsychotic effects but do not produce catalepsy — most notably clozapine. It is also applied to drugs that are potentially more effective (particularly against depressive, negative, or cognitive symptoms) or better tolerated (especially causing fewer extrapyramidal side effects) than conventional antipsychotics or have a different pharmacological profile (such as blockade of serotonin 5-HT2 receptors). No definition is wholly satisfactory, partly because the term atypical is relative rather than absolute. We use the term simply to refer to clozapine and all the novel antipsychotics introduced in the past decade.
We conducted a systematic review of the effectiveness and tolerability of atypical versus conventional antipsychotics in the treatment of schizophrenia to inform the development of a clinical practice guideline. The primary outcomes we investigated were control of psychotic symptoms and overall acceptability, although we also looked at the possibility of studying outcomes such as quality of life and rates of specific adverse effects. We decided beforehand to examine the influence of the dose of the conventional drug, because common side effects (such as extrapyramidal side effects and sedation) are dose related, whereas efficacy reaches a plateau.2 The recommended optimal dose is 6-12 mg/day haloperidol or its equivalent,3 although higher doses are still commonly used.4 Evaluation of the relative efficacy and tolerability of conventional and atypical antipsychotics must, therefore, take into account the comparator dose.
Systematic reviews of individual atypical antipsychotic drugs (clozapine,5 olanzapine,6,7 quetiapine,7,8 risperidone,7,9,10 and sertindole7) exist but were either unavailable or out of date at the time we were developing the guideline. Furthermore, they do not formally assess the effect of dose or allow evaluation of atypical antipsychotics as a group.
Methods
Inclusion criteria
We included randomised trials of atypical antipsychotics (amisulpride, clozapine, olanzapine, quetiapine, risperidone, and sertindole) against conventional antipsychotics or alternative atypical antipsychotics in patients with schizophrenia or related disorders for which data on efficacy or drop out were available.
Search strategy
We used optimally sensitive search strategies, based on a combination of text and index terms of Medline, Embase, PsychLIT, and the Cochrane Controlled Trial Register to locate randomised trials comparing the effectiveness of atypical and conventional antipsychotic drugs in the treatment of schizophrenia and related disorders (further details of the search strategies and the trials included are available on the BMJ 's website). Searches were limited to compounds licensed in the United Kingdom. The expert knowledge and experience of group members was used to augment the findings of the search strategies, and we requested information on all comparative trials of the atypical drugs from the relevant pharmaceutical companies. We included identified trials up to a cut off date of 1 December 1998.
For each trial, we identified the inclusion and exclusion criteria, length of follow up, main outcome measures, and patient characteristics. Data on overall symptom scores (brief psychiatric rating scale or positive and negative syndrome scale (box)), quality of life, drop out, side effects, and costs were collected. We appraised the quality of each study by assessing features empirically associated with bias, including concealment of allocation,11 loss to follow up, and level of blinding (open, single, or double).12 If data on main outcomes were missing or trials were unpublished, we requested data from the authors and sponsors of trials and sent reminders after one month. In dose ranging studies, only data on doses of atypical antipsychotic drugs within the licensed therapeutic ranges were included.
Scales of symptom reduction commonly used in randomised trials
Brief psychiatric rating scale13
16 item, 7 point severity scale; 5 positive, 2 negative, and 9 general symptom items
Completed by clinician
Range of possible scores 16-112
Patients with schizophrenia typically score about 33 at entry to trialw30
Positive and negative syndrome scale14 (derived from brief psychiatric rating scale)
30 item, 7 point severity scale; 7 positive, 7 negative, and 16 general psychopathology symptom items
Completed by clinician
Range of possible scores 30-210 (positive and negative symptom groups are often reported separately; both score 7-49)
Patients with schizophrenia typically score about 91 at entry to trialw6 w31
Statistical analyses
For the primary analyses of continuous outcome measures, we calculated standardised effect sizes.15 The effect size is a measure of the overlap in the distributions of scores on the outcome between the two treatment groups; we represent this in the results as the percentage of patients treated with comparator drug who did less well than the average of the group given an atypical antipsychotic.
The primary method of analysis was a fixed effects model, by the methods of Smith et al.16 Fixed effects approaches assume a single underlying treatment effect across the studies, but systematic differences may exist in treatment effects (heterogeneity). Random effects approaches to meta-analyses have been developed which take the heterogeneity of treatment effects into account.16,17 The random effects model allows for heterogeneity in both the estimate of treatment effect and the width of the confidence intervals. As heterogeneity reduces, the random effects approach moves asymptotically towards a fixed effects model. An important advantage of the methods of Smith et al is that this “full random effects method” takes into account not just observed heterogeneity, as is the case with standard methods, but also sampling error in the observed differences.16 Thus, heterogeneity and its uncertainty is represented by the width of the confidence intervals for the random effects estimates.
When important heterogeneity was identified, we formally investigated its causes using meta-regression.16,18 In particular, we used the method to test our hypothesis about the effect of the dose of comparator antipsychotic. We examined the predictive value of the dose of haloperidol (or chlorpromazine) on outcome, modelling the coefficient describing the predictive value of haloperidol and the overall treatment effect on outcome, using fixed effects because of the small number of trials contributing to the analysis.16 We also examined the potential importance of the choice of atypical antipsychotic drug.
Results
We identified 52 trials, including 12 649 patients, meeting the inclusion criteria. Most were short term (median follow up 6.5 weeks), although five trials provided follow up data for one year or more (see tables 1–21 on BMJ 's website).w1-w6 Most trials compared the effectiveness of atypical antipsychotics with haloperidol. Occasionally, chlorpromazine was also used, and flupenthixol, perphenazine, and zuclopenthixol were the comparators in one trial each. There was substantial drop out in most trials from each group. This made interpretation of the commonly used “last observation carried forward” analyses problematic as it introduced uncertainty about the actual clinical state of the patients after they left the study.
The results presented for individual drugs depend on the data available from the trials and mainly concern reduction in symptom score and drop out (see table 22 on BMJ 's website). For trials that detected a significant effect we also give an estimate of the magnitude of the effect in terms of points on the brief psychiatric rating scale. Measurement of secondary outcomes (such as extrapyramidal side effects) was not standardised between studies and so they are reported separately in the text. There were few data on quality of life, specific side effects, or cost effectiveness, and we have therefore not included these outcomes in this report.
Amisulpride
We identified four short term trials examining the effectiveness of amisulpride compared with haloperidolw7-w9 and flupenthixol.w10 The standardised weighted mean difference was −0.35 (95% confidence interval −0.52 to −0.18) in favour of amisulpride, indicating that about 64% of patients given a conventional antipsychotic had higher (worse) symptom scores after treatment than the average patient treated with amisulpride. The fixed pooled odds ratio for drop out was 0.55 (0.38 to 0.79) and the random effects pooled odds ratio was 0.54 (0.33 to 0.85). The standardised weighted mean difference estimate of the effect on the Simpson Angus scale (a scale measuring extrapyramidal side effects (range 0-4) was −0.44 (−0.26 to −0.61).
Clozapine
We identified 12 trials providing data on efficacyw4-w6 w11-w18 and 20 trials providing data on tolerability.w4 w11-w27 Two long term trials were identified.w4-w6 Ten trials compared clozapine with haloperidolw6 w11 w13 w14 w17 w18 w20-w24 and 10 with chlorpromazine.w12 w13 w15 w16 w19 w22 w25-w27 One trial compared clozapine with an individually based choice of conventional antipsychotic.w4 w5 Several trials focused on treatment resistant schizophrenia,w4-w6 w12 w13 w15-w17 one of which included children and adolescents.w17 The standardised weighted mean difference for the overall symptom score in short term trials was −0.68 (−0.82 to −0.55) in favour of clozapine, signifying that about 75% of patients given a conventional antipsychotic had higher symptom scores after treatment than the average patient treated with clozapine. Patients allocated to clozapine were less likely to drop out (odds ratio 0.52 (0.40 to 0.67) with fixed effects model), although the odds ratio became non-significant with the random effects model (0.69 (0.45 to 1.19)).
The two long term trials gave a less consistent picture.w4-w6 Both were conducted in the United States on patients with treatment resistant schizophrenia. In the trial by Rosenheck et al the comparator was haloperidol (mean (SD) 28 (5.3) mg daily, maximum 30 mg).w6 In the trial by Essock et al the comparator was an alternative antipsychotic chosen by the clinician at mean 1386 mg/day chlorpromazine equivalent.w4 w5 Rosenheck et al described a benefit of about 6.9 points on the positive and negative syndrome scale for clozapine compared with haloperidol, whereas Essock et al reported a disadvantage of about 2.7 points on the brief psychiatric rating scale for clozapine.
Olanzapine
We identified four short term trials comparing olanzapine with haloperidolw28-w30 or chlorpromazine.w31 The standardised weighted mean difference was −0.22 (−0.30 to −0.14) in favour of olanzapine, indicating that about 59% of patients taking a conventional antipsychotic had higher symptom scores after treatment than the average patient taking olanzapine. The pooled odds ratio for drop out was 0.52 (0.44 to 0.61) with the fixed effects model and 0.63 (0.40 to 1.17) with the random effect model.
There was a 4.8% (3.1% to 6.5%) reduction in dystonia and a 14.1% (11.0% to 17.2%) reduction in akathisia with olanzapine. Olanzapine was associated with a 12% (8% to 15%) increase in excessive appetite compared with haloperidol.w29
Quetiapine
Two short term trials compared quetiapine with chlorpromazinew32 or haloperidol.w33 There was no difference in the overall symptom score between quetiapine and the conventional antipsychotic (standardised weighted mean difference −0.03 (−0.23 to 0.18)), nor was there any reliable evidence of a reduced rate of drop out with quetiapine (odds ratio 0.70 (0.46 to 1.06)).
Risperidone
Six short termw34-w39 and two long term trialsw1 w2 comparing therapeutic doses of risperidone with a conventional antipsychotic provided data on overall symptom score. The short term trials all compared risperidone with various regimens of haloperidol. The two long term trials were naturalistic in design, comparing the decision to use risperidone with the decision to use a conventional antipsychotic chosen at the discretion of the psychiatrist.
In the short term trials, the fixed effects standardised weighted mean difference was −0.15 (−0.27 to −0.04) in favour of risperidone and the random effects standardised weighted mean difference was −0.16 (−0.47 to 0.16). We observed substantial heterogeneity between trials, reflected in the random effects estimate and its wide confidence intervals (which include the point of no difference between the treatments). Potential explanations for this heterogeneity include interactions between the use of risperidone and specific patient groups and variability in the comparators.
Data on dropout rates were provided by ten short term trials.w34-w43 The comparator antipsychotics were haloperidol,w34-w39 w42 w43 perphenazine,w40 and zuclopenthixol.w41 The fixed effects pooled odds ratio for drop out from risperidone was 0.59 (0.46 to 0.74), and the random effects odds ratio was 0.62 (0.31 to 1.34).
Benefits from risperidone were observed on the Parkinson, dyskinesia, and dystonia symptom scales. The standardised weighted mean difference was −0.39 (−0.51 to −0.27) for the Parkinson scale, −0.26 (−0.39 to −0.12) for the dystonia scale, and −0.16 (−0.28 to −0.04) for the dyskinesia scale.
In two long term, naturalistic, intention to treat trials, 840 patients were randomised to risperidone or conventional antipsychotics.w1 w2 Patients could switch between groups and between conventional antipsychotics at the discretion of the psychiatrist. In the study of Bouchard et al the mean daily comparator dose of conventional antipsychotics was a chlorpromazine equivalent of 1006 (SD 1348) mg daily, median 551 mg daily.w2 The mean dose of haloperidol used in the risperidone outcome of effectiveness (ROSE) trial was 16.1 (13.3) mg daily.w1 At 12 months, the pooled standardised weighted mean difference for the overall positive and negative syndrome scale score was −0.40 (−0.27 to −0.54), indicating that about 66% of patients treated with conventional antipsychotics had higher symptom scores after treatment than the average patient treated with risperidone.
Sertindole
Lundbeck voluntarily suspended marketing for sertindole in the United Kingdom on 2 December 1998 because of reports of cardiac arrhythmias and sudden death. This occurred during the late stages of the development of this clinical practice guideline. We identified four trials including 1549 patients comparing sertindole with haloperidol.w3 w44-w46 One trial had one year of randomised follow up.w3 The other trials lasted eight weeks. Doses of haloperidol ranged from 4 mg to 16 mg daily. The trial results were included in the meta-regression but are not given here because the drug is not currently available.
Effect on positive and negative symptoms
Overall, no evidence was identified to suggest that any individual atypical antipsychotic had a specific effect on either positive or negative symptoms. Instead, benefits seemed evenly spread between them (tables 1–21 on BMJ 's website).
Effect of dose of comparator antipsychotic (haloperidol or chlorpromazine) on outcome
The dose of haloperidol significantly affected outcome in the 23 trials in which it was used. Within the range of mean doses of haloperidol reported (about 6-22.5 mg daily), meta-regression identified a significant advantage for atypical antipsychotics as the dose of haloperidol increased; the coefficient describing the effect of dose was −0.021 (−0.003 to −0.038). The observed advantage in favour of the atypical drug disappeared as the dose of haloperidol decreased. A similar effect was seen for chlorpromazine, which was used in seven trials. Within the range of mean doses of chlorpromazine (about 375-1000 mg daily), meta-regression identified a significant advantage for atypical antipsychotics as the dose of chlorpromazine increased (−1.14 (−1.68 to −0.58)).
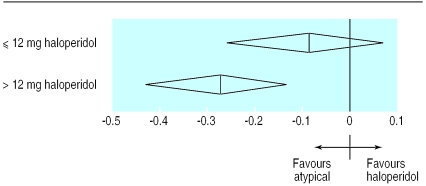
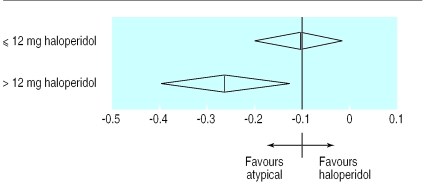
As a further method of examining the clinical implications of this finding, and based on previous recommendations that a dose of the equivalent of 6-12 mg of haloperidol is optimal,2 3 we compared trials using 12 mg or less of haloperidol with those that used a higher dose. In the trials in which the mean haloperidol dose was ⩽12mg/day, the random effects standardised weighted mean difference was −0.09 (−0.07 to −0.26), whereas in those with a mean haloperidol dose of >12 mg/day it was −0.28 (−0.13 to −0.44) (fig 1). There was no difference in dropout rates between atypical antipsychotics and haloperidol in the trials that used ⩽12 mg/day haloperidol (pooled risk difference was −0.1% (−4.6% to 4.4%) with the random effects model, but the pooled drop out in trials using >12 mg/day haloperidol was −8.3% (−1.3% to 15.2%) (fig 2). In other words, the advantages of atypical antipsychotics in terms of efficacy and dropout rates are not seen if haloperidol is used at doses of 12mg/day or less. These results from the meta-regression analysis were unaffected by the removal of trials including treatment resistant patients, those taking sertindole, or long term trials (data not shown).
Figure 1.
Overall symptom score by dose of comparator drug in trials of patients with schizophrenia or related disorders (standardised weighted mean difference and 95% confidence intervals)
Figure 2.
Drop out rates by dose of comparator drug in trials of patients with schizophrenia or related disorders (risk difference and 95% confidence intervals)
We examined whether the lower incidence of extrapyramidal side effects with atypical antipsychotics was dose related. The trials used a range of measures to describe side effects, making meta-analysis and meta-regression problematic. Two large trials describe the side effects experienced by patients receiving risperidonew39 or sertindolew46 compared with haloperidol at a fixed dose of 10mg daily. Peushens et al used a questionnaire based assessment tool to assess extrapyramidal side effects and reported a significant benefit for risperidone (4-16 mg) over haloperidol for dystonia (P=0.0004) and dyskinesia (P=0.0499).w39 In the trials of sertindole versus 10 mg haloperidol we noted a 16% (10% to 22%) reduction in the incidence of akathisia attributable to sertindole.w46 Thus, reduced extrapyramidal side effects remain apparent at lower doses of comparator drugs examined in the available trials.
Comparison between atypical antipsychotics
We found no difference in pooled efficacy in two trials comparing olanzapine and risperidone.w47 w48 Both trials showed olanzapine was better tolerated, equivalent to about a 7% (0.4% to 13.6%) difference in drop out. The two trials comparing risperidone and clozapine randomised a total of only 146 patients and had insufficient power to provide useful data.w49 w50 Indirect comparison in meta-regression models did not identify any individual atypical antipsychotic as more or less effective when dose of comparator drug was taken into account.
Discussion
Two main conclusions can be drawn from this review. Firstly, taking the trial results at face value, atypical antipsychotics are slightly more effective and better tolerated in patients with schizophrenia. Atypical antipsychotics also have a significantly lower risk of causing extrapyramidal side effects. We found no reliable evidence of differential effects between atypical antipsychotics and we have therefore grouped them together in this discussion. Secondly, when we controlled for the higher than recommended dose of conventional antipsychotics used in some trials, a modest advantage in favour of atypical antipsychotics in terms of extrapyramidal side effects remains, but the differences in efficacy and overall tolerability disappear, suggesting that many of the perceived benefits of atypical antipsychotics are really due to excessive doses of the comparator drug used in the trials. Taking these points into account, we think it inappropriate to advocate the first line use of a new drug without clear evidence of overall superior efficacy or tolerability.
However, for patients who do not response adequately to a standard dose of a conventional antipsychotic or experience severe extrapyramidal side effects it is appropriate to use atypical antipsychotics. Because conventional antipsychotics have limited effectiveness and tolerability, a large proportion of patients will, by this criterion, be prescribed atypical antipsychotics, often relatively early on in treatment. One of the most important results of our meta-regression is the confirmation that using conventional drugs at excessive doses reduces efficacy and increases adverse effects.2 Pharmacokinetic variability means that the optimal dose for individual patients will vary, but doses above 12 mg haloperidol a day (or equivalent) do not normally seem appropriate. This point may prove as important to the overall care of patients with schizophrenia as the intrinsic benefits of atypical antipsychotics.
Debate around our conclusion that a conventional antipsychotic should normally be prescribed first is likely to centre on two factors. The first is the negative consequences on patient compliance as a result of extrapyramidal side effects. In other words, if initial treatment produces worse side effects, then the patient may be unwilling to try a second drug. However, our analysis shows that patients taking atypical antipsychotics have no lower dropout rates and no better response than patients taking the optimal dose of conventional antipsychotics. This suggests that the lower risk of extrapyramidal side effects seen with the atypical antipsychotics may be counterbalanced by their greater propensity to cause other side effects and highlights the importance of early detection and treatment of adverse effects. A common side effect of atypical antipsychotic drugs is appreciable weight gain,19 and there are also rarer but serious side effects such as agranulocytosis with clozapine. In addition, concordance with treatment is probably not determined solely, or even primarily, by specific drug side effects, but by many factors, including those related to the disorder and the therapeutic relationship. Secondly, it may be that the lower incidence of extrapyramidal side effects translates into a lower long term risk of tardive dyskinesia.20 However, to date, the evidence on this issue is preliminary and inconclusive.21
Cost issues
Atypical antipsychotics are more expensive than the conventional drugs. Inevitably, therefore, cost has become part of the controversy concerning their prescribing. We believe that cost is not a crucial issue at present because the evidence and analyses described above indicate that the new drugs have no unequivocal advantages for first line use that would need to be set against their greater acquisition costs.
Potential publication bias
As in all systematic overviews, the results are only as good as the studies that contributed to the analyses. Publication is a major source of systematic bias in overviews, where trials with positive results are more likely to be published than those with neutral or negative results, especially if the trials are small. We went to considerable lengths to obtain data on trials that had not been published and trials that were incompletely reported. There is no way of estimating the magnitude of or potential for publication bias, although since the direction of such a bias would normally be in favour of the newer drugs, its existence would not undermine the results presented here.
Beyond the randomised evidence
This review shows that in several key areas, evidence is insufficient or absent. The trials have a median length of six weeks, yet antipsychotic drugs are often used for many years — and the conventional drugs are often used in depot form. The trials exclude a large proportion of patients who are treated with these drugs (including those with comorbid disorders). With the exception of extrapyramidal side effects, there is little consistent reporting of adverse events. There are few data on quality of life or clinically relevant functional outcomes and few reliable data on the cost effectiveness of atypical antipsychotics — none in the United Kingdom.
What is already known on this topic
Antipsychotic drugs have a central role in the treatment of schizophrenia
Newer, atypical antipsychotics are increasingly considered superior to conventional drugs
What this study adds
Atypical antipsychotics have a similar effect on symptoms to conventional antipsychotics at an average dose of ⩽12 mg haloperidol or equivalent
Atypical antipsychotics cause fewer extrapyramidal side effects, but overall tolerability is similar to conventional drugs
Conventional drugs should remain the first treatment, although atypical antipsychotic drugs are a valuable addition to treatment options, especially when extrapyramidal side effects are a problem
Longer term trials that concentrate on prevention of relapses rather than treatment of acute episodes do not yet provide a large or clear body of evidence on which to base recommendations. The case in favour of atypical antipsychotics may be strengthened once good, long term data are available on efficacy (especially in terms of other important outcomes such as reduction in suicide rates or improvement in cognitive functioning), tolerability, and safety. This review will need to be updated regularly as evidence in each of these areas emerges over the next few years.
Evidence and concordance
Implicit in the above considerations, as with all evidence based recommendations, is the importance of an informed relationship between doctor and patient in which treatment decisions can be based on the likely beneficial and adverse effects of atypical and conventional antipsychotics, patient preference, and clinical judgment. Given the equivocal nature of the evidence, deviations from these recommendations may, and should, occur. For example, antipsychotic drugs clearly have different side effect profiles. The broader choice of drugs now available increases the chance of finding a drug for an individual patient that is tolerated as well as effective and thus makes adherence more likely. In the near future it may also be possible to take predictors of treatment response, such as pharmacogenomic considerations, into account when deciding which antipsychotic to prescribe.
Supplementary Material
Acknowledgments
We thank the investigators and sponsors who provided unpublished information to aid our work. The work described in this paper was done as part of a programme of guideline development undertaken by the Royal College of Psychiatrists Research Unit and the Centre for Outcomes Research and Effectiveness of the British Psychological Society. The recommendations presented in this article are the views of the technical development group and should not be considered to represent the views of the Royal College of Psychiatrists, the British Psychological Society, or the National Institute of Clinical Excellence.
Editorial by Kapur and Remington
Footnotes
Funding: English Department of Health as part of a larger programme of schizophrenia management guidelines to be undertaken by the National Institute of Clinical Excellence.
Competing interests: JG, as director of the Centre for Evidence Based Mental Health, has run workshops around the United Kingdom, organised independently, but often sponsored by pharmaceutical companies. The centre has therefore indirectly received fees and expenses from several of the companies who manufacture antipsychotic drugs. NF has received funds for research, fees, and expenses from several pharmaceutical companies who manufacture antipsychotic drugs and from the Department of Health in England. PH has received support from pharmaceutical companies to attend conferences. He has also received fees for educational lectures to psychiatrists on the psychopharmcology of schizophrenia and on the work described in this paper. PB has received fees for presentations at meetings sponsored by various pharmaceutical companies who manufacture typical and atypical antipsychotics. In addition he is one of the lead investigators of the European schizophrenia cohort funded by Lundbeck.
Further data and members of the National Schizophrenia Guideline Development Group are available on the BMJ's website
References
- 1.Kane JM, McGlashan TH. Treatment of schizophrenia. Lancet. 1995;346:820–825. doi: 10.1016/s0140-6736(95)91630-x. [DOI] [PubMed] [Google Scholar]
- 2.Bollini P, Pampallona S, Orza MJ, Adams ME, Chalmers TC. Antipsychotic drugs: is more worse? A meta-analysis of the published randomized control trials. Psychol Med. 1994;24:307–316. doi: 10.1017/s003329170002729x. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Washington, DC: APA; 1997. [Google Scholar]
- 4.Lehman AF, Steinwachs DM. Patterns of usual care for schizophrenia: initial results from the schizophrenia patient outcomes research team (PORT) client survey. Schizophr Bull. 1998;24:11–20. doi: 10.1093/oxfordjournals.schbul.a033303. [DOI] [PubMed] [Google Scholar]
- 5.Wahlbeck K, Cheine M, Essali MA. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Clozapine versus typical neuroleptic medication for schizophrenia In: Cochrane Collaboration. [DOI] [PubMed] [Google Scholar]
- 6.Duggan L, Fenton M, Dardennes RM, El-Dosoky A, Indran S. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Olanzapine for schizophrenia In: Cochrane Collaboration. [Google Scholar]
- 7.Leucht S, Pitschel-Walz G, Abraham D, Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- 8.Srisurapanont M, Disayavanish C, Taimkaew K Cochrane Collaboration, editors. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Quetiapine for schizophrenia. [DOI] [PubMed] [Google Scholar]
- 9.Song F. Risperidone in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 1997;11:65–71. doi: 10.1177/026988119701100116. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy E, Song F, Hunter R, Clark A, Gilbody S Cochrane Collaboration, editors. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Risperidone versus typical antipsychotic medication for schizophrenia. [Google Scholar]
- 11.Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 12.Eccles M, Freemantle N, Mason JM. Methods of developing guidelines for efficient drug use in primary care: North of England evidence-based guidelines development project. BMJ. 1998;316:1232–1235. doi: 10.1136/bmj.316.7139.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- 14.Kay SR, Fiszbein S, Opler LA. The positive and negative syndrome scale for schizophrenia. Schizophrenia Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Hedges LV, Olkin I. Statistical methods for meta-analysis. London: Academic Press; 1985. [Google Scholar]
- 16.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14:2685–2699. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Sutton AJ, Jones DR, Abrams K, Sheldon TA, Song F. Systematic reviews of randomised trials. In: Black N, Brazier J, Fitzpatrick R, Reeves B, editors. Health Services Research Methods. London: BMJ; 1998. [Google Scholar]
- 19.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 20.Beasley CM, Dellva MA, Tamura RN, Morgenstern H, Glazer WM, Ferguson K, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry. 1999;74:23–30. doi: 10.1192/bjp.174.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Beasley CM. Olanzapine and tardive dyskinesia. Br J Psychiatry. 1999;175:392. doi: 10.1192/bjp.175.4.391b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.